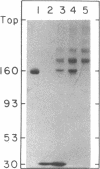
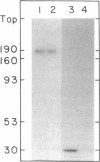
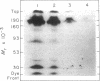
Abstract
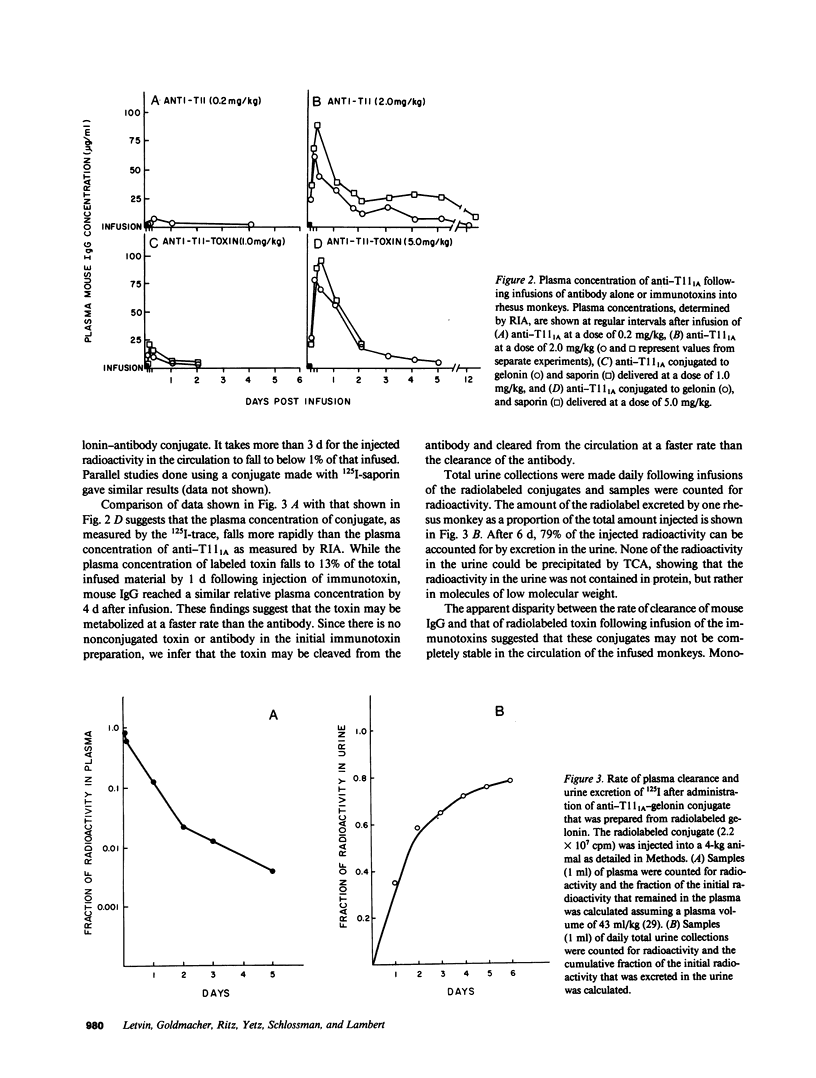
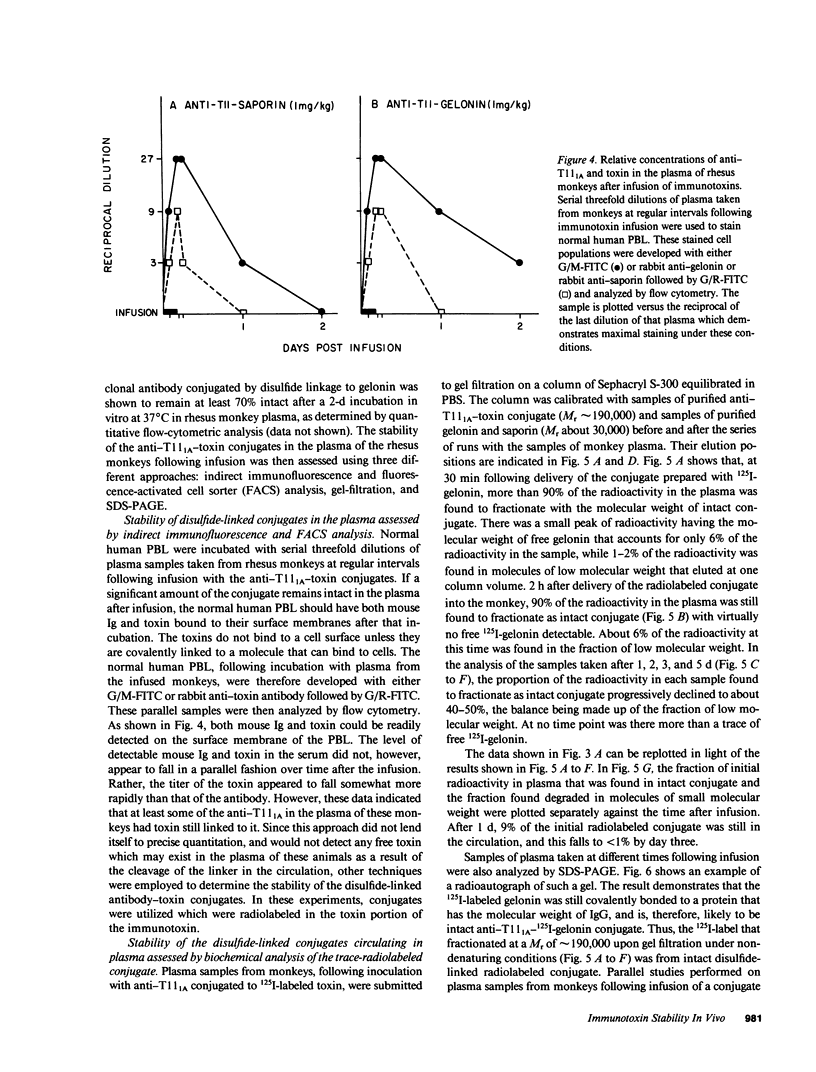
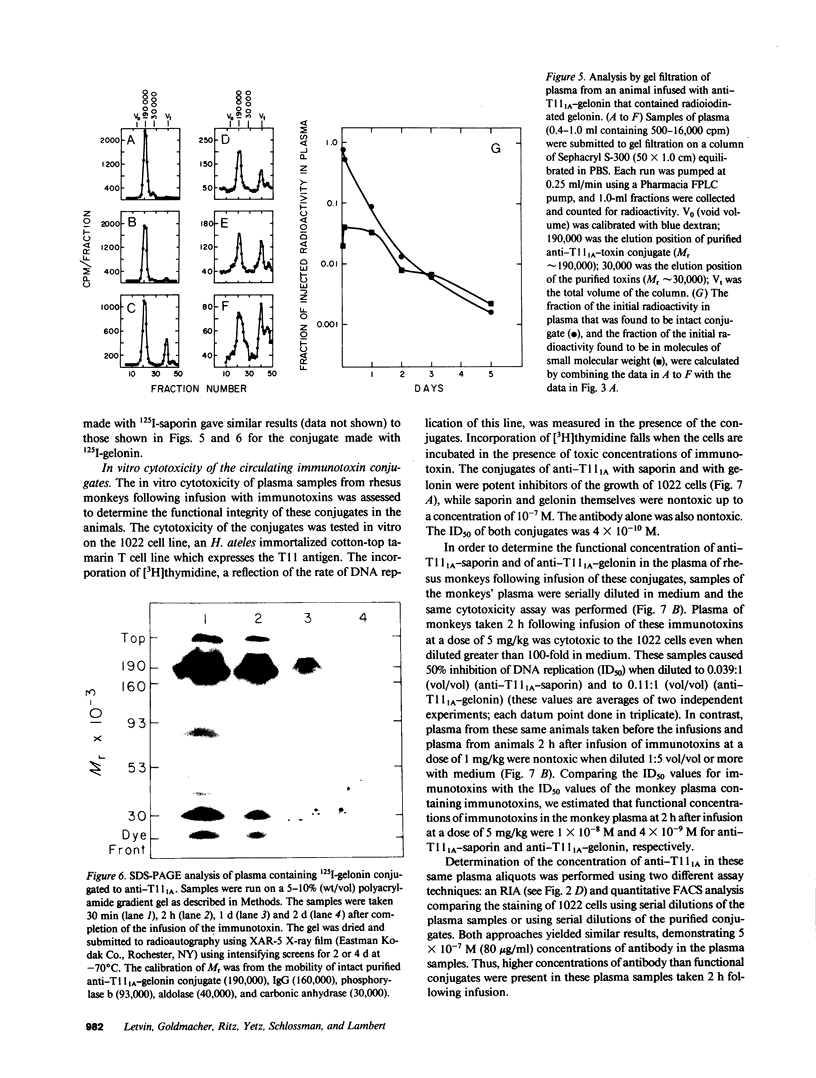
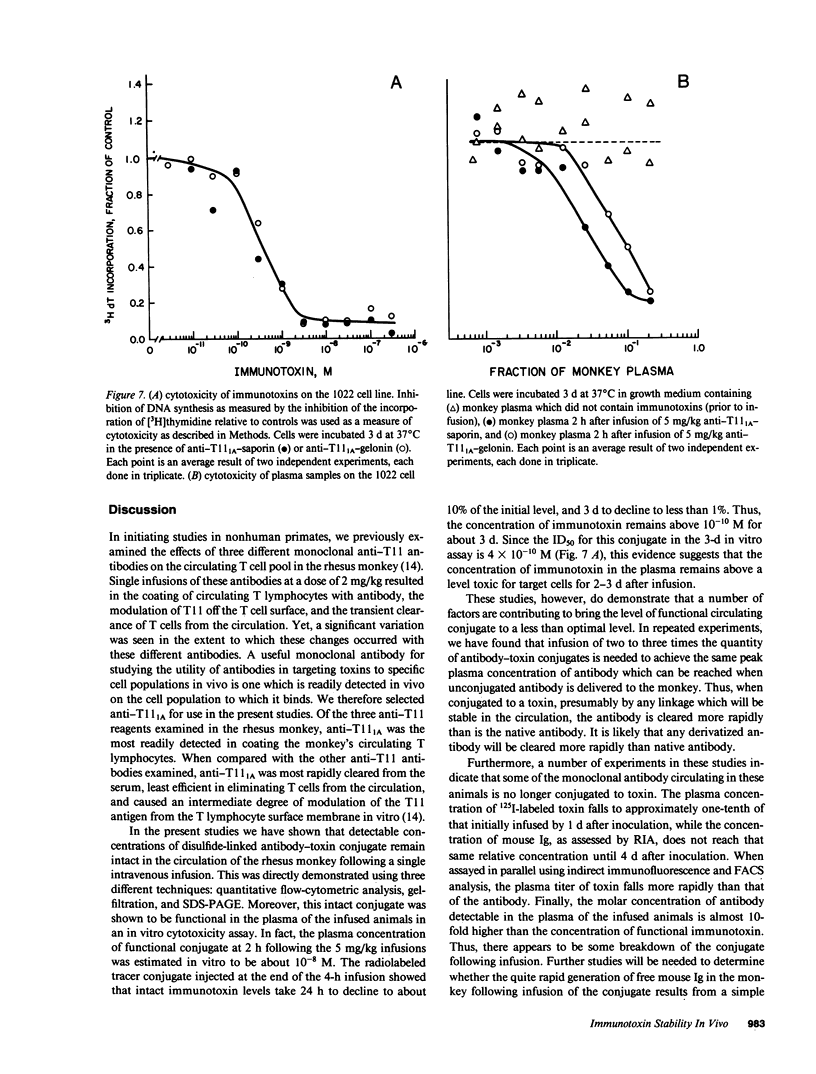
The stability in vivo and circulatory clearance of immunotoxins were assessed in rhesus monkeys. The immunotoxins studied were T cell-specific monoclonal anti-T11 antibodies conjugated by disulfide linkage to ribosome-inactivating toxins. Intact immunotoxin was detectable in the circulation of the monkeys following a single intravenous infusion. This was demonstrated by quantitative flow-cytometric analysis, gel-filtration, and sodium dodecyl sulfate-gel electrophoresis. This intact conjugate was shown to be functional in the plasma of the infused animals in an in vitro cytotoxicity assay. However, a number of factors contributed to bring the level of circulating immunotoxin to a less than optimal level. When conjugated to a ribosome-inactivating toxin, the antibody was cleared more rapidly than was the native antibody. Furthermore, following infusion, some breakdown of the conjugate occurred, resulting in the generation of detectable levels of circulating free antibody. The present data indicate the feasibility of using immunotoxins as therapeutic tools in man.
