Significance
The daily environmental changes in light and dark set the pace of the circadian clock, which at the molecular level is composed of transcription-based feedback loops. It is well known that temperature fluctuations affect the circadian clock, but the molecules involved remain largely undefined. We found that the heat shock transcription factor HsfB2b works in the repression of the morning clock gene PRR7. We tested the properties of the circadian clock, and growth behavior, in the hsfB2b mutant and in transgenics with altered HsfB2b expression. Our results reveal that HsfB2b expression is important for accurate circadian rhythms following elevated temperature and/or salt treatment. Our work provides evidence of a molecular entry point for temperature signaling to the plant circadian clock.
Keywords: circadian clock, Hsf, heat compensation, salt tolerance
Abstract
The circadian clock perceives environmental signals to reset to local time, but the underlying molecular mechanisms are not well understood. Here we present data revealing that a member of the heat shock factor (Hsf) family is involved in the input pathway to the plant circadian clock. Using the yeast one-hybrid approach, we isolated several Hsfs, including HEAT SHOCK FACTOR B2b (HsfB2b), a transcriptional repressor that binds the promoter of PSEUDO RESPONSE REGULATOR 7 (PRR7) at a conserved binding site. The constitutive expression of HsfB2b leads to severely reduced levels of the PRR7 transcript and late flowering and elongated hypocotyls. HsfB2b function is important during heat and salt stress because HsfB2b overexpression sustains circadian rhythms, and the hsfB2b mutant has a short circadian period under these conditions. HsfB2b is also involved in the regulation of hypocotyl growth under warm, short days. Our findings highlight the role of the circadian clock as an integrator of ambient abiotic stress signals important for the growth and fitness of plants.
The circadian clock is an endogenous timing mechanism that ensures that daily rhythmic processes are synchronized with the environment. The circadian oscillator runs with a period of ∼24 h. It entrains to environmental signals, such as light and temperature, and in this way is able to anticipate daily environmental changes (1). Molecular genetics and modeling have revealed that the circadian system comprises several interconnected feedback loops involving multiple phase-specific gene products (2).
In the model plant Arabidopsis, the morning feedback loops are composed of the major oscillator proteins CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), which peak in abundance at dawn and activate the expression of early-late morning genes PSEUDO RESPONSE REGULATOR 9 (PRR9) and PRR7 (3). Pseudo-response regulator (PRR) proteins are transcriptional repressors, and PRR9 and PRR7 close the morning loops by binding to the CCA1 and LHY promoters (4). The transcription of PRR9 is acutely activated by light, a feature that is not shared by PRR7 (3, 5). Disruption of either locus results in a mild clock phenotype, including an increased clock period of ∼1 h and an elongated hypocotyl (6–8). The double loss-of-function mutant exhibits a strong synergistic phenotype with free-running period lengths of up to 35 h and loss of temperature entrainment and compensation, indicating overlapping roles for PRR9 and PRR7 (3, 9, 10).
The ambient environment influences the circadian clock in a number of different ways. Although the most well-studied input cue is light, temperature is a major zeitgeber as well (11). Brief pulses (from minutes to hours, depending on the intensity) result in acute responses from the clock. Importantly, the severity of such responses is time-dependent and gated by the oscillator (12–14). Longer durations of zeitgeber change can function as an entrainment signal and generally follow the diurnal pattern of the light–dark cycle. In this way, clock phase is adjusted to match seasonal changes. In addition, the clock is temperature-compensated so that a ∼24-h period is maintained despite fluctuations in temperature.
The heat shock factor (Hsf) family of transcription factors is large in plants compared with fungi and animals, and the Arabidopsis genome encodes 21 Hsfs (15, 16). Many are activated on heat stress, but specific Hsfs respond to other abiotic stresses, including oxidative and drought stress (17). The function of many Hsfs remains to be characterized.
Hsfs bind to the heat shock element (HSE) repeat, which is an inverted repeat of the nucleotides nGAAn (17, 18). HsfB1 and HsfB2b have been confirmed to function in transcriptional repression, and their overexpression in the nucleus results in cell death (19, 20). HsfB2b is induced after heat stress (21) and has redundant functions with HsfB1 in the repression of HsfA2 and HEAT SHOCK PROTEIN (Hsp) genes (22).
Here we have identified HsfB2b as a novel repressor of the morning clock gene PRR7. HsfB2b binds a conserved HSE site in the PRR7 promoter and HsfB2b-ox plants have reduced PRR7 expression, have elongated hypocotyls, and are late-flowering. We characterize HsfB2b as a circadian gene, and the loss-of-function mutant exhibits compromised temperature compensation under high temperature. HsfB2b also has a role in growth under warm conditions, along with the compensation of circadian rhythms under salt stress. Finally, the expression of PRR7 target genes is altered by the hsfB2b-1 mutation. We propose that HsfB2b-mediated repression is important for the appropriate expression pattern of PRR7 and its target gene network involved in plant growth and stress responses.
Results
Identification of HsfB2b Binding to the PRR7 Promoter.
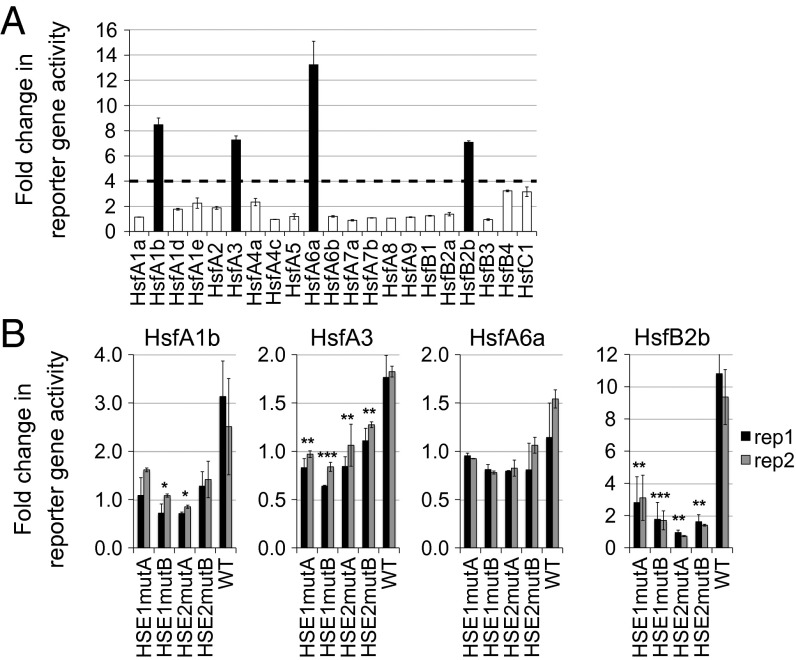
We performed a yeast one-hybrid screen with an arrayed Arabidopsis transcription factor library (23) using the PRR7 promoter (proPRR7) as bait. We screened the 1-kb region upstream of ATG and observed binding by several Hsf factors, including HsfA1b, HsfA3, HsfA6a, and HsfB2b, to a specific fragment of the promoter (−673/−328 bp; Fig. 1 and Fig. S1). On mutation of the critical G nucleotides in either of the two HSEs in proPRR7 (termed HSE1mut or HSE2mut; Materials and Methods), Hsf binding was lost (Fig. 1B), supporting that Hsfs bind proPRR7 in an HSE-dependent manner. In this validation of the Hsf candidates, we note that HSE1mut and HSE2mut most dramatically affected the fold change of HsfB2b binding to proPRR7. Thus, we focused follow-up studies on this Hsf isoform.
