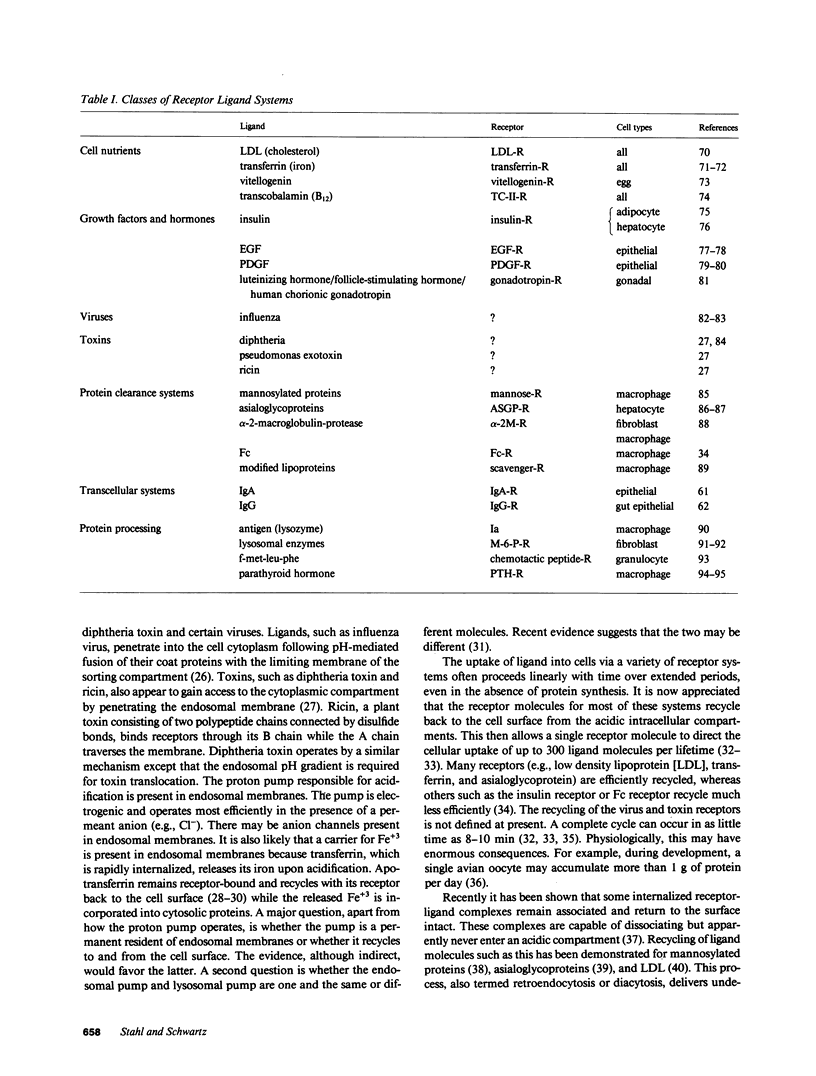
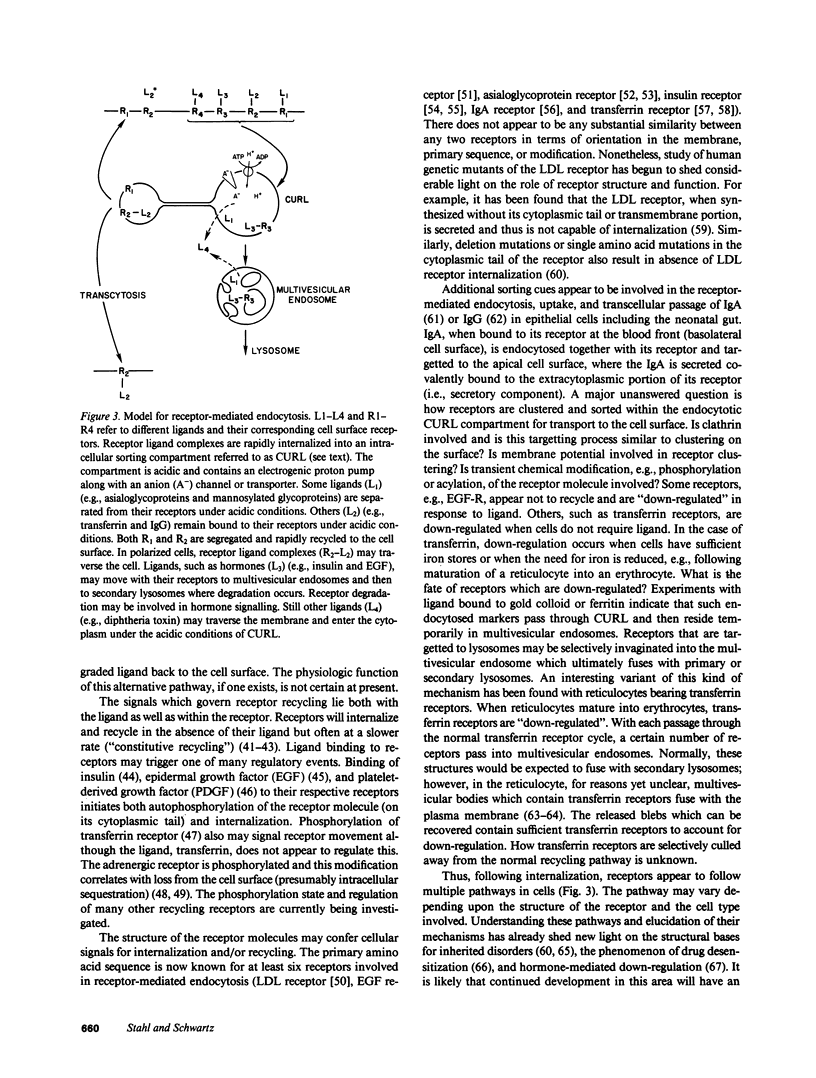
Full text
PDF


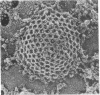
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Rodewald R. Evidence for the sorting of endocytic vesicle contents during the receptor-mediated transport of IgG across the newborn rat intestine. J Cell Biol. 1981 Oct;91(1):270–280. doi: 10.1083/jcb.91.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Anderson R. G., Brown M. S., Goldstein J. L. Inefficient internalization of receptor-bound low density lipoprotein in human carcinoma A-431 cells. J Cell Biol. 1981 Feb;88(2):441–452. doi: 10.1083/jcb.88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Brown M. S., Goldstein J. L. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977 Mar;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Falck J. R., Goldstein J. L., Brown M. S. Visualization of acidic organelles in intact cells by electron microscopy. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4838–4842. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. Localization of low density lipoprotein receptors on plasma membrane of normal human fibroblasts and their absence in cells from a familial hypercholesterolemia homozygote. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2434–2438. doi: 10.1073/pnas.73.7.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M., Puett D. Inhibition of the degradation of receptor-bound human choriogonadotropin by lysosomotropic agents, protease inhibitors, and metabolic inhibitors. J Biol Chem. 1978 Nov 10;253(21):7832–7838. [PubMed] [Google Scholar]
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Carpenter G. Properties of the receptor for epidermal growth factor. Cell. 1984 Jun;37(2):357–358. doi: 10.1016/0092-8674(84)90365-9. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Pearse B. M. Assembly and packing of clathrin into coats. J Cell Biol. 1981 Dec;91(3 Pt 1):790–797. doi: 10.1083/jcb.91.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. A., Roth T. F. Changes in specific sequestration of protein during transport into the developing oocyte of the chicken. Biochim Biophys Acta. 1973 Apr 16;298(4):951–955. doi: 10.1016/0005-2736(73)90398-2. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S. Platelet-derived growth factor. Structure, function, and roles in normal and transformed cells. J Clin Invest. 1984 Sep;74(3):669–676. doi: 10.1172/JCI111482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Receptor-mediated internalization and degradation of diphtheria toxin by monkey kidney cells. J Biol Chem. 1979 Nov 25;254(22):11337–11342. [PubMed] [Google Scholar]
- Drickamer K., Mamon J. F., Binns G., Leung J. O. Primary structure of the rat liver asialoglycoprotein receptor. Structural evidence for multiple polypeptide species. J Biol Chem. 1984 Jan 25;259(2):770–778. [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud B., Huet C., Louvard D. Assembled and unassembled pools of clathrin: a quantitative study using an enzyme immunoassay. J Cell Biol. 1985 Feb;100(2):521–527. doi: 10.1083/jcb.100.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P., St Clair R. W. Retroendocytosis of low density lipoprotein. Effect of lysosomal inhibitors on the release of undegraded 125I-low density lipoprotein of altered composition from skin fibroblasts in culture. J Biol Chem. 1984 Feb 10;259(3):1703–1713. [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Levy M. A., Stahl P. Morphological analysis of ligand uptake and processing: the role of multivesicular endosomes and CURL in receptor-ligand processing. Eur J Cell Biol. 1985 Mar;36(2):230–238. [PubMed] [Google Scholar]
