There are two main threads in biomedical research: One is to discover how biological systems work, basic science; and the other is to use knowledge obtained from basic research in medicine, translation. The availability of genome sequences has brought an unprecedented opportunity for discovery (1). As one of several initial efforts toward the interpretation of the human and mouse genomes, Hogenesch and coworkers built the Gene Atlas in 2004 (2) in which the protein-coding genes were profiled for their expression against a panel of over 100 human and mouse tissues and cells. In the current study by Zhang et al. a decade later (3), Hogenesch and his team have now added the temporal dimension to construct the Circadian Gene Atlas in which gene expression is profiled across 12 different mouse tissues at different times of the day. For more than a decade, circadian researchers have examined the cycling transcriptomes in many tissues (reviewed in ref. 4). Notably, this new dataset represents by far the most comprehensive profiling of protein-coding and noncoding genes and provides not only a panoramic view of temporal gene expression patterns at the genome-wide level, but also a nucleotide view of any gene. As you delve into the data and its analysis, it is easy to see that circadian biology is not just for chronobiologists; it is important for all biologists and has important implications in medicine.
The circadian clock in mammals regulates many aspects of physiology and behavior, and its disruption is associated with a host of pathological and disease states (5). The hallmark of circadian timekeeping is the rhythmic expression of genes. In this study, the team used high-throughput DNA microarray and RNA-sequencing (RNA-seq) analyses to quantify all of the gene transcripts in 12 different mouse organs, representing diverse physiologies. Each organ was profiled every 2 h by arrays and every 6 h by RNA-seq to provide a temporal expression profile. This high spatial and temporal resolution design allowed for more thorough and accurate detection of circadian genes (4). So how many genes are oscillating in individual organs and throughout the organism? Among all of the ∼20,000 protein-coding genes, 43% oscillated in at least one organ, with the liver having the most circadian genes (>3,000). The power of deep RNA-seq also enabled detection of noncoding RNAs (ncRNAs): >300 of the 1,016 mouse–human conserved ncRNAs were rhythmic, whereas relatively few nonconserved ncRNAs oscillated. Importantly, there is a remarkable scope of tissue specificity in the circadian genes. Although many oscillated in multiple organs and 10 genes (mostly core clock factors) were ubiquitously rhythmic in all 12 organs, the vast majority showed organ-specific rhythms. Taking into consideration tissue-specific physiologies and cell type heterogeneity of organ systems, the authors estimate that >50% of all genes in the mouse genome oscillate somewhere in the body!
The study also revealed a remarkable synchrony among circadian genes and various phase relationships both within and between the organs. First, almost all of the 12 organs had two prominent peaks of transcriptional activities, appropriately termed as predawn and predusk “rush hours.” These peaks likely reflect the two frantic times of behavioral and physiological transitions. Second, although several genes oscillated synchronously across all organs, including the core clock genes, >1,700 displayed phase differences of 6 to 12 h in different organs, adding another form of tissue specificity. Third, many important biological pathways are enriched for circadian genes and interesting phase relationships exist: in-phase, out-of-phase, and even antiphase within and across organs. These spatiotemporal phase relationships of circadian genes point to physiological importance of timing for their correct function.
Closer inspection of the circadian genes revealed some common genomic characteristics: Compared with noncircadian genes, the circadian genes tend to physically cluster in the genome, tend to be longer, and tend to have more spliceforms. Do circadian genes have more regulatory capacity than noncircadian genes? From the evolutionary point of view, it is a plausible proposition. These genomic characteristics are a signature of adaptive radiation on both shallow and deep time scales and are in line with and in support of the theory of facilitated variation (6). According to this theory, genetic variation is not purely random but biased to facilitate natural selection. In this context, circadian genes and networks could represent a major source of conserved core elements and processes (an evolutionary toolbox) from which new functions can be elaborated through regulatory changes in different contexts and to different degrees depending on time, place, and condition (adaptive novelty). These genes might have played a more important role in evolution than noncircadian genes and had more opportunity to evolve, acquiring regulatory add-ons for their appropriate function.