Fig. 1.
Hsfs bind the PRR7 promoter. (A) Hsf binding to the PRR7 promoter in yeast one-hybrid assay. Twenty-one Hsf family members were tested. Data are the average of three technical replicates. Error bars represent SEM. The fold change in reporter activity is relative to the background in the pEXP502 negative control strain. (B) Mutation of HSE1 or HSE2 abolishes Hsf binding to proPRR7 in yeast. The fold change of β-galactosidase activity of each HSE-mutated strain was obtained by normalizing to the negative control. Black and gray bars denote biological replicates, and error bars represent the SEM of the technical replicates. *P < 0.05; **P < 0.01; ***P < 0.001 (Student t test).
HsfB2b Is a Repressor of PRR7, and Its Basal Expression Is Circadian.
To analyze the role of HsfB2b in regulating PRR7 expression, we generated plants with altered Hsf expression and screened for phenotypes of the transgenic plants (Hsf-ox) in the Col-0 PRR7:LUCIFERASE (LUC) background. We used a construct encoding a GFP C-terminal tag under the control of the UBIQUITIN10 (UBQ10) promoter (proUBQ10:Hsf-GFP), which has intermediate strength compared with the popular 35S constitutive promoter. The 35S sequence was reported to contain potential HSE elements, and the overexpression of HsfB2b can lead to cell death (20, 24).
Interestingly, we found that independent HsfB2b-ox lines resulted in late flowering and loss of PRR7:LUC luminescence (Fig. 2A and Fig. S2). Under long days, HsfB2b-ox flowered with approximately double the number of leaves of WT Col-0. The prr7-3 mutant flowered slightly late, at the ∼20-leaf stage, whereas the hsfB2b-1 mutant had WT flowering time (Fig. 2A). The delay in flowering time for HsfB2b-ox was more extreme than the delayed flowering of the prr7-3 null mutant under long days (8). This stronger phenotype of HsfB2b-ox suggests that additional flowering time regulators besides PRR7 are targeted (22).
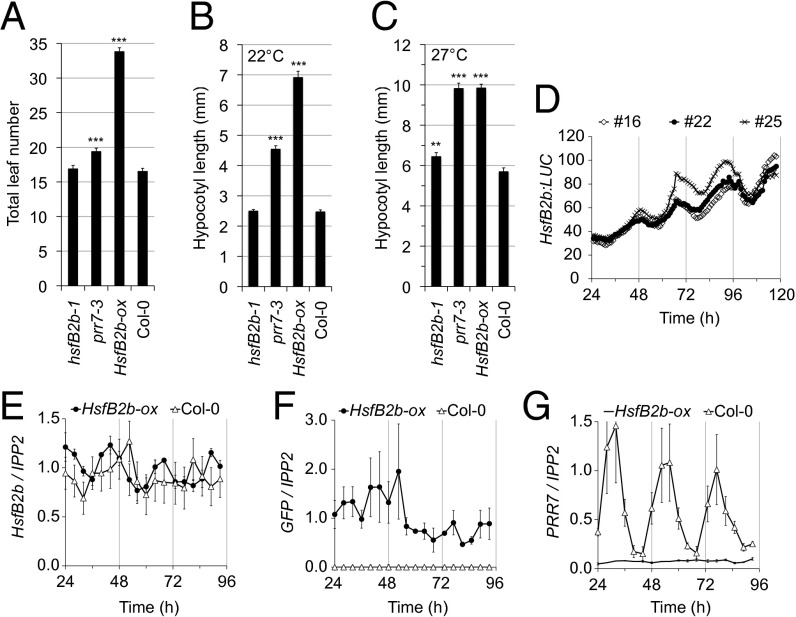
Fig. 2.
HsfB2b alters growth and is a circadian gene. (A) Delayed flowering time of HsfB2b-ox. Plants were grown in soil under long-day conditions (16L:8D), and the total leaf number was determined at the time of bolting. The experiment was repeated twice, with similar results; n > 18. Error bars represent SD. (B) Hypocotyl length of 6-d-old hsfB2b-1, prr7-3, HsfB2b-ox, and Col-0 seedlings grown under short-day photoperiods at 22 °C. Error bars represent SEM of technical replicates; n > 18. (C) Hypocotyl length of 6-d-old seedlings after growth under constantly warm short days. Seedlings were grown at 22 °C for the first 3 d and then transferred to warm short days (27 °C). Error bars represent the SEM of technical replicates; n > 23. **P < 0.01; ***P < 0.001 (Student t test). (D) HsfB2b:LUC activity under LL. Seedlings were monitored for 5 d under LL after entrainment in 12L:12D for 1 wk. Three independent T2 lines are shown. Error bars represent SEM of technical replicates; n = 12. (E–G) Gene expression in HsfB2b-ox (proUBQ10:HsfB2b-GFP). The values are relative to IPP2 and measured by qPCR. (E) Endogenous HsfB2b, amplicon spanning the 3′ UTR. (F) GFP. Note that this line is in Col-0 WT background and thus has two HsfB2b transcripts (with and without GFP). (G) PRR7. Samples were obtained every 3 h on days 2–4 in LL after 12L:12D entrainment for 1 wk. The experiments were repeated at least twice. Error bars represent the SEM of biological replicates.
For the experiments reported herein, we chose HsfB2b-ox #23 as the representative line. The late flowering of HsfB2b-ox was also observed under short days (Fig. S3). Similar to the prr7-3 mutant, HsfB2b-ox seedlings maintained under short photoperiods exhibited elongated hypocotyls (Fig. 2B). Interestingly, hypocotyl growth of HsfB2b-ox was increased on transfer to warm temperature in a similar manner to prr7-3, and, notably, the relative increase of HsfB2b-ox was less than that of Col-0, suggesting heat insensitivity (Fig. 2 B and C). In addition, the hsfB2b-1 mutant had a heat-dependent, long hypocotyl phenotype (Fig. 2C). Taken together, our results suggest that HsfB2b has a role in regulating growth.