Full text
PDF
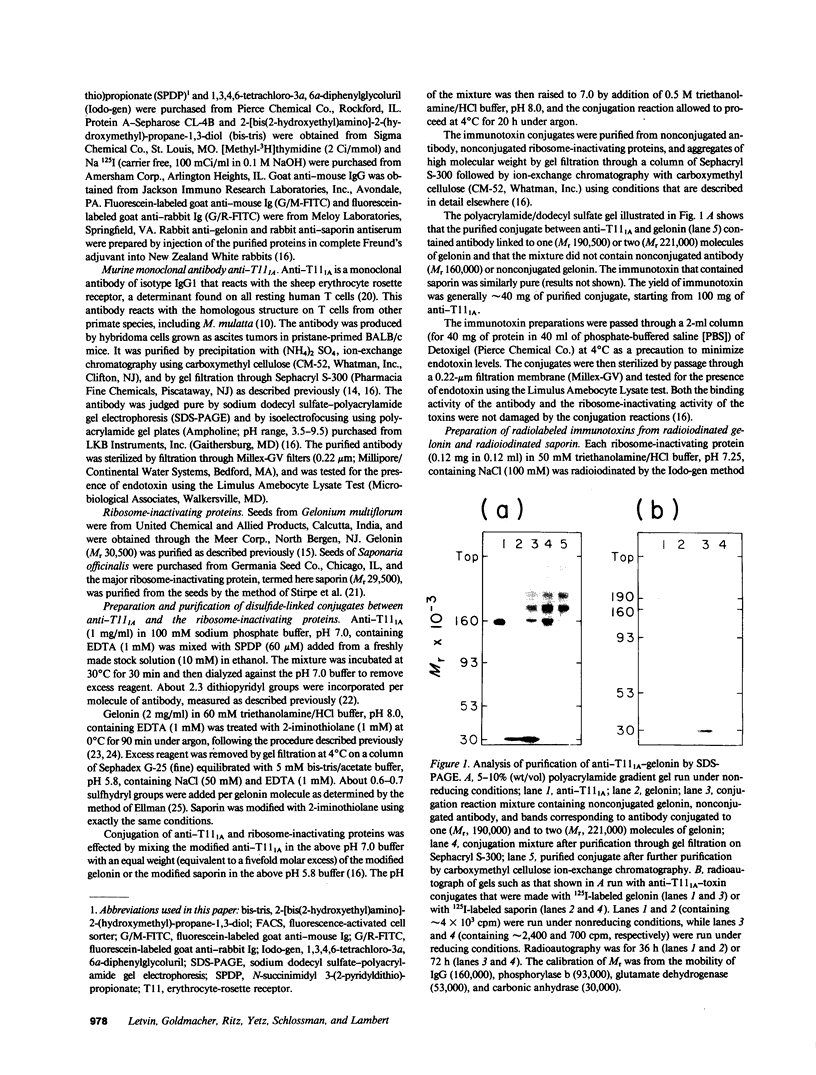


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Falk L., Wright J., Wolfe L., Deinhardt F. Herpesvirus ateles: transformation in vitro of marmoset splenic lymphocytes. Int J Cancer. 1974 Aug 15;14(2):244–251. doi: 10.1002/ijc.2910140213. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Dowell D. L., Hensley L. L., Gore I., Metzgar R. S. Human T cell antigen expression by primate T cells. Science. 1982 Jan 15;215(4530):298–300. doi: 10.1126/science.6171885. [DOI] [PubMed] [Google Scholar]
- Kirkman R. L., Araujo J. L., Busch G. J., Carpenter C. B., Milford E. L., Reinherz E. L., Schlossman S. F., Strom T. B., Tilney N. L. Treatment of acute renal allograft rejection with monoclonal anti-T12 antibody. Transplantation. 1983 Dec;36(6):620–626. doi: 10.1097/00007890-198336060-00005. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. M., Jue R., Traut R. R. Disulfide cross-linking of Escherichia coli ribosomal proteins with 2-iminothiolane (methyl 4-mercaptobutyrimidate): evidence that the cross-linked protein pairs are formed in the intact ribosomal subunit. Biochemistry. 1978 Dec 12;17(25):5406–5416. doi: 10.1021/bi00618a014. [DOI] [PubMed] [Google Scholar]
- Lambert J. M., Senter P. D., Yau-Young A., Blättler W. A., Goldmacher V. S. Purified immunotoxins that are reactive with human lymphoid cells. Monoclonal antibodies conjugated to the ribosome-inactivating proteins gelonin and the pokeweed antiviral proteins. J Biol Chem. 1985 Oct 5;260(22):12035–12041. [PubMed] [Google Scholar]
- Letvin N. L., Aldrich W. R., Thorley-Lawson D. A., Schlossman S. F., Nadler L. M. Surface antigen changes during B-lymphocyte activation in primates. Cell Immunol. 1984 Mar;84(1):163–170. doi: 10.1016/0008-8749(84)90087-x. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., King N. W., Reinherz E. L., Hunt R. D., Lane H., Schlossman S. F. T lymphocyte surface antigens in primates. Eur J Immunol. 1983 Apr;13(4):345–347. doi: 10.1002/eji.1830130414. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Ritz J., Guida L. J., Yetz J. M., Lambert J. M., Reinherz E. L., Schlossman S. F. In vivo administration of lymphocyte-specific monoclonal antibodies in nonhuman primates: I. Effects of anti-T11 antibodies on the circulating T cell pool. Blood. 1985 Oct;66(4):961–966. [PubMed] [Google Scholar]
- Letvin N. L., Todd R. F., 3rd, Palley L. S., Schlossman S. F., Griffin J. D. Conservation of myeloid surface antigens on primate granulocytes. Blood. 1983 Feb;61(2):408–410. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Palley L. S., Schlossman S. F., Letvin N. L. Common tree shrews and primates share leukocyte membrane antigens. J Med Primatol. 1984;13(2):67–71. [PubMed] [Google Scholar]
- Ramakrishnan S., Houston L. L. Immunological and biological stability of immunotoxins in vivo as studied by the clearance of disulfide-linked pokeweed antiviral protein-antibody conjugates from blood. Cancer Res. 1985 May;45(5):2031–2036. [PubMed] [Google Scholar]
