Abstract
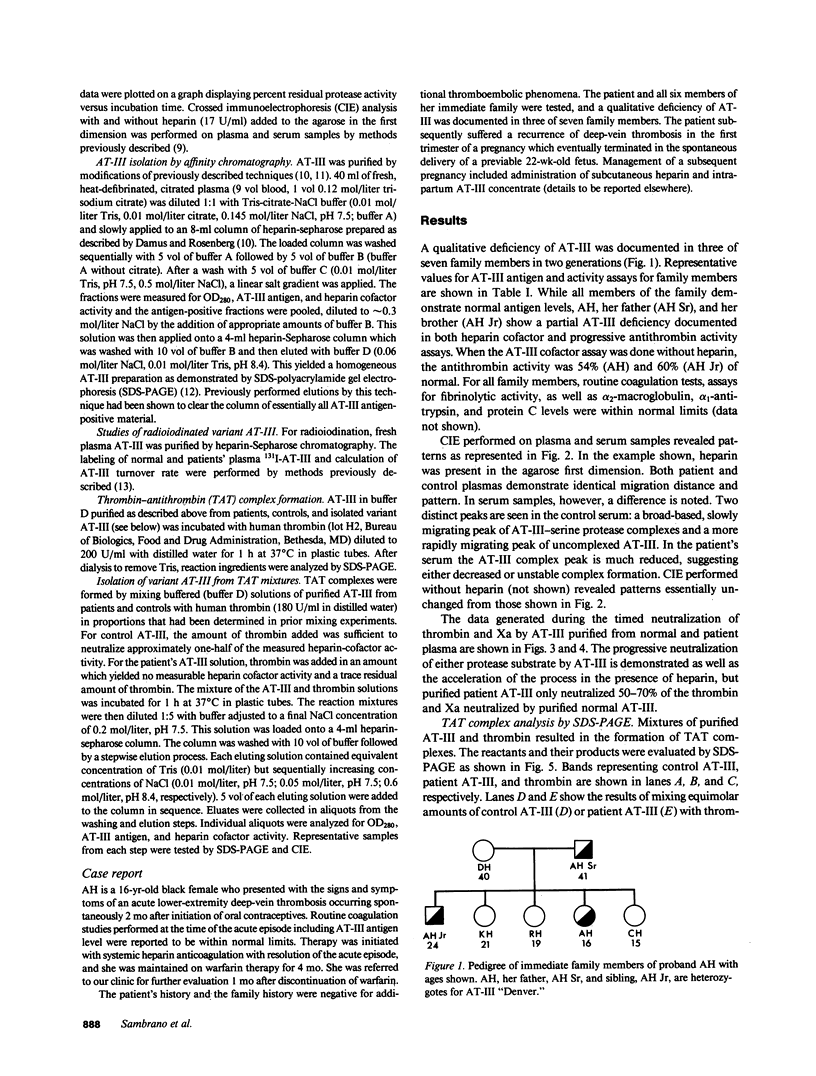
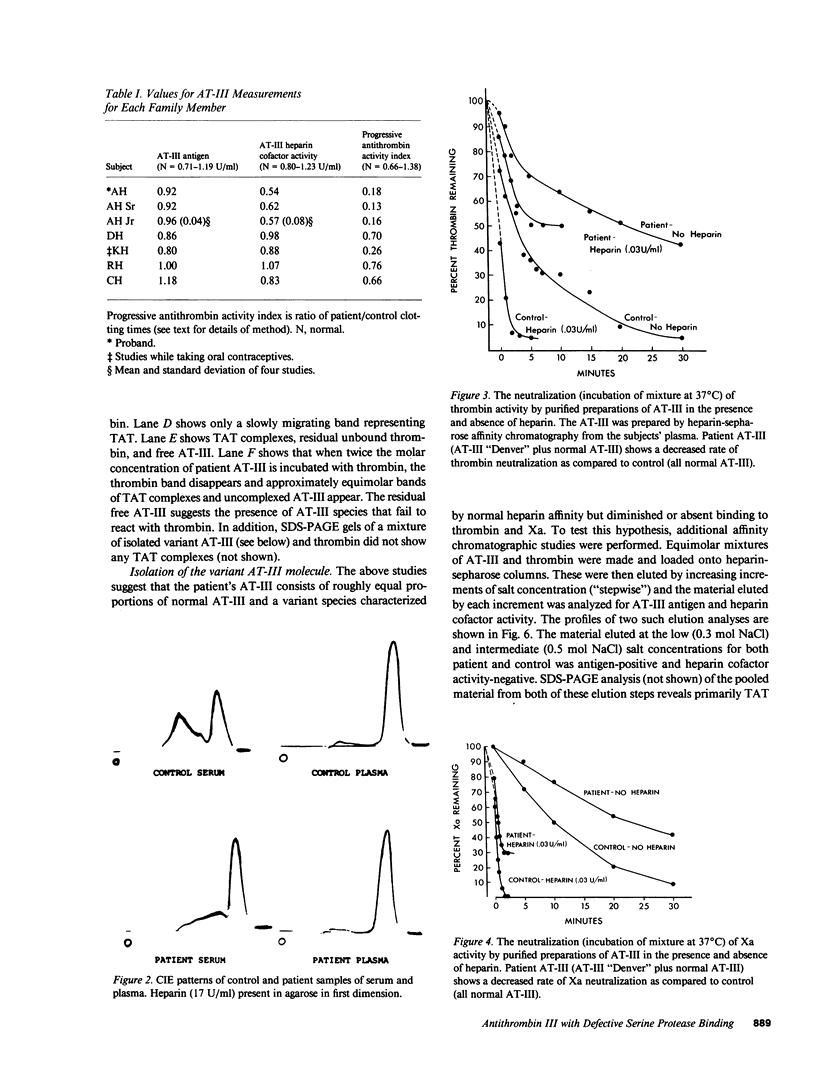
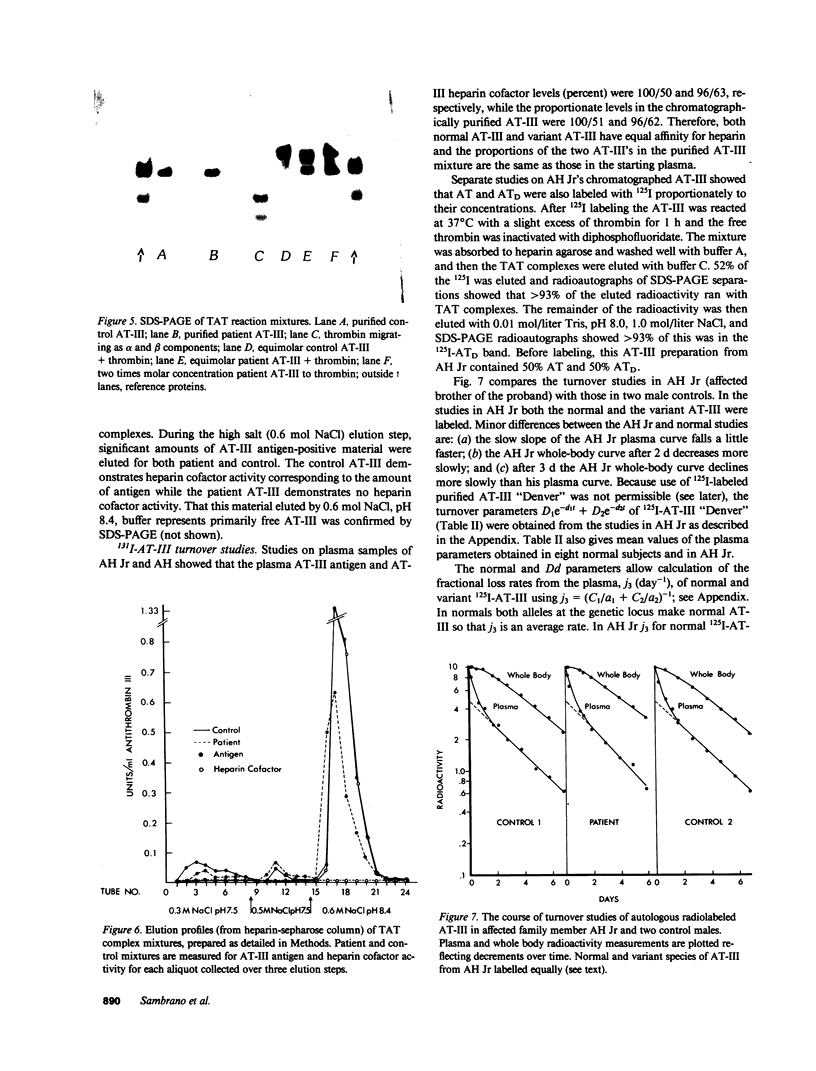
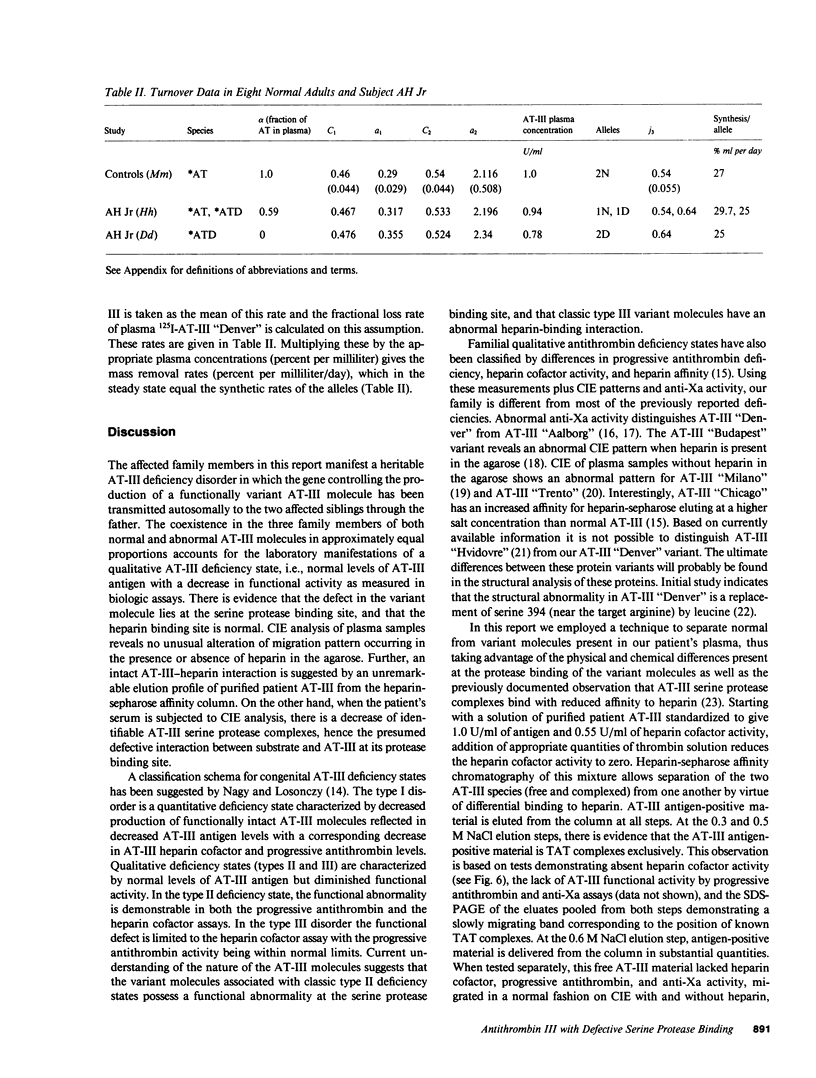
A hereditary (three family members) deficiency of antithrombin III (AT-III) in which AT-III antigen (AT-III ag) is normal in spite of low heparin cofactor and antithrombin activity is described. Plasma levels were: AT-III ag, 0.92-0.96 U/ml; AT-III heparin cofactor activity, 0.54-0.62 U/ml; progressive antithrombin activity index, 0.13-0.18; anti-Xa activity, 0.50-0.56 U/ml. Plasma crossed immunoelectrophoresis (CIE) patterns performed with and without added heparin were normal, but serum CIE revealed a decreased complex peak. Purification of the patient's plasma AT-III by heparin-sepharose affinity chromatography showed a normal protein recovery and elution profile, but the purified AT-III fraction showed only 50% of the normal progressive thrombin neutralization and anti-Xa activity. When thrombin-antithrombin (TAT) complexes were formed by incubating with excess thrombin, SDS-polyacrylamide gel electrophoresis (PAGE) analysis revealed that half the patient AT-III formed TAT complexes while the remainder migrated as free AT-III. All the control AT-III formed TAT complexes. The patient's nonreacting AT-III (AT-III "Denver"), isolated by affinity chromatography, showed CIE and SDS-PAGE migration patterns characteristic of normal AT-III but failed to bind thrombin or Xa. Calculations from