Abstract
A differential solute clearance technique was used to evaluate glomerular capillary wall function in 20 patients with membranous glomerulopathy and massive proteinuria. The clearance of inulin, the filtration fraction, and the fractional clearance of uncharged dextrans of a radius of 28-48 A were depressed significantly below control values in 20 healthy volunteers (P less than 0.01). In contrast, the fractional clearance of dextrans of radius greater than 50 A was elevated markedly. A theoretical model of solute transport that depicts the major portion of the glomerular capillary wall as an isoporous membrane and the minor portion as a nondiscriminatory shunt pathway revealed the calculated glomerular ultrafiltration coefficient to be five times lower and mean pore radius of the major membrane component to be 4 A smaller than control values. However, the fraction of filtrate volume permeating the shunt pathway was three- to fourfold above control values and correlated strongly in individual patients with the fractional clearance of albumin (r = 0.76) and of IgG (r = 0.80). Lowering renal plasma flow by 24% during indomethacin therapy in seven patients resulted in a 74% reduction in proteinuria accompanied by a corresponding diminution of filtrate formed through the shunt pathway. Morphometric analysis of glomerular ultrastructure revealed the magnitude of depression of the glomerular filtration rate and of urinary protein leakage to be related strongly to changes in the epithelial layer of the glomerular capillary wall, but not to the density of subepithelial immune deposits. We conclude that glomerular capillaries in membranous glomerulopathy are characterized by a loss of ultrafiltration capacity and of barrier size-selectivity, and that subepithelial immune deposits do not provide a structural basis for these functional alterations.