- Ramakrishnan S., Houston L. L. Prevention of growth of leukemia cells in mice by monoclonal antibodies directed against Thy 1.1 antigen disulfide linked to two ribosomal inhibitors:pokeweed antiviral protein or ricin A chain. Cancer Res. 1984 Apr;44(4):1398–1404. [PubMed] [Google Scholar]
- Reinherz E. L., Geha R., Rappeport J. M., Wilson M., Penta A. C., Hussey R. E., Fitzgerald K. A., Daley J. F., Levine H., Rosen F. S. Reconstitution after transplantation with T-lymphocyte-depleted HLA haplotype-mismatched bone marrow for severe combined immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6047–6051. doi: 10.1073/pnas.79.19.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Gasperi-Campani A., Barbieri L., Falasca A., Abbondanza A., Stevens W. A. Ribosome-inactivating proteins from the seeds of Saponaria officinalis L. (soapwort), of Agrostemma githago L. (corn cockle) and of Asparagus officinalis L. (asparagus), and from the latex of Hura crepitans L. (sandbox tree). Biochem J. 1983 Dec 15;216(3):617–625. doi: 10.1042/bj2160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Olsnes S., Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980 Jul 25;255(14):6947–6953. [PubMed] [Google Scholar]
- Takahashi H., Okazaki H., Terasaki P. I., Iwaki Y., Kinukawa T., Taguchi Y., Chia D., Hardiwidjaja S., Miura K., Ishizaki M. Reversal of transplant rejection by monoclonal antiblast antibody. Lancet. 1983 Nov 19;2(8360):1155–1158. doi: 10.1016/s0140-6736(83)91212-6. [DOI] [PubMed] [Google Scholar]
- Thorpe P. E., Brown A. N., Bremner J. A., Jr, Foxwell B. M., Stirpe F. An immunotoxin composed of monoclonal anti-Thy 1.1 antibody and a ribosome-inactivating protein from Saponaria officinalis: potent antitumor effects in vitro and in vivo. J Natl Cancer Inst. 1985 Jul;75(1):151–159. [PubMed] [Google Scholar]
- Thorpe P. E., Brown A. N., Ross W. C., Cumber A. J., Detre S. I., Edwards D. C., Davies A. J., Stirpe F. Cytotoxicity acquired by conjugation of an anti-Thy1.1 monoclonal antibody and the ribosome-inactivating protein, gelonin. Eur J Biochem. 1981 Jun 1;116(3):447–454. doi: 10.1111/j.1432-1033.1981.tb05356.x. [DOI] [PubMed] [Google Scholar]
- Uhr J. W. Immunotoxins: harnessing nature's poisons. J Immunol. 1984 Dec;133(6):i–x. [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Immunotoxins: redirecting nature's poisons. Cell. 1985 Jul;41(3):653–654. doi: 10.1016/s0092-8674(85)80042-8. [DOI] [PubMed] [Google Scholar]
- Waldmann H., Polliak A., Hale G., Or R., Cividalli G., Weiss L., Weshler Z., Samuel S., Manor D., Brautbar C. Elimination of graft-versus-host disease by in-vitro depletion of alloreactive lymphocytes with a monoclonal rat anti-human lymphocyte antibody (CAMPATH-1). Lancet. 1984 Sep 1;2(8401):483–486. doi: 10.1016/s0140-6736(84)92564-9. [DOI] [PubMed] [Google Scholar]