The study raises many interesting questions. Do circadian ncRNAs have functional significance? How can a handful of core clock genes generate such wide ranges of phases of gene expression in a given organ? For a given gene, how are the drastically different phases in different organs achieved from the core clock genes that have same phases across all organs? How are the different amplitudes achieved from organ to organ? How important is rhythmicity with respect to gene function? Steps toward answering the phase problem will include identifying the missing links between the core clock genes and the outputs, i.e., tissue-specific mediators with which to propagate the circadian signal to achieve specificity and temporal separation for their targets. Future studies should extend the ChIP-sequencing effort in the liver (7) to include several other organs to gain insights into the transcriptional logic at the systems level. It is not all about transcription; future studies will need to address the interplay between transcriptional and posttranscriptional mechanisms.
Circadian clock research stands as one of the great success stories in biology. It started out with descriptive studies of behavior at the organism level and later matured into the elaboration of cellular and molecular mechanisms (reviewed in refs. 8 and 9). However, the circadian timekeeping system must be robust against perturbation and responsive to local physiology. Functional sophistication requires a complex system. Indeed, in addition to the thousands of circadian genes (3), hundreds of modulators functioning in various cellular pathways impact clock function (10). It is likely that most if not all major cellular networks are integrated with the circadian clock—a complex system, indeed! This systems complexity allows us to better appreciate the heroic and at-times fortuitous efforts of the pioneers in the field that led to the identification of the core clock genes and the illustration of the basic negative feedback mechanism.
Overt circadian rhythms in the organism emerge from oscillatory behavior in cells and cellular oscillations emerge from a handful of core clock components. These emerging properties raise outstanding questions concerning circadian timekeeping at the systems level (11), which remains a fundamental question in biology at large in the postgenome era. The overarching goal of systems biology is to understand how genes and networks culminate in emergent properties (12). The circadian system represents one of the most tractable models for providing a complete understanding of systems biology. We are close to having a fairly accurate idea about the components of the system (i.e., core clock genes, clock
The current study revealed that more than 50% of all drugs target circadian genes/proteins.
modulators, and all circadian genes) and how they work together, especially in the liver and U2OS cellular model. It is expected that results from future network analysis, experimental perturbation, and computational modeling will help uncover design principles of circadian oscillations, an emergent systems property.
Given the prevalence of circadian genes, circadian biology is surprisingly pervasive. We can envisage that every laboratory is working on circadian genes, knowingly or unknowingly. Thus, it is vital that all researchers take into consideration the circadian properties of the genes in a particular physiology. We hope a case is made here that circadian biology is for circadian and noncircadian biologists alike. To facilitate future work in the broader research community, the team built a high temporal and genome-space resolution, multiorgan expression dataset called “Circadian Gene Atlas” or “CIRCA,” which is freely accessible to any researcher. Researchers can access CIRCA to find out where and when genes of interest are expressed and what their respective phase and amplitude are.