We next investigated for a circadian aspect of HsfB2b. Interestingly, although HsfB2b is a heat-induced gene (21), in the DIURNAL database HsfB2b is a rhythmic gene under most regimes, with a peak during the daytime (25). We fused the HsfB2b promoter to LUC and confirmed the circadian activity of the HsfB2b promoter under constant light (LL) conditions (Fig. 2D). In addition, we performed a circadian time course with samples obtained every 3 h and determined relative mRNA expression levels using quantitative RT-PCR (qPCR). Indeed, expression of HsfB2b was rhythmic under LL (Fig. 2E). HsfB2b-ox had similar amplitude of HsfB2b expression as the WT, but with a shifted phase (Fig. 2E). Specifically, the HsfB2b-GFP transgene profile, as measured by quantification of the GFP amplicon, indicated loss of rhythmicity of the HsfB2b-GFP transcript (Fig. 2F). Strikingly, and in accordance with the loss of PRR7:LUC bioluminescence (Fig. S2), we found very low levels of the PRR7 transcript in HsfB2b-ox, revealing a strong repressor activity of HsfB2b (Fig. 2G).
We also analyzed the hsfB2b-1 mutant, which has a T-DNA insertion in the first exon and confers a loss-of-function mutation (26). We detected subtle increases and phase advances of PRR7 transcript levels (full-length and alternatively spliced PRR7 transcripts, AS1 and AS3) in this background compared with WT, specifically an increase in PRR7 expression during the first subjective day, followed by a gradual decrease on days 2 and 3, indicative of a PRR7 negative feedback (Fig. S4). Collectively, these phenotypes are in agreement with HsfB2b repressor function and the redundancy among multiple factors in heat-signaling pathways (22, 27).
HsfB2b Affects Temperature Compensation and Clock Resetting.
The morning genes of the circadian clock are involved in temperature compensation of the circadian system (10). To investigate a role for HsfB2b in compensation of the circadian clock after heat exposure, we determined the free-running period length of the clock at 22 °C compared with 27 °C. To this end, we crossed the PRR7:LUC reporter into the hsfB2b-1 mutant, and because the luminescence of PRR7:LUC was absent in HsfB2b-ox, we crossed the CCA1:LUC reporter into the HsfB2b-ox background. We found that hsfB2b-1 failed to sufficiently compensate period length and displayed a 1- to 2-h shorter period compared with WT, and that this temperature defect was absent in HsfB2b-ox (Fig. 3A).
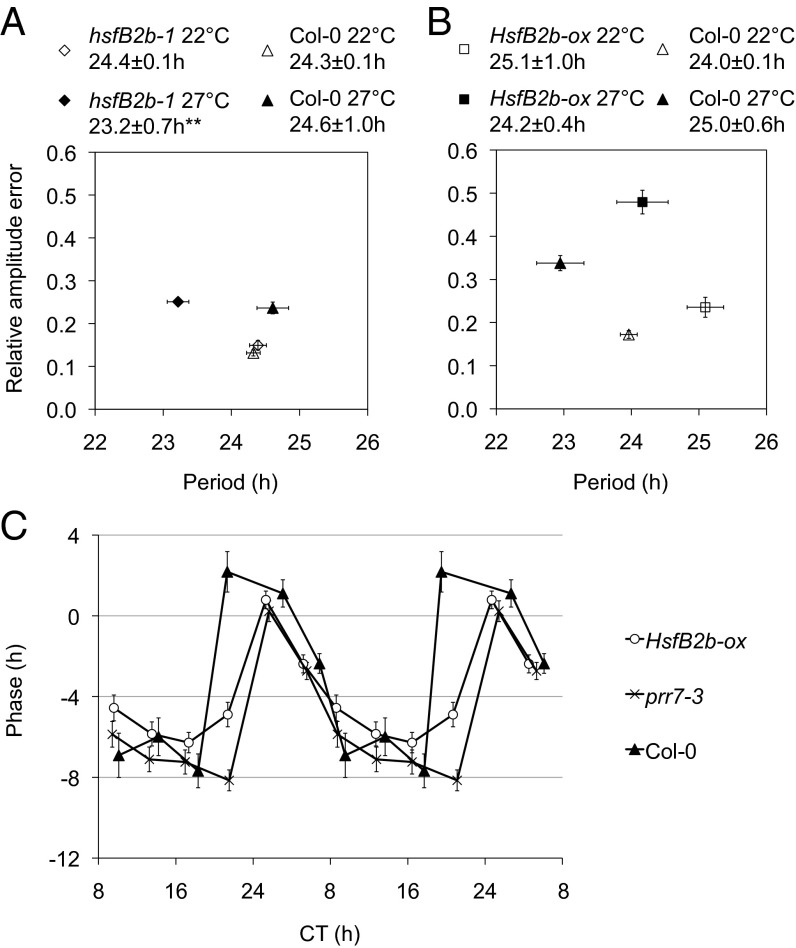
Fig. 3.
HsfB2b is important for sustenance of period under high temperature. (A and B) Temperature compensation of hsfB2b-1 PRR7:LUC and HsfB2b-ox CCA1:LUC at 22 °C and 27 °C. Seedlings were entrained under 12L:12D at 22 °C for 1 wk and transferred to constant conditions at ZT0. The graphs show the mean period estimate of single seedlings vs. the mean RAE of the estimates. Low RAE indicates robust rhythmicity. Shown are RAE-weighted period means and SEM at 22 °C and 27 °C for hsfB2b-1 PRR7:LUC (A) and HsfB2b-ox CCA1:LUC (B). **P < 0.01 (Student t test) for 22 °C compared with 27 °C; n = 10–20. (C) tPRCs of HsfB2b-ox, prr7-3, and Col-0. Seedlings harbored the CCA1:LUC reporter and were entrained for one week (under 12L:12D at 22 °C) and then released in LL at 22 °C. Phase resetting pulses of 38 °C were applied for 3h at different times during the circadian cycle (circadian time, CT; corrected for period length). The circadian phase changes were measured based on the circadian phase of nonpulsed plants. Error bars represent SEM of technical replicates; n = 15–26.