- Harding C., Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983 Jun 15;113(2):650–658. doi: 10.1016/0006-291x(83)91776-x. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Kirchhausen T. Clathrin, cages, and coated vesicles. Cell. 1983 Jul;33(3):650–652. doi: 10.1016/0092-8674(83)90007-7. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol. 1980 Mar;84(3):560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoflack B., Kornfeld S. Lysosomal enzyme binding to mouse P388D1 macrophage membranes lacking the 215-kDa mannose 6-phosphate receptor: evidence for the existence of a second mannose 6-phosphate receptor. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4428–4432. doi: 10.1073/pnas.82.13.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983 Nov;35(1):321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Korkor A., Martin K., Slatopolsky E. Peripheral metabolism of intact parathyroid hormone. Role of liver and kidney and the effect of chronic renal failure. J Clin Invest. 1981 Mar;67(3):885–892. doi: 10.1172/JCI110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E., Rector F. C., Jr Proton transport and cell function. J Clin Invest. 1984 Feb;73(2):285–290. doi: 10.1172/JCI111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. I. Binding of alpha-macroglobulin . protease complexes to rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7323–7328. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G., Stiles G. L. Mechanisms of membrane-receptor regulation. Biochemical, physiological, and clinical insights derived from studies of the adrenergic receptors. N Engl J Med. 1984 Jun 14;310(24):1570–1579. doi: 10.1056/NEJM198406143102406. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S. Kinetics of insulin receptor internalization and recycling in adipocytes. Shunting of receptors to a degradative pathway by inhibitors of recycling. J Biol Chem. 1985 Apr 10;260(7):4136–4144. [PubMed] [Google Scholar]
- McClelland A., Kühn L. C., Ruddle F. H. The human transferrin receptor gene: genomic organization, and the complete primary structure of the receptor deduced from a cDNA sequence. Cell. 1984 Dec;39(2 Pt 1):267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984 Apr;98(4):1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merion M., Sly W. S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J Cell Biol. 1983 Mar;96(3):644–650. doi: 10.1083/jcb.96.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Pan B. T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983 Jul;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett G. V., Knutson V. P., Lane M. D. Insulin-induced down-regulation of insulin receptors in 3T3-L1 adipocytes. Altered rate of receptor inactivation. J Biol Chem. 1982 Apr 25;257(8):4285–4291. [PubMed] [Google Scholar]
- Russell D. W., Schneider W. J., Yamamoto T., Luskey K. L., Brown M. S., Goldstein J. L. Domain map of the LDL receptor: sequence homology with the epidermal growth factor precursor. Cell. 1984 Jun;37(2):577–585. doi: 10.1016/0092-8674(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Schmid S. L., Braell W. A., Rothman J. E. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Schwartz A. L., Bolognesi A., Fridovich S. E. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J Cell Biol. 1984 Feb;98(2):732–738. doi: 10.1083/jcb.98.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Lodish H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982 Apr 25;257(8):4230–4237. [PubMed] [Google Scholar]