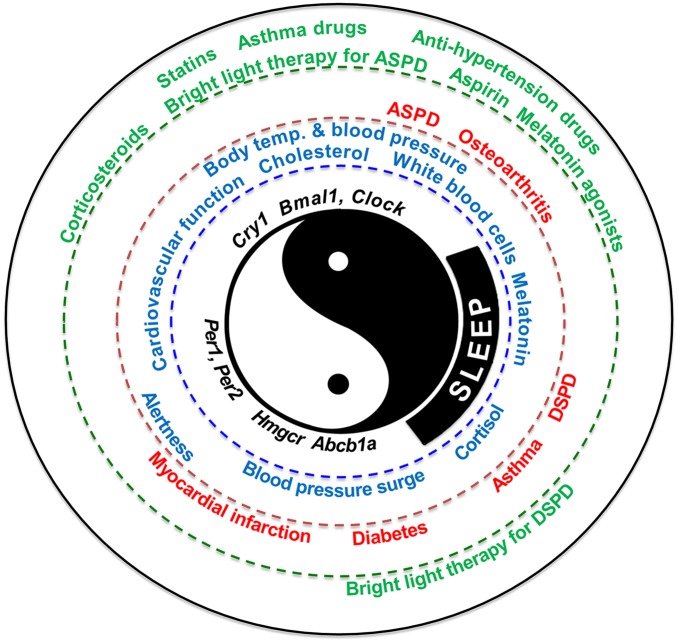
Last, but not least, circadian biology is of great importance to medicine. Although the circadian phenomenon has been generally accepted, this concept is all too often overlooked in the practice of medicine, which is largely based on the concept of physiological homeostasis or constancy. Our bodily functions display best times under normal physiology and, expectedly, worst times under pathological states (Fig. 1). Thus, the thought of administering drugs in tune with their targets to elicit maximum response makes perfect sense. In this regard, the current study revealed that more than 50% of all drugs target circadian genes/proteins, including 56 of the 100 best sellers in the United States and 119 of the WHO’s list of essential medicines. It is of utmost importance that the body’s clock be taken into consideration during diagnosis and treatment. Many drugs have relatively short half-lives and would be grossly ineffective if administered at times when their targets are at the nadir. Chronotherapeutics is the purposeful delivery of medications at biologically opportune times, which may be achieved by simply practicing time-of-day dependent dosing with existing drugs. Chronotherapeutics has great potential to maximize effectiveness, optimize outcomes, and minimize/avoid adverse effects. Indeed, chronotherapy has made significant progresses in recent years in the treatment of asthma, cancer, and hypertension (13, 14). Thus, the circadian gene profiles of drug targets will be extremely useful in predictive medicine. The clock keeps ticking, a notion that biologists, physicians, and pharmacologists should be well aware of. Personalized medicine just been given a new dimension: circadian time!
Fig. 1.
A holistic view of the human biological clock, illustrating the peak times of selected biological variables, including genes (in black), physiology (best times appear in blue), pathology and disease (worst times are red), and treatment (chronotherapy show in green). The yin–yang symbol represents dynamic interactions of seemingly contrary forces in a coherent system. The sleep/wake cycle is the most obvious manifestation of circadian rhythms. The core clock genes are Bmal1, Clock, Cry1, Per1, and Per2. Hmgcr and Abcb1a2 are two circadian gene examples. ASPD, advanced sleep phase disorder; DSPD, delayed sleep phase disorder.
Footnotes
The authors declare no conflict of interest.
See companion article on page 16219.
References
- 1.Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470(7333):187–197. doi: 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- 2.Su AI, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101(16):6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ptitsyn AA, Gimble JM. True or false: All genes are rhythmic. Ann Med. 2011;43(1):1–12. doi: 10.3109/07853890.2010.538078. [DOI] [PubMed] [Google Scholar]
- 5.Richards J, Gumz ML. Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol. 2013;304(12):R1053–R1064. doi: 10.1152/ajpregu.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhart J, Kirschner M. The theory of facilitated variation. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8582–8589. doi: 10.1073/pnas.0701035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi JS. Finding new clock components: Past and future. J Biol Rhythms. 2004;19(5):339–347. doi: 10.1177/0748730404269151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang EE, et al. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell. 2009;139(1):199–210. doi: 10.1016/j.cell.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogenesch JB, Ueda HR. Understanding systems-level properties: Timely stories from the study of clocks. Nat Rev Genet. 2011;12(6):407–416. doi: 10.1038/nrg2972. [DOI] [PubMed] [Google Scholar]
- 12.Nurse P, Hayles J. The cell in an era of systems biology. Cell. 2011;144(6):850–854. doi: 10.1016/j.cell.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Levi F, Schibler U. Circadian rhythms: Mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- 14.Hermida RC, et al. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol. 2013;9(6):358–368. doi: 10.1038/nrneph.2013.79. [DOI] [PubMed] [Google Scholar]