Temperature serves as an entrainment cue for the circadian clock. To test the effect of heat as an entrainment signal, we performed a temperature phase response curve (tPRC). We note that the shape of the warm tPRC (transfer from 18 °C to 22 °C) is similar to the cold tPRC (transfer from 22 °C to 12 °C); however, the break point (switch from phase delays to advances) is ∼CT6 (circadian time, CT) for the cold tPRC and ∼CT18 for warm tPRC (28, 29). We tested the resetting behavior of CCA1:LUC to heat pulses (38 °C) applied in LL after entrainment to 12-h light:12-h dark (12L:12D) cycles and constant 22 °C. In agreement with an earlier report (29), Col-0 seedlings displayed phase delays from subjective late day to middle of the night, and advances from late night to late morning (i.e., exhibiting little resetting during the day). Under these conditions, HsfB2b-ox displayed no phase advances compared with Col-0, along with a later breakpoint (Fig. 3C). Interestingly, we found a similar phenotype for the prr7-3 mutant (Fig. 3C), in agreement with a role for HsfB2b-mediated control of PRR7 expression in heat-responsive resetting.
HsfB2b Buffers the Clock Period After Salt Treatment.
OsHsfB2b, the HsfB2b ortholog in rice, was recently reported to be involved in salt and drought tolerance (30). Thus, we tested the effect of salt on circadian phenotypes. To this end, we transferred seedlings to salt-containing medium and monitored the bioluminescence rhythms under LL at 22 °C. WT seedlings maintained robust circadian oscillations except for an ∼1-h phase delay compared with control medium (Fig. S5). In contrast, the hsfB2b-1 mutant displayed short-period rhythms after transfer to salt (0.7 h shorter than Col-0 on salt and 0.7 h shorter than the hsfB2b-1 control on no salt). HsfB2b-ox maintained WT period length on average; however, we observed a greater variation in period length and less robust rhythms, as indicated by higher relative amplitude error (RAE) values (Figs. 3 A and B and 4 A and B).
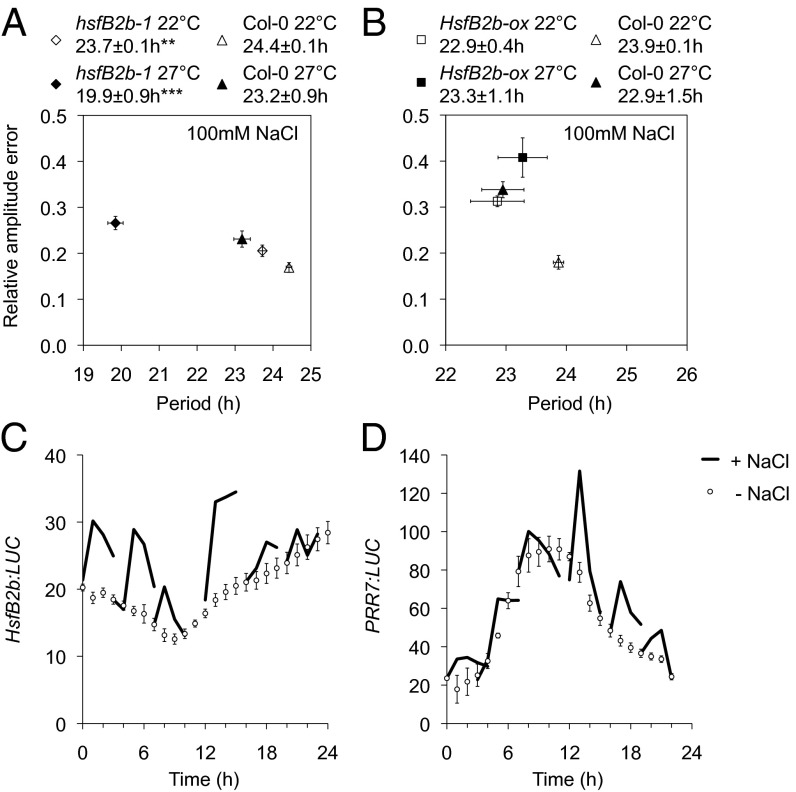
Fig. 4.
Circadian and HsfB2b-mediated responses on salt exposure. (A and B) Compensation response of circadian rhythms to salt. Shown are RAE-weighted period means and SEM for hsfB2b-1 PRR7:LUC (A) and HsfB2b-ox CCA1:LUC (B) on 100 mM NaCl at 22 °C and 27 °C. **P < 0.01; ***P < 0.001 (Student t test) for 22 °C compared with 27 °C; n = 11–19. (C and D) The HsfB2b and PRR7 promoters have a gated response to salt exposure. One-week-old seedlings were sprayed with 5 M NaCl at 4-h intervals under LL, and luminescence was monitored for ∼6 h and compared with control seedlings. The luminescence at each time point was normalized to the luminescence before treatment. Error bars represent SEM of three biological replicates for HsfB2b:LUC and two biological replicates for PRR7:LUC; n = 9–18.
In nature, several stress conditions often occur simultaneously (31). We tested the circadian rhythms of hsfB2b-1 and HsfB2b-ox seedlings under the combination of salt and higher temperature (27 °C). Interestingly, the heat-dependent short period of the hsfB2b-1 mutant was enhanced by salt (Figs. 3A and 4A). HsfB2b-ox exhibited no major change in period, but had less robust rhythms compared with the control plants (Fig. 4B). Collectively, HsfB2b expression is required to buffer the detrimental effects of drought and heat on the clock system.
The circadian clock gates acute effects on stress. To assess the acute salt response, we used the HsfB2b:LUC and PRR7:LUC reporter lines and measured their activity after salt application at different times of the day. We found that both reporters had a gated response to salt uptake and, interestingly, were inversely correlated, in agreement with a repressor function for HsfB2b on PRR7 expression (Fig. 4C). We note that the acute response to salt for PRR7 is similar to the response of the desiccation-responsive gene RD29A (32).
An Upstream Role for HsfB2b in Hypocotyl Elongation Growth.
To investigate the effect of the hsfB2b-1 mutation in a PRR7-sensitized background, we crossed the hsfB2b-1 mutation into proPRR7:HA-PRR7 (PRR7 minigene, 7MG), which features elevated PRR7 levels and a shortened circadian period (33). Because HsfB2b is involved in growth (Fig. 2), we compared hypocotyls of seedlings grown under short days at 22 °C and seedlings grown at 27 °C. Both lines displayed elongated hypocotyls after heat exposure, but the hsfB2b-1 7MG seedlings grew more than the single 7MG line (Fig. S6), indicating a role for HsfB2b-mediated repression of PRR7 after heat exposure.