Full text
PDF

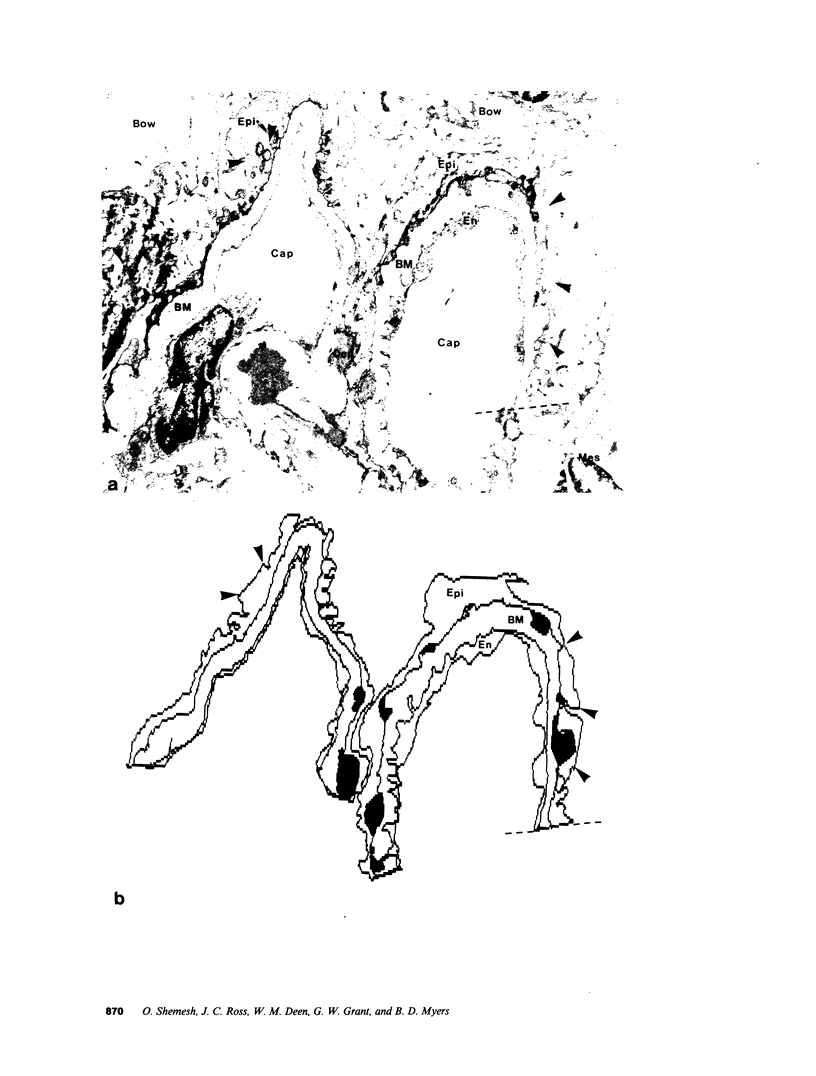

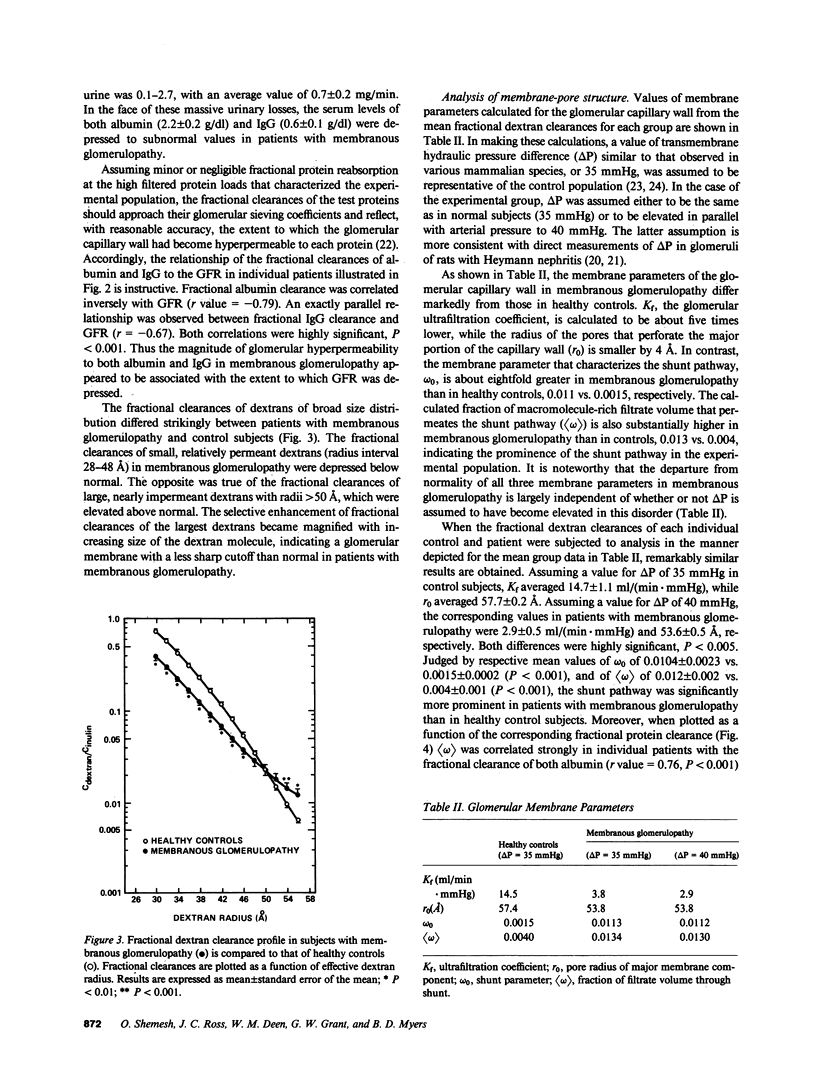
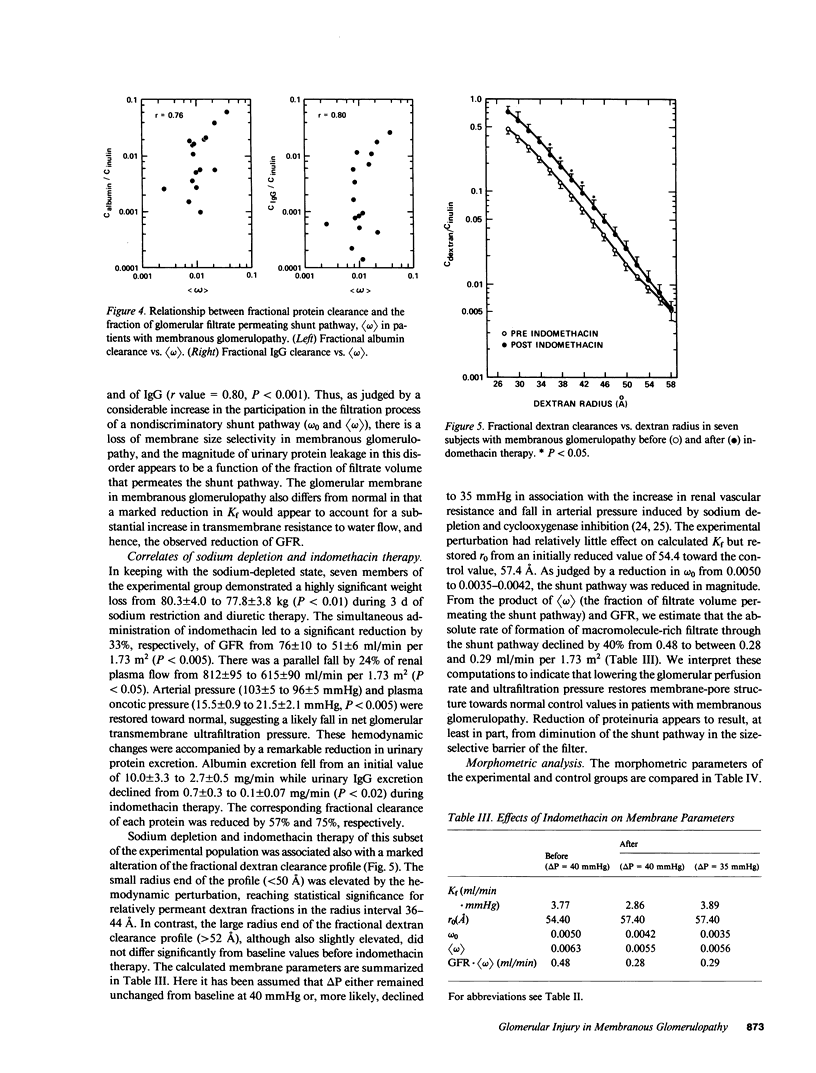
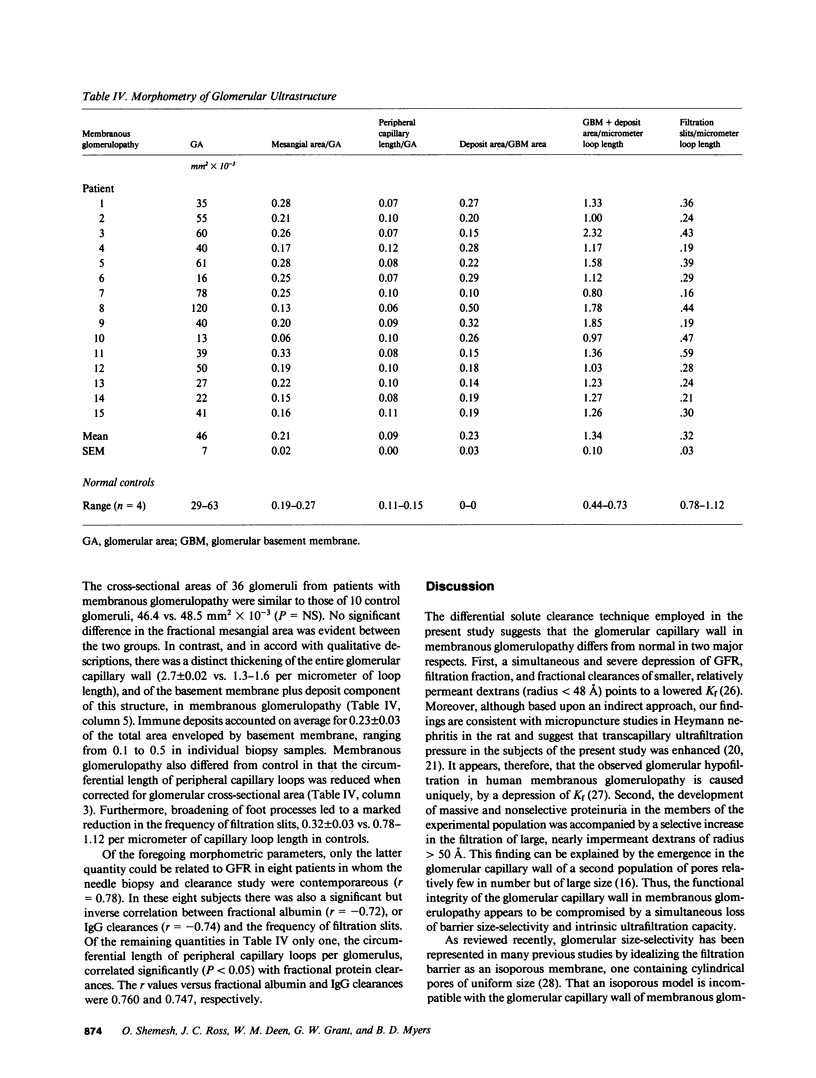



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. E., Wilson C. B., Gottschalk C. W. Pathophysiology of experimental glomerulonephritis in rats. J Clin Invest. 1974 May;53(5):1402–1423. doi: 10.1172/JCI107689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGSTROM J., BUCHT H., EK J., JOSEPHSON B., SUNDELL H., WERKO L. The renal extraction of para-aminohippurate in normal persons and in patients with diseased kidneys. Scand J Clin Lab Invest. 1959;11:361–375. doi: 10.3109/00365515909060466. [DOI] [PubMed] [Google Scholar]
- BRADLEY S. E., BRADLEY G. P., TYSON C. J., CURRY J. J., BLAKE W. D. Renal function in renal diseases. Am J Med. 1950 Dec;9(6):766–798. doi: 10.1016/0002-9343(50)90292-0. [DOI] [PubMed] [Google Scholar]
- BRODWALL E. K. RENAL EXTRACTION OF PAH IN RENAL DISEASE. Scand J Clin Lab Invest. 1964;16:12–20. doi: 10.3109/00365516409060477. [DOI] [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Bennett C. M., Glassock R. J., Brenner B. M., Troy J. L., Ueki I. F., Rasmussen B. Permselectivity of of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using neutral dextran. J Clin Invest. 1976 May;57(5):1272–1286. doi: 10.1172/JCI108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Ueki I. F., Troy J. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys J. 1975 Sep;15(9):887–906. doi: 10.1016/S0006-3495(75)85863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Ueki I. F., Troy J. