Abstract
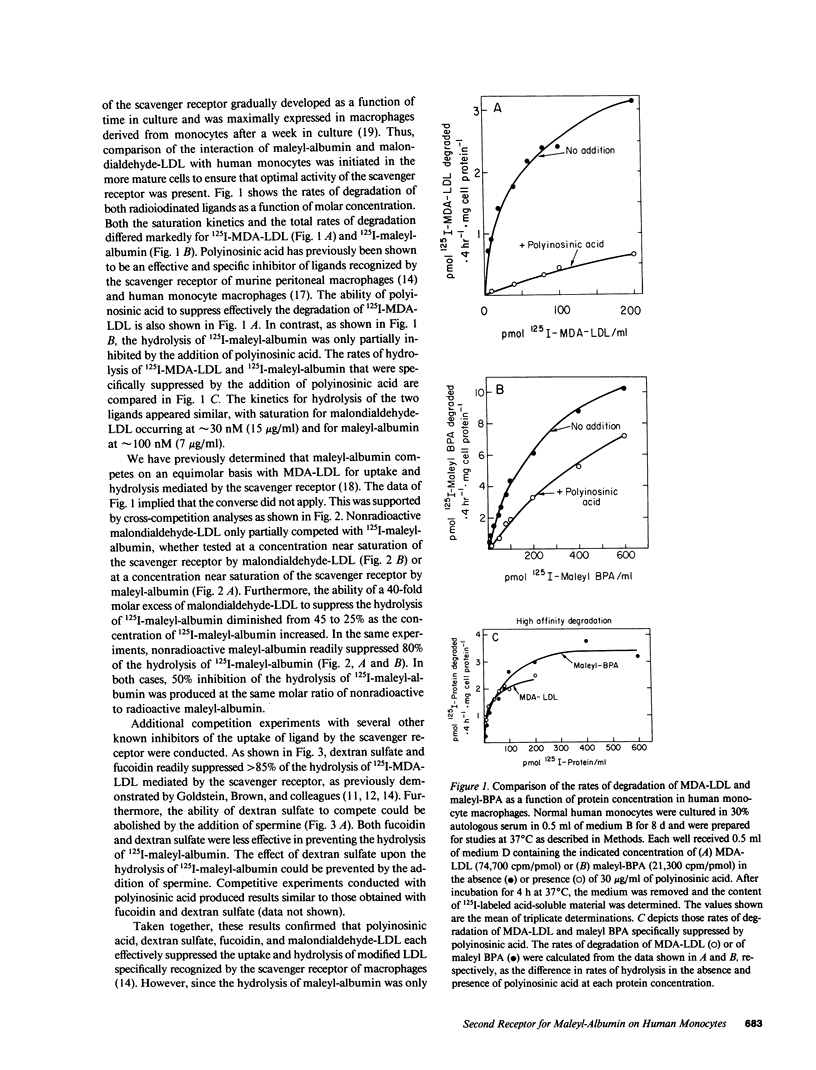
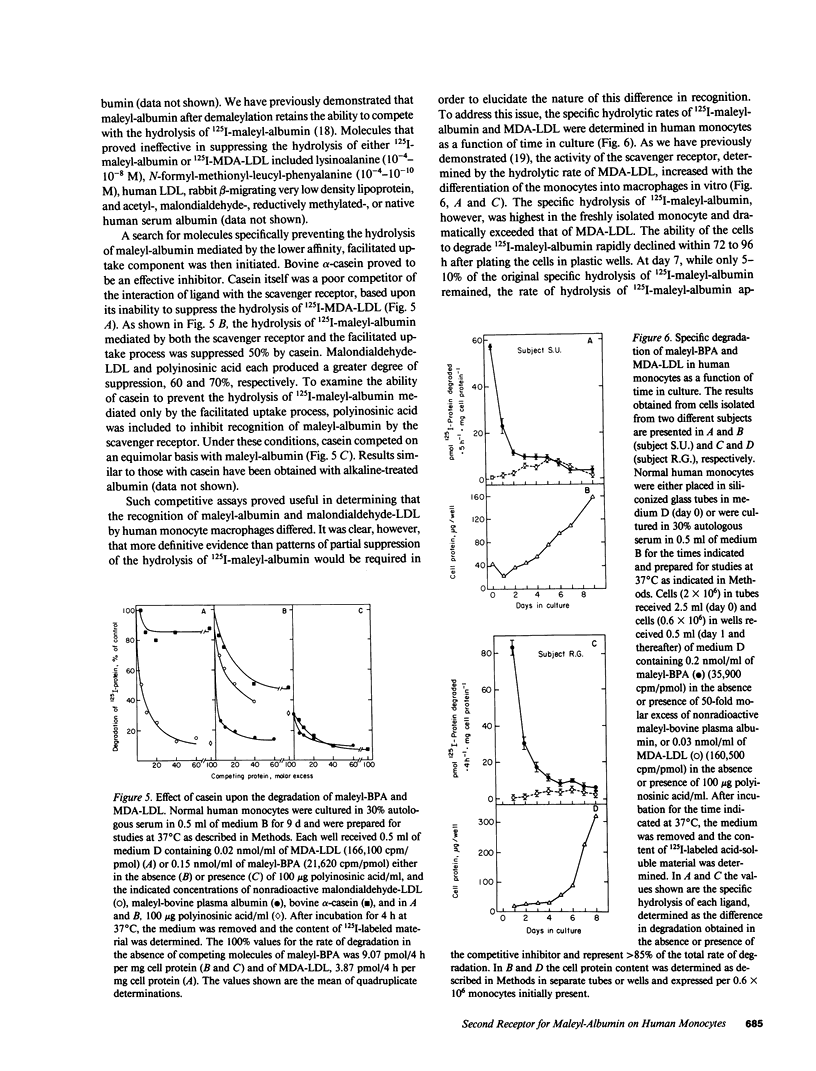
A comparison of the receptor-mediated interaction of malondialdehyde-low density lipoprotein and maleyl-albumin has been examined in human monocytes during differentiation in vitro. The recognition of both ligands by the scavenger receptor of these cells has been confirmed. We now report that human monocytes express a second cellular surface receptor for maleyl-albumin that is distinct from the scavenger receptor. The activity of the maleyl-albumin receptor, determined by both binding and lysosomal hydrolytic assays, substantially exceeds that of the scavenger receptor in freshly isolated monocytes. A dramatic and rapid decline in the activity of the maleyl-albumin receptor occurs within 72 to 96 h during differentiation in vitro. At day 7, while only 5-10% of the original activity of the maleyl-albumin receptor remains, it is similar to that of the maximally expressed scavenger receptor. Both the binding and hydrolysis of ligand mediated by the maleyl-albumin receptor are specifically inhibited by alpha-casein and alkaline-treated albumin; neither of these proteins is recognized by the scavenger receptor. The occurrence of the exceptionally active maleyl-albumin receptor on freshly isolated human monocytes suggests that it participates in processes necessary to the function of the cells that diminish in importance after differentiation of the monocytes into macrophages in vitro. Furthermore, while maleyl-albumin is a useful adjunct to studies of cellular events mediated by the scavenger receptor, the presence of a second receptor for maleyl-albumin must be taken into account as a potential contributing and complicating event.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Buja L. M., Kovanen P. T., Bilheimer D. W. Cellular pathology of homozygous familial hypercholesterolemia. Am J Pathol. 1979 Nov;97(2):327–357. [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Edmond J., Seager J., Popják G. Abnormal induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in leukocytes from subjects with heterozygous familial hypercholesterolemia. J Biol Chem. 1975 Mar 25;250(6):2045–2055. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brunschede G. Y., Brown M. S. Inhibition of proteolytic degradation of low density lipoprotein in human fibroblasts by chloroquine, concanavalin A, and Triton WR 1339. J Biol Chem. 1975 Oct 10;250(19):7854–7862. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M., Edwards P. A. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M. Scavenger receptor-mediated recognition of maleyl bovine plasma albumin and the demaleylated protein in human monocyte macrophages. Proc Natl Acad Sci U S A. 1985 May;82(9):2693–2697. doi: 10.1073/pnas.82.