PRR7 is a transcriptional repressor and is associated with several genomic loci at ZT12 (34). To identify PRR7-regulated genes involved in the thermosensory regulation of growth, we looked at the diurnal expression of PRR7 target genes (PRR7-ChIP dataset) using available microarray data (DIURNAL database) (25, 34). Specifically, we reasoned that genes with increased expression in short-day photoperiods compared with long-day photoperiods were important, because the clock controls the phase of growth (35, 36). We identified 47 genes that were phase-advanced (1–9 h; Table S1). Apart from core clock genes (CCA1, LUX, and PRR7), many genes included in this list are known to be involved in hypocotyl growth, including PHYTOCHROME INTERACTING FACTOR4 (PIF4), PIF5, REVEILLE2 (RVE2), RVE7, ELONGATED HYPOCOTYL5 (HY5), HY5 HOMOLOG (HYH), and members of the B-BOX (BBX) family (BBX24/STO, BBX25/STH, and BBX29) (35–41).
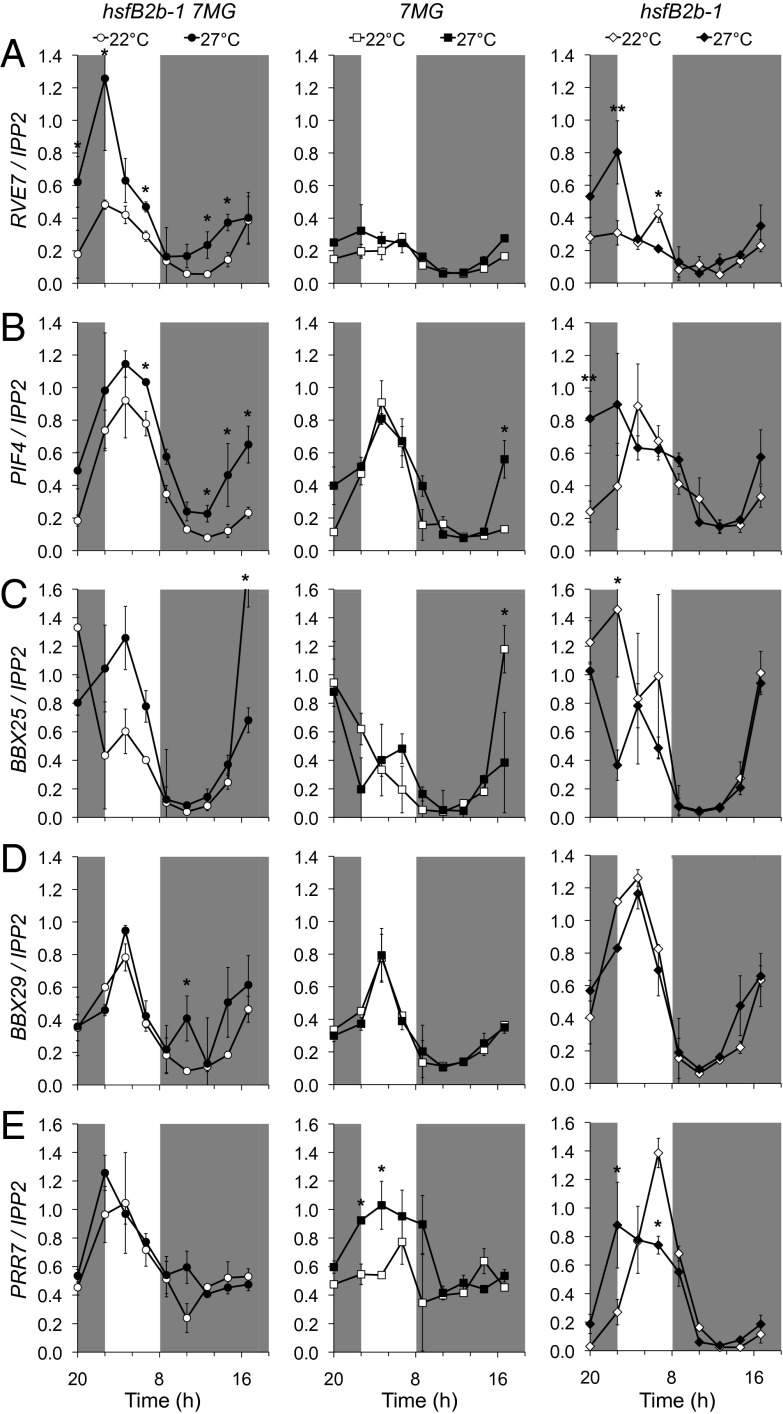
A subset of these genes (RVE7, PIF4, BBX25, and BBX29) exhibited elevated expression at certain time points at 27 °C compared with 22 °C in hsfB2b-1 7MG, a temperature phenotype that was not observed in the 7MG line (Fig. 5 and Fig. S7). We note that the overexpression of PIF4, BBX25, or BBX29 leads to elongated hypocotyls (35, 42) (Fig. S8), whereas RVE7 may be a negative regulator of hypocotyl growth (37, 43).
Fig. 5.
Loss of HsfB2b in the proPRR7:HA-PRR7 (7MG) background derepresses gene expression related to growth under warm short days. Shown are RVE7 (A), PIF4 (B), BBX25 (C), BBX29 (D), and PRR7 (E) expression in hsfB2b-1 7MG, 7MG, and hsfB2b-1 under 22 °C and 27 °C short days. The seedlings were grown under short-day photoperiods at room temperature (22 °C) or under warm short days at 27 °C. For the latter, seedlings were grown at 22 °C for the first 3 d and then transferred to 27 °C. The seedlings were harvested on day 7. Error bars represent the SD of two biological replicates. *P < 0.05 (Student t test) for 22 °C compared with 27 °C.
We propose two possible scenarios for HsfB2b regulation of growth. First, HsfB2b may act through the repression of PRR7 after a temperature increase. Second, HsfB2b may function independent of PRR7 to regulate common target genes. Supporting the latter scenario, RVE7 is derepressed at 27 °C in the hsfB2b-1 single mutant, and PRR7 levels are unchanged in hsfB2b-1 7MG and 7MG (Fig. 5 and Fig. S7). Collectively, the output genes PIF4, BBX25, BBX29, and RVE7 are candidate members of the HsfB2b- and PRR7-mediated pathway(s) controlling thermosensory hypocotyl growth.
Discussion
In this work, we positioned HsfB2b in the temperature input pathway to the circadian clock by transcriptional regulation of PRR7. A study of Hsf1’s action on the circadian clock in mammalian cells was reported recently; however, the molecular mechanism remains unknown (44, 45). In general, Hsfs have been described to target HSP genes via multiple HSE repeats in their promoters, and few studies exist where Hsfs regulate non-HSP genes (46–48). Importantly, mammalian Hsfs are known to control the expression of various developmental genes in addition to HSPs, and it recently has become clear that the stress-independent expression of HSF1 is crucial in oncogenesis (49, 50). Thus, it appears that the low basal circadian expression of HsfB2b (DIURNAL; Fig. 2E) may have important functions, specifically in maintaining the transcriptional state of the circadian clock. In future studies, it would be interesting to determine the degree to which Hsf-mediated input to the circadian system is evolutionarily conserved.