turnover studies in one patient and normal subjects with autologous 131I-AT-III suggested that AT-III "Denver" is removed from the plasma slightly more rapidly than normal. These studies indicate that the patients' variant AT-III molecule was characterized by normal heparin interaction but defective binding and inhibition of thrombin and Xa. These characteristics allow isolation of the nonreactive variant molecule by heparin-sepharose affinity chromatography.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Jacobson L. J., Hathaway W. E. Inherited antithrombin III deficiency and cerebral thrombosis in a child. Pediatrics. 1980 Jan;65(1):125–131. [PubMed] [Google Scholar]
- Ambruso D. R., Leonard B. D., Bies R. D., Jacobson L., Hathaway W. E., Reeve E. B. Antithrombin III deficiency: decreased synthesis of a biochemically normal molecule. Blood. 1982 Jul;60(1):78–83. [PubMed] [Google Scholar]
- Andersson L. O., Engman L., Henningsson E. Crossed immunoelectrophoresis as applied to studies on complex formation. The binding of heparin to antithrombin III and the antithrombin III--thrombin complex. J Immunol Methods. 1977;14(3-4):271–281. doi: 10.1016/0022-1759(77)90138-7. [DOI] [PubMed] [Google Scholar]
- Bauer K. A., Ashenhurst J. B., Chediak J., Rosenberg R. D. Antithrombin "Chicago": a functionally abnormal molecule with increased heparin affinity causing familial thrombophilia. Blood. 1983 Dec;62(6):1242–1250. [PubMed] [Google Scholar]