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Olch C. L., Folgelman A. M. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984 Sep 25;259(18):11305–11311. [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. J., Pizzo S. V., Imber M. J., Adams D. O. Receptors for maleylated proteins regulate secretion of neutral proteases by murine macrophages. Science. 1982 Nov 5;218(4572):574–576. doi: 10.1126/science.6289443. [DOI] [PubMed] [Google Scholar]
- King T. P., Spencer M. Structural studies and organic ligand-binding properties of bovine plasma albumin. J Biol Chem. 1970 Nov 25;245(22):6134–6148. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKenzie H. A. Milk proteins. Adv Protein Chem. 1967;22:55–234. doi: 10.1016/s0065-3233(08)60041-8. [DOI] [PubMed] [Google Scholar]
- Musson R. A., Shafran H., Henson P. M. Intracellular levels and stimulated release of lysosomal enzymes from human peripheral blood monocytes and monocyte-derived macrophages. J Reticuloendothel Soc. 1980 Sep;28(3):249–264. [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Shechter I., Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981 Jan;22(1):63–71. [PubMed] [Google Scholar]
- Steele J. C., Jr, Reynolds J. A. Molecular weight and hydrodynamic properties of apolipoprotein B in guanidine hydrochloride and sodium dodecyl sulfate solutions. J Biol Chem. 1979 Mar 10;254(5):1639–1643. [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Jackson R. L., Shapiro S., Haberland M. E., Edwards P. A. Receptor-mediated uptake of remnant lipoproteins by cholesterol-loaded human monocyte-macrophages. J Biol Chem. 1985 Jul 25;260(15):8783–8788. [PubMed] [Google Scholar]
- Via D. P., Dresel H. A., Cheng S. L., Gotto A. M., Jr Murine macrophage tumors are a source of a 260,000-dalton acetyl-low density lipoprotein receptor. J Biol Chem. 1985 Jun 25;260(12):7379–7386. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Wilkinson P. C., Allan R. B. Binding of protein chemotactic factors to the surfaces of neutrophil leukocytes and its modification with lipid-specific bacterial toxins. Mol Cell Biochem. 1978 Jun 15;20(1):25–40. doi: 10.1007/BF00229452. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Bradley G. R. Chemotactic and enzyme-releasing activity of amphipathic proteins for neutrophils. A possible role for protease in chemotaxis on substratum-bound protein gradients. Immunology. 1981 Apr;42(4):637–648. [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Ackerman S. K., Douglas S. D. Long-term human peripheral blood monocyte cultures: establishment, metabolism and morphology of primary human monocyte-macrophage cell cultures. Immunology. 1979 Oct;38(2):401–411. [PMC free article] [PubMed] [Google Scholar]



