Abstract
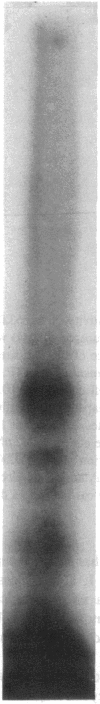
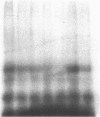
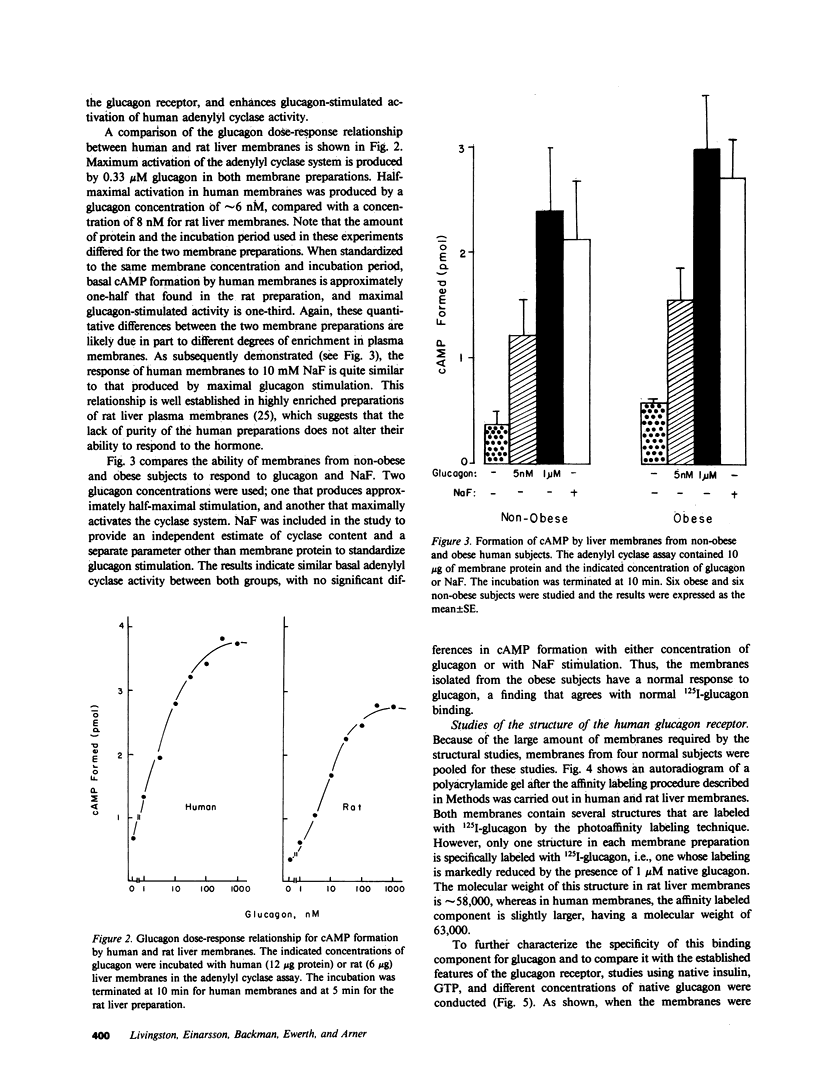
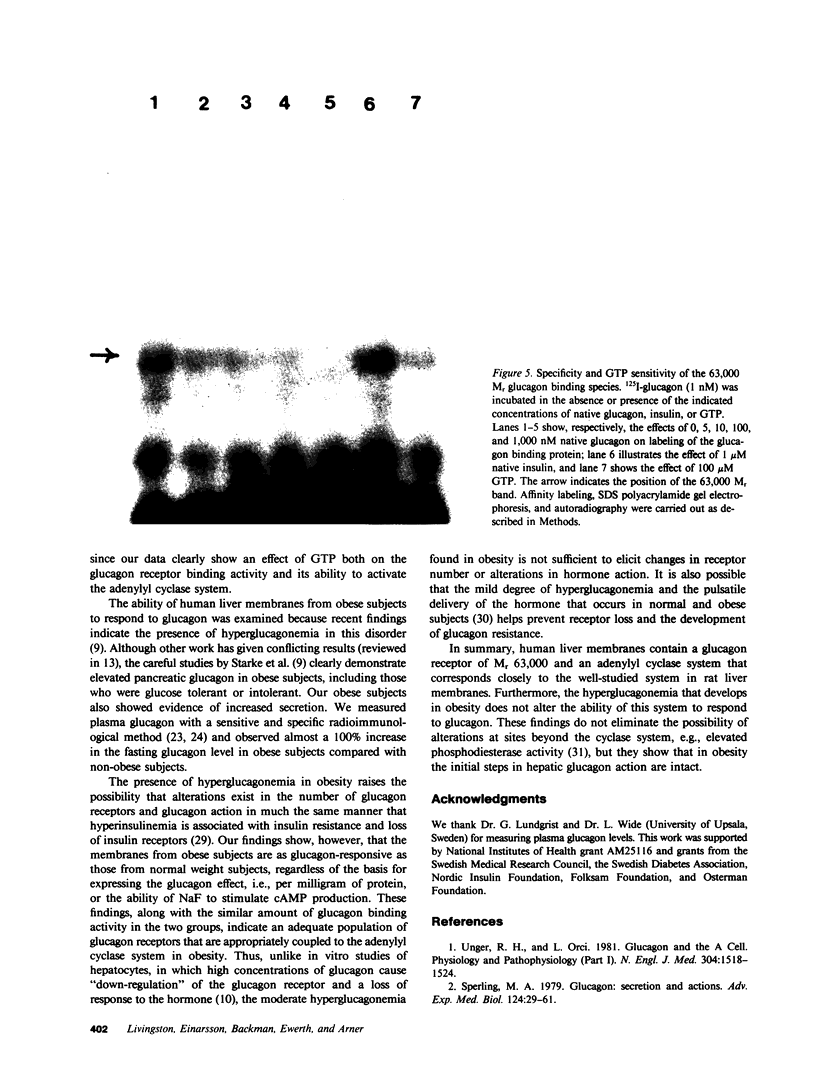
The glucagon receptor and the adenylyl cyclase system of human liver membranes were studied in six non-obese and six obese subjects who had elevated insulin and plasma glucagon levels. Analysis of specific glucagon binding by the method of Scatchard demonstrated a linear (monocomponent) plot with a dissociation constant of 2-3 nM, and the binding at low hormone concentrations was sensitive to guanosine triphosphate (GTP). The molecular weight of the glucagon receptor was 63,000 D as determined by an affinity labeling procedure and sodium dodecyl sulfate gel electrophoresis. Affinity labeling of this structure was specific for glucagon and inhibited by GTP. Glucagon stimulated the production of cyclic adenosine monophosphate (cAMP) by human membranes with half-maximal activation elicited by 6 nM hormone. The human cyclase system required GTP to facilitate an optimal glucagon response. NaF (10 mM) also activated the cyclase system and produced the same magnitude of response as maximum glucagon activation. A comparison of the liver adenylyl cyclase system of non-obese and obese subjects was made using glucagon (5 nM and 1 microM) and NaF (10 mM). No significant differences in cAMP production were noted between the two groups, regardless of the agent used to activate the enzyme. These findings agree with the glucagon binding studies that showed similar amounts of binding activity in the membranes from the two groups. Also, there was no influence of either age or sex of the subjects on the adenylyl cyclase response. In conclusion, human liver membranes contain a glucagon receptor and an adenylyl cyclase system that correspond closely to the well-studied system in animal liver. This system in human obesity is not altered by the approximately twofold elevation in plasma glucagon that occurs in this metabolic disorder.