- Schwartz A. L., Strous G. J., Slot J. W., Geuze H. J. Immunoelectron microscopic localization of acidic intracellular compartments in hepatoma cells. EMBO J. 1985 Apr;4(4):899–904. doi: 10.1002/j.1460-2075.1985.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett C., Rosenberg L. E., Mellman I. S. Transmembrane transport of cobalamin in prokaryotic and eukaryotic cells. Annu Rev Biochem. 1981;50:1053–1086. doi: 10.1146/annurev.bi.50.070181.005201. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Stahl P. D., Bernd P., Rabinovitch M. Receptor-mediated entry of beta-glucuronidase into the parasitophorous vacuoles of macrophages infected with Leishmania mexicana amazonensis. J Exp Med. 1983 May 1;157(5):1471–1482. doi: 10.1084/jem.157.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Caron M. G., Lefkowitz R. J. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. J Biol Chem. 1985 Apr 10;260(7):3883–3886. [PubMed] [Google Scholar]
- Simmons C. F., Jr, Schwartz A. L. Cellular pathways of galactose-terminal ligand movement in a cloned human hepatoma cell line. Mol Pharmacol. 1984 Nov;26(3):509–519. [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Spiess M., Schwartz A. L., Lodish H. F. Sequence of human asialoglycoprotein receptor cDNA. An internal signal sequence for membrane insertion. J Biol Chem. 1985 Feb 25;260(4):1979–1982. [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Immunotoxins: redirecting nature's poisons. Cell. 1985 Jul;41(3):653–654. doi: 10.1016/s0092-8674(85)80042-8. [DOI] [PubMed] [Google Scholar]
- Ward J. H., Jordan I., Kushner J. P., Kaplan J. Heme regulation of HeLa cell transferrin receptor number. J Biol Chem. 1984 Nov 10;259(21):13235–13240. [PubMed] [Google Scholar]
- Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985 Feb;100(2):633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wileman T., Boshans R. L., Schlesinger P., Stahl P. Monensin inhibits recycling of macrophage mannose-glycoprotein receptors and ligand delivery to lysosomes. Biochem J. 1984 Jun 15;220(3):665–675. doi: 10.1042/bj2200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Boshans R., Stahl P. Uptake and transport of mannosylated ligands by alveolar macrophages. Studies on ATP-dependent receptor-ligand dissociation. J Biol Chem. 1985 Jun 25;260(12):7387–7393. [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Formation of receptosomes from plasma membrane coated pits during endocytosis: analysis by serial sections with improved membrane labeling and preservation techniques. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5617–5621. doi: 10.1073/pnas.80.18.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. W., Roth T. F. A specific subunit of vitellogenin that mediates receptor binding. Biochemistry. 1984 Nov 20;23(24):5774–5780. doi: 10.1021/bi00319a016. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J., Lauffenburger D. A. Kinetic analysis of chemotactic peptide receptor modulation. J Cell Biol. 1982 Jan;92(1):34–43. doi: 10.1083/jcb.92.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zull J. E., Chuang J. Characterization of parathyroid hormone fragments produced by cathepsin D. J Biol Chem. 1985 Feb 10;260(3):1608–1613. [PubMed] [Google Scholar]