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys J. 1975 Sep;15(9):887–906. doi: 10.1016/S0006-3495(75)85863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Bridges C. R., Brenner B. M. Biophysical basis of glomerular permselectivity. J Membr Biol. 1983;71(1-2):1–10. doi: 10.1007/BF01870670. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Bridges C. R., Brenner B. M., Myers B. D. Heteroporous model of glomerular size selectivity: application to normal and nephrotic humans. Am J Physiol. 1985 Sep;249(3 Pt 2):F374–F389. doi: 10.1152/ajprenal.1985.249.3.F374. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Erwin D. T., Donadio J. V., Jr, Holley K. E. The clinical course of idiopathic membranous nephropathy. Mayo Clin Proc. 1973 Oct;48(10):697–712. [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuren G., Grond J., Hoedemaeker P. J. In situ formation of subepithelial glomerular immune complexes in passive serum sickness. Kidney Int. 1980 May;17(5):631–637. doi: 10.1038/ki.1980.74. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Jennings R. B., Earle D. P. Membranous glomerulonephritis: long-term serial observations on clinical course and morphology. Kidney Int. 1973 Jul;4(1):36–56. doi: 10.1038/ki.1973.78. [DOI] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Hoyer J. R., Seiler M. W., Brenner B. M. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982 Jan;69(1):185–198. doi: 10.1172/JCI110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Cavallo T. Glomerular permeability. Ultrastructural studies in New Zealand black/white mice using polyanionic ferritin as a molecular probe. Lab Invest. 1977 Sep;37(3):265–275. [PubMed] [Google Scholar]