Temperature compensation is a key property of the circadian clock. It has been shown that the morning genes of the circadian system, including PRR7, were important for maintenance of period length under divergent temperatures (10). We found that the hsfB2b-1 mutant was uncompensated and had a shorter period than WT specifically at the higher temperature (Fig. 3 A and B). Repression of PRR7 by HsfB2b may maintain PRR7 levels, and this correlates with period length; that is, it is known that 7MG lines can have a short period and the prr7-3 mutant can have a long period under LL (3, 33). Gating of acute inputs to the circadian system is another important feature of the circadian clock. We found that the HsfB2b and PRR7 promoters responded acutely to the application of salt, and that the salt-induced patterns had a circadian profile. Possibly, the gated profile of HsfB2b expression is directly connected to its circadian expression (Figs. 2 D and E and 4 C and D). It was previously shown that RD29A had a gated induction by salt, and that this likely is connected to GI, a core circadian protein involved in flowering time (32). Future studies will help determine whether there is a direct connection between PRR7 and GI activities regarding circadian compensation of salt and drought responses.
In the mammalian clock system, Hsf1 has a key role in clock resetting to temperature (44, 45). We found that HsfB2b-ox altered the resetting to warm temperature in Arabidopsis (Fig. 3C). The tPRC for HsfB2b-ox was not identical to the tPRC for the prr7-3 mutant, possibly because HsfB2b also represses other genes involved in temperature perception (e.g., Hsp), which may provide feedback for the regulation of circadian rhythms (22, 51). Of note, misexpression due to the ubiquitous tissue expression of proUBQ10:HsfB2b-GFP could explain the strong growth phenotypes observed in HsfB2b-ox, given that endogenous HsfB2b expression is strongest in roots and floral organs and overlaps with PRR7 expression only in these tissues (52). PRR7 is expressed in all organs except siliques (52). We also note here that under short days, HsfB2b-ox flowered later than prr7-3, and that the heat-dependent acceleration of flowering at 27 °C compared with at 22 °C was diminished for HsfB2b-ox (Fig. S2). Hsp90 is involved in the proteostasis of clock proteins, and down-regulation of Hsp90 using RNAi resulted in late-flowering plants, a phenotype similar to HsfB2b-ox (53, 54).
Hsf genes are abundant in plants compared with other organisms, and the activity of certain Hsfs are specific to different biotic or abiotic stresses (16). Thellungiella salsuginea, an extremophile closely related to Arabidopsis, has 28 Hsfs, supporting the importance of this transcription factor family in abiotic stress responses (55). A previous transcriptomic study revealed the involvement of HsfB2b in the necrotrophic pathogen response, but the direct molecular target of HsfB2b is in the corresponding signaling pathway(s) remains unclear (26). Another microarray study revealed that HsfB2b has overlapping functions with HsfB1 in thermotolerance, and that the Hsp genes are misregulated in the double-mutant background (22). In rice, OsHsfB2b is a negative regulator of drought and salt tolerance (30). We found that HsfB2b has a positive role in maintaining the circadian period under salt stress (Fig. 4). Future experiments will reveal to what degree the function of HsfB2b is conserved between monocotyledonous and dicotyledonous plants.
The circadian clock controls rhythmic hypocotyl growth, which occurs at the end of the night. With regard to PRR7 target genes known to be involved in growth, PIF4 has been characterized extensively (35, 36, 56, 57). In addition to PIF4, we found increased levels of RVE7, BBX25, and BBX29 at 27 °C compared with 22 °C in the hsfB2b-1 7MG double transgenic, an increase not seen in the single 7MG line (Fig. 5). Our finding of several genes with altered expression in this analysis is hardly a surprise; for example, PRR5 also has RVE7 and BBX29 as direct targets (58), and BBX25 is working with HY5 in a signaling pathway specific to UV-B responses, whereas PIF4 is involved in growth regarding red light, temperature, and GA signaling (56, 59). There is cross-talk between these pathways; for example, it was recently shown that the PIFs and HY5 share binding sites in their target promoters (60), and there are feedback loops within the circadian clock involving PRR7 and PRR5. RVE7 is an interesting candidate because its promoter contains HSE repeats, and no HSE repeats are found in the promoters of the other PRR7 targets that we investigated here (www.arabidopsis.org). Future studies will help determine the molecular mode of action of the transcription factor complexes controlling hypocotyl growth, and identify whether the PRR7-mediated repression of the four candidate target loci is temperature-regulated.
Here we have provided further proof of concept that the Arabidopsis TF ORF clone collection is a sensitized genomic tool for identifying key transcriptional regulators in the expanding network of the circadian system (23). Our discovery that HsfB2b is involved in heat and salt input signaling to the circadian clock provides a molecular target for improving stress tolerance in plants.
Materials and Methods
Promoter Analysis.
Details are provided in SI Materials and Methods.
Plant Materials and Growth Conditions.
All Arabidopsis lines used were in the Col-0 background (reporter lines PRR7:LUC for Hsf-ox; CCR2:LUC for 7MG lines). Mutant and transgenic lines were prr7-3 (3), hsfB2b-1 (SALK-047291) (26), proPRR7:HA-PRR7 prr7-3 CCR2:LUC #151 (7MG) (33), and CCA1:LUC (61). Hsf-ox transgenic lines were generated in the PRR7:LUC background using A. tumefaciens-mediated transformation (floral dip) (62). HsfB2b-ox CCA1:LUC was generated by crossing HsfB2b-ox #23 to CCA1:LUC. For flowering time measurements, seeds were sown in soil (Sunshine Mix #3), fertilized once monthly (15–16-17 Peat Lite Special; Everris) and grown under a controlled environment (Conviron) with the indicated photoperiod at ∼20 °C. The total leaf number was scored at the time of bolting. For hypocotyl measurement, seeds were stratified for 3 d and then grown on 1× MS (no sucrose) vertical plates under short days in a growth chamber (Percival) at ∼20 μE intensity at the indicated temperature. The seedlings were scanned on day 6, and the hypocotyls were measured using ImageJ software. For RNA isolation, seedlings were grown in growth chambers (Percival) with lights at 60–80 μE intensity. Flowering and growth experiments were performed two or three times, yielding similar results.
Molecular Cloning and Constructs.