- Carlström A. S., Liedén K., Björk I. Decreased binding of heparin to antithrombin following the interaction between antithrombin and thrombin. Thromb Res. 1977 Dec;11(6):785–797. doi: 10.1016/0049-3848(77)90107-4. [DOI] [PubMed] [Google Scholar]
- Damus P. S., Rosenberg R. D. Antithrombin-heparin cofactor. Methods Enzymol. 1976;45:653–669. doi: 10.1016/s0076-6879(76)45056-5. [DOI] [PubMed] [Google Scholar]
- EGEBERG O. INHERITED ANTITHROMBIN DEFICIENCY CAUSING THROMBOPHILIA. Thromb Diath Haemorrh. 1965 Jun 15;13:516–530. [PubMed] [Google Scholar]
- Girolami A., Marafioti F., Rubertelli M., Vicarioto M. A., Cappellato G., Mazzuccato M. Antithrombin III Trento. A 'new' congenital AT III abnormality with a peculiar crossed-immunoelectrophoretic pattern in the absence of heparin. Acta Haematol. 1984;72(2):73–82. doi: 10.1159/000206364. [DOI] [PubMed] [Google Scholar]
- Goodnight S. H., Jr, Schaeffer J. L., Sheth K. Measurement of antithrombin III in normal and pathologic states using chromogenic substrate S-2238. Comparison with immunoelectrophoretic and factor Xa inhibition assays. Am J Clin Pathol. 1980 May;73(5):639–647. doi: 10.1093/ajcp/73.5.639. [DOI] [PubMed] [Google Scholar]
- Jørgensen M., Petersen L. C., Thorsen S. Purification and characterization of hereditary abnormal antithrombin III with impaired thrombin binding. J Lab Clin Med. 1984 Aug;104(2):245–256. [PubMed] [Google Scholar]