- Madaio M. P., Salant D. J., Adler S., Darby C., Couser W. G. Effect of antibody charge and concentration on deposition of antibody to glomerular basement membrane. Kidney Int. 1984 Oct;26(4):397–403. doi: 10.1038/ki.1984.188. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Steffes M. W., Ellis E. N., Sutherland D. E., Brown D. M., Goetz F. C. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984 Oct;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. D., Okarma T. B., Friedman S., Bridges C., Ross J., Asseff S., Deen W. M. Mechanisms of proteinuria in human glomerulonephritis. J Clin Invest. 1982 Oct;70(4):732–746. doi: 10.1172/JCI110669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar L. G., Bell P. D., White R. W., Watts R. L., Williams R. H. Evaluation of the single nephron glomerular filtration coefficient in the dog. Kidney Int. 1977 Aug;12(2):137–149. doi: 10.1038/ki.1977.91. [DOI] [PubMed] [Google Scholar]
- Noel L. H., Zanetti M., Droz D., Barbanel C. Long-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patients. Am J Med. 1979 Jan;66(1):82–90. doi: 10.1016/0002-9343(79)90486-8. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Kirschbaum B. B., Landwehr D. M. Micropuncture studies of the mechanisms of normal and pathologic albuminuria. Contrib Nephrol. 1981;24:1–7. doi: 10.1159/000395223. [DOI] [PubMed] [Google Scholar]
- Pessina A. C., Hulme B., Peart W. S. Renin induced proteinuria and the effects of adrenalectomy. II. Morphology in relation to function. Proc R Soc Lond B Biol Sci. 1972 Jan 18;180(1058):61–71. doi: 10.1098/rspb.1972.0005. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Venkatachalam M. A. Glomerular permeability of macromolecules. Effect of molecular configuration on the fractional clearance of uncharged dextran and neutral horseradish peroxidase in the rat. J Clin Invest. 1979 Apr;63(4):713–717. doi: 10.1172/JCI109354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row P. G., Cameron J. S., Turner D. R., Evans D. J., White R. H., Ogg C. S., Chantler C., Brown C. B. Membranous nephropathy. Long-term follow-up and association with neoplasia. Q J Med. 1975 Apr;44(174):207–239. [PubMed] [Google Scholar]
- Salant D. J., Belok S., Madaio M. P., Couser W. G. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980 Dec;66(6):1339–1350. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E. E., Grupe W. E. The ultrastructure of the glomerular slit diaphragm in autologous immune complex nephritis. Lab Invest. 1976 Mar;34(3):298–305. [PubMed] [Google Scholar]
- Schneeberger E. E., O'Brien A., Grupe W. E. Altered glomerular permeability in Munich-Wistar rats with autologous immune complex nephritis. Lab Invest. 1979 Feb;40(2):227–235. [PubMed] [Google Scholar]
- Schneeberger E. E., Stavrakis G., McCarthy K. Alterations in glomerular anionic sites in autologous immune complex nephritis. Lab Invest. 1983 Oct;49(4):445–452. [PubMed] [Google Scholar]
- Schor N., Ichikawa I., Brenner B. M. Mechanisms of action of various hormones and vasoactive substances on glomerular ultrafiltration in the rat. Kidney Int. 1981 Oct;20(4):442–451. doi: 10.1038/ki.1981.160. [DOI] [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]
- de Heer E., Daha M. R., Bhakdi S., Bazin H., van Es L. A. Possible involvement of terminal complement complex in active Heymann nephritis. Kidney Int. 1985 Feb;27(2):388–393. doi: 10.1038/ki.1985.21. [DOI] [PubMed] [Google Scholar]