For the Y1H screen, the −673/−328-bp region of the PRR7 promoter was subcloned in pENTR/TOPO-D (Gateway; Invitrogen) and then cloned in pGLacZi (63). Primers are listed in Table S2. The primers for site-directed mutagenesis of pENTR-proPRR7 were designed using QuikChange Primer Design (Agilent Technologies), and PCR was performed with PfuTurbo DNA polymerase (Agilent Technologies), followed by DpnI digestion and cloning. The HSEmut constructs were verified by sequencing. The full-length promoter of HsfB2b (3,082 bp, including the 5′ UTR) and PRR7 (1,096 bp, including the 5′ UTR), respectively, was amplified with ExTaq polymerase (TaKaRa) from gDNA, subcloned in pENTR/TOPO-D, and fused to LUC in the Gateway-compatible destination vector pFLASH (64). The proUBQ10:Hsf-GFP (Hsf-ox) constructs were generated by multisite cloning in R4pGWB504 (65), the Hsf ORFs subcloned in pENTR/TOPO-D and proUBQ10 (646 bp upstream of ATG; At4g05320) in pDONRP4P1R (Invitrogen).
Luciferase Imaging.
Details are provided in SI Materials and Methods.
Gene Expression Analysis.
Details are provided in SI Materials and Methods.
Yeast One-Hybrid Screen.
The pGLacZi-proPRR7 and HSEmut constructs were integrated into the genome of the yeast strain YM4271 using NcoI. The yeast one-hybrid screen and HSEmut assays were performed as described previously (23, 66). Ortho-nitrophenyl-β-galactoside (ONPG) served as the substrate for measurement of β-galactosidase activity.
Arabidopsis Gene Identifiers.
Arabidopsis gene identifiers were as follows: BBX25, At2g31380; BBX29, At5g54470; CCA1, At2g46830; HsfB2b, At4g11660; PIF4, At2g43010; PRR7, At5g02810; RVE7, At1g18330; UBQ10, At4g02310.
Supplementary Material
Acknowledgments
We thank E. M. Farre for the PRR7:LUC reporter line, E. E. Hamilton for BBX29-ox seeds, and M. Tien for technical assistance. We also thank M. A. Nohales-Zafra, S. E. Sanchez, P. Tripathi, and former group members for helpful discussions. The research reported in this paper was supported by a postdoctoral fellowship from Deutsche Forschungsgemeinschaft (to E.K.) and by National Institute of General Medical Sciences Grants R01 GM056006, R01 GM067837, and RC2 GM092412 (to S.A.K.) and R01 GM056006 (to J.L.P.-P. as a coinvestigator).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418483111/-/DCSupplemental.
References
- 1.Rensing L, Ruoff P. Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol Int. 2002;19(5):807–864. doi: 10.1081/cbi-120014569. [DOI] [PubMed] [Google Scholar]
- 2.Nagel DH, Kay SA. Complexity in the wiring and regulation of plant circadian networks. Curr Biol. 2012;22(16):R648–R657. doi: 10.1016/j.cub.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol. 2005;15(1):47–54. doi: 10.1016/j.cub.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 4.Nakamichi N, et al. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell. 2010;22(3):594–605. doi: 10.1105/tpc.109.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makino S, Matsushika A, Kojima M, Oda Y, Mizuno T. Light response of the circadian waves of the APRR1/TOC1 quintet: When does the quintet start singing rhythmically in Arabidopsis? Plant Cell Physiol. 2001;42(3):334–339. doi: 10.1093/pcp/pce036. [DOI] [PubMed] [Google Scholar]
- 6.Michael TP, et al. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science. 2003;302(5647):1049–1053. doi: 10.1126/science.1082971. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson ME, Hanano S, Southern MM, Hall A, Millar AJ. Response regulator homologues have complementary, light-dependent functions in the Arabidopsis circadian clock. Planta. 2003;218(1):159–162. doi: 10.1007/s00425-003-1106-4. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y, et al. Comparative genetic studies on the APRR5 and APRR7 genes belonging to the APRR1/TOC1 quintet implicated in circadian rhythm, control of flowering time, and early photomorphogenesis. Plant Cell Physiol. 2003;44(11):1119–1130. doi: 10.1093/pcp/pcg148. [DOI] [PubMed] [Google Scholar]
- 9.Salomé PA, McClung CR. PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell. 2005;17(3):791–803. doi: 10.1105/tpc.104.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salomé PA, Weigel D, McClung CR. The role of the Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. Plant Cell. 2010;22(11):3650–3661. doi: 10.1105/tpc.110.079087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClung CR, Davis SJ. Ambient thermometers in plants: From physiological outputs towards mechanisms of thermal sensing. Curr Biol. 2010;20(24):R1086–R1092. doi: 10.1016/j.cub.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 12.McWatters HG, Bastow RM, Hall A, Millar AJ. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature. 2000;408(6813):716–720. doi: 10.1038/35047079. [DOI] [PubMed] [Google Scholar]
- 13.Nagano AJ, et al. Deciphering and prediction of transcriptome dynamics under fluctuating field conditions. Cell. 2012;151(6):1358–1369. doi: 10.1016/j.cell.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Millar AJ, Kay SA. Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA. 1996;93(26):15491–15496. doi: 10.1073/pnas.93.26.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nover L, et al. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones. 2001;6(3):177–189. doi: 10.1379/1466-1268(2001)006<0177:aathst>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scharf K-D, Berberich T, Ebersberger I, Nover L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim Biophys Acta. 2012;1819(2):104–119. doi: 10.1016/j.bbagrm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 17.von Koskull-Döring P, Scharf KD, Nover L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007;12(10):452–457. doi: 10.1016/j.tplants.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Franco-Zorrilla JM, et al. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci USA. 2014;111(6):2367–2372. doi: 10.1073/pnas.1316278111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda M, Ohme-Takagi M. A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 2009;50(5):970–975. doi: 10.1093/pcp/pcp048. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X, Thalor SK, Takahashi Y, Berberich T, Kusano T. An inhibitory effect of the sequence-conserved upstream open-reading frame on the translation of the main open-reading frame of HsfB1 transcripts in Arabidopsis. Plant Cell Environ. 2012;35(11):2014–2030. doi: 10.1111/j.1365-3040.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- 21.Busch W, Wunderlich M, Schöffl F. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 2005;41(1):1–14. doi: 10.1111/j.1365-313X.2004.02272.x. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 2011;157(3):1243–1254. doi: 10.1104/pp.111.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruneda-Paz JL, et al. A genome-scale resource for the functional characterization of Arabidopsis transcription factors. Cell Reports. 2014;8(2):622–632. doi: 10.1016/j.celrep.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharti K, et al. Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB-binding protein ortholog HAC1. Plant Cell. 2004;16(6):1521–1535. doi: 10.1105/tpc.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael TP, et al. Network discovery pipeline elucidates conserved time-of-day–specific cis-regulatory modules. PLoS Genet. 2008;4(2):e14. doi: 10.1371/journal.pgen.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar M, et al. Heat shock factors HsfB1 and HsfB2b are involved in the regulation of Pdf1.2 expression and pathogen resistance in Arabidopsis. Mol Plant. 2009;2(1):152–165. doi: 10.1093/mp/ssn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biochem Sci. 2012;37(3):118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Michael TP, Salomé PA, McClung CR. Two Arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proc Natl Acad Sci USA. 2003;100(11):6878–6883. doi: 10.1073/pnas.1131995100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thines B, Harmon FG. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proc Natl Acad Sci USA. 2010;107(7):3257–3262. doi: 10.1073/pnas.0911006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang J, et al. Heat shock factor OsHsfB2b negatively regulates drought and salt tolerance in rice. Plant Cell Rep. 2013;32(11):1795–1806. doi: 10.1007/s00299-013-1492-4. [DOI] [PubMed] [Google Scholar]