- Odegård O. R., Lie M., Abildgaard U. Antifactor Xa activity measured with amidolytic methods. Haemostasis. 1976;5(5):265–275. doi: 10.1159/000214145. [DOI] [PubMed] [Google Scholar]
- Reeve E. B., Leonard B., Wentland S. H., Damus P. Studies with 131I-labelled antithrombin III in dogs. Thromb Res. 1980 Nov 15;20(4):375–389. doi: 10.1016/0049-3848(80)90277-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Sas G., Blaskó G., Bánhegyi D., Jákó J., Pálos L. A. Abnormal antithrombin III (antithrombin III "Budapest") as a cause of a familial thrombophilia. Thromb Diath Haemorrh. 1974 Sep 30;32(1):105–115. [PubMed] [Google Scholar]
- Sas G., Pepper D. S., Cash J. D. Further investigations on antithrombin III in the plasmas of patients with the abnormality of antithrombin III Budapest. Thromb Diath Haemorrh. 1975 Jun 30;33(3):564–572. [PubMed] [Google Scholar]
- Sørensen P. J., Dyerberg J., Stoffersen E., Jensen M. K. Familial functional antithrombin III deficiency. Scand J Haematol. 1980 Feb;24(2):105–109. doi: 10.1111/j.1600-0609.1980.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Sørensen P. J., Sas G., Petó I., Blaskó G., Kremmer T., Samu A. Distinction of two pathologic antithrombin III molecules: antithrombin III "Aalborg' and antithrombin III "Budapest'. Thromb Res. 1982 May 1;26(3):211–219. doi: 10.1016/0049-3848(82)90142-6. [DOI] [PubMed] [Google Scholar]
- Von Kaulla E., Von Kaulla K. N. Antithrombin 3 and diseases. Am J Clin Pathol. 1967 Jul;48(1):69–80. doi: 10.1093/ajcp/48.1.69. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]