- 31.Colmenero-Flores JM, Rosales MA. Interaction between salt and heat stress: When two wrongs make a right. Plant Cell Environ. 2014;37(5):1042–1045. doi: 10.1111/pce.12229. [DOI] [PubMed] [Google Scholar]
- 32.Kim W-Y, et al. Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis. Nat Commun. 2013;4(1352):1352. doi: 10.1038/ncomms2357. [DOI] [PubMed] [Google Scholar]
- 33.Farré EM, Kay SA. PRR7 protein levels are regulated by light and the circadian clock in Arabidopsis. Plant J. 2007;52(3):548–560. doi: 10.1111/j.1365-313X.2007.03258.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Carlsson J, Takeuchi T, Newton L, Farré EM. Direct regulation of abiotic responses by the Arabidopsis circadian clock component PRR7. Plant J. 2013;76(1):101–114. doi: 10.1111/tpj.12276. [DOI] [PubMed] [Google Scholar]
- 35.Nozue K, et al. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448(7151):358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 36.Nusinow DA, et al. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature. 2011;475(7356):398–402. doi: 10.1038/nature10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ang LH, et al. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell. 1998;1(2):213–222. doi: 10.1016/s1097-2765(00)80022-2. [DOI] [PubMed] [Google Scholar]
- 38.Jiang L, et al. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 2012;22(6):1046–1057. doi: 10.1038/cr.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangappa SN, Botto JF. The BBX family of plant transcription factors. Trends Plant Sci. 2014;19(7):460–470. doi: 10.1016/j.tplants.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, et al. Constitutive expression of CIR1 (RVE2) affects several circadian-regulated processes and seed germination in Arabidopsis. Plant J. 2007;51(3):512–525. doi: 10.1111/j.1365-313X.2007.03156.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuno N, et al. The novel MYB protein EARLY-PHYTOCHROME-RESPONSIVE1 is a component of a slave circadian oscillator in Arabidopsis. Plant Cell. 2003;15(10):2476–2488. doi: 10.1105/tpc.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gangappa SN, et al. The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell. 2013;25(4):1243–1257. doi: 10.1105/tpc.113.109751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitsuda N, Umemura Y, Ikeda M, Shikata M. FioreDB: A database of phenotypic information induced by the chimeric repressor silencing technology (CRES-T) in Arabidopsis and floricultural plants. Plant Biotechnol. 2008;25(1):37–41. [Google Scholar]
- 44.Buhr ED, Yoo S-H, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinke H, et al. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008;22(3):331–345. doi: 10.1101/gad.453808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishizawa-Yokoi A, Yoshida E, Yabuta Y, Shigeoka S. Analysis of the regulation of target genes by an Arabidopsis heat shock transcription factor, HsfA2. Biosci Biotechnol Biochem. 2009;73(4):890–895. doi: 10.1271/bbb.80809. [DOI] [PubMed] [Google Scholar]
- 47.Schramm F, et al. The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol Biol. 2006;60(5):759–772. doi: 10.1007/s11103-005-5750-x. [DOI] [PubMed] [Google Scholar]
- 48.Hwang JE, et al. Overexpression of Arabidopsis dehydration-responsive element-binding protein 2C confers tolerance to oxidative stress. Mol Cells. 2012;33(2):135–140. doi: 10.1007/s10059-012-2188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendillo ML, et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150(3):549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan J, Wang H, Liu Y, Shao C. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLOS Comput Biol. 2008;4(10):e1000193. doi: 10.1371/journal.pcbi.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter D, et al. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE. 2007;2(8):e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim T-S, et al. HSP90 functions in the circadian clock through stabilization of the client F-box protein ZEITLUPE. Proc Natl Acad Sci U S A. 2011;108(40):16843–16848. doi: 10.1073/pnas.1110406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sangster TA, et al. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS ONE. 2007;2(7):e648. doi: 10.1371/journal.pone.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H-J, et al. Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc Natl Acad Sci USA. 2012;109(30):12219–12224. doi: 10.1073/pnas.1209954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar SV, et al. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature. 2012;484(7393):242–245. doi: 10.1038/nature10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nomoto Y, et al. A circadian clock- and PIF4-mediated double coincidence mechanism is implicated in the thermosensitive photoperiodic control of plant architectures in Arabidopsis thaliana. Plant Cell Physiol. 2012;53(11):1965–1973. doi: 10.1093/pcp/pcs141. [DOI] [PubMed] [Google Scholar]
- 58.Nakamichi N, et al. Transcriptional repressor PRR5 directly regulates clock-output pathways. Proc Natl Acad Sci USA. 2012;109(42):17123–17128. doi: 10.1073/pnas.1205156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Lucas M, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451(7177):480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 60.Toledo-Ortiz G, et al. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 2014;10(6):e1004416. doi: 10.1371/journal.pgen.1004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pruneda-Paz JL, Breton G, Para A, Kay SA. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science. 2009;323(5920):1481–1485. doi: 10.1126/science.1167206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 63.Helfer A, et al. LUX ARRHYTHMO encodes a nighttime repressor of circadian gene expression in the Arabidopsis core clock. Curr Biol. 2011;21(2):126–133. doi: 10.1016/j.cub.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gendron JM, et al. Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc Natl Acad Sci USA. 2012;109(8):3167–3172. doi: 10.1073/pnas.1200355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakagawa T, Ishiguro S, Kimura T. Gateway vectors for plant transformation. Plant Biotechnol. 2009;26(3):275–284. [Google Scholar]
- 66.Chow BY, et al. Transcriptional regulation of LUX by CBF1 mediates cold input to the circadian clock in Arabidopsis. Curr Biol. 2014;24(13):1518–1524. doi: 10.1016/j.cub.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.