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Einarsson K., Backman L., Nilsell K., Lerea K. M., Livingston J. N. Studies of liver insulin receptors in non-obese and obese human subjects. J Clin Invest. 1983 Nov;72(5):1729–1736. doi: 10.1172/JCI111132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhathena S. J., Voyles N. R., Smith S., Recant L. Decreased glucagon receptors in diabetic rat hepatocytes. Evidence for regulation of glucagon receptors by hyperglucagonemia. J Clin Invest. 1978 Jun;61(6):1488–1497. doi: 10.1172/JCI109069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman M. D., Levy D. Labeling of glucagon binding components in hepatocyte plasma membranes. Biochem Biophys Res Commun. 1977 Sep 23;78(2):584–590. doi: 10.1016/0006-291x(77)90219-4. [DOI] [PubMed] [Google Scholar]
- De Santis R. A., Gorenstein T., Livingston J. N., Lockwood D. H. Role of phosphodiesterase in glucagon resistance of large adipocytes. J Lipid Res. 1974 Jan;15(1):33–38. [PubMed] [Google Scholar]
- Demoliou-Mason C., Epand R. M. Identification of the glucagon receptor by covalent labeling with a radiolabeled photoreactive glucagon analogue. Biochemistry. 1982 Apr 27;21(9):1996–2004. doi: 10.1021/bi00538a004. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Kitabchi A. E. Insulin and glucagon degradation by the same enzyme. Diabetes. 1974 Jun;23(6):536–543. doi: 10.2337/diab.23.6.536. [DOI] [PubMed] [Google Scholar]
- Fisher M., Sherwin R. S., Hendler R., Felig P. Kinetics of glucagon in man: effects of starvation. Proc Natl Acad Sci U S A. 1976 May;73(5):1735–1739. doi: 10.1073/pnas.73.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B. C., Jen K. C., Belbez Pek S., Wolfe R. A. Rapid oscillations in plasma insulin, glucagon, and glucose in obese and normal weight humans. J Clin Endocrinol Metab. 1982 Apr;54(4):785–792. doi: 10.1210/jcem-54-4-785. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of pancreatic and gut glucagon in plasma. Diabetologia. 1971 Feb;7(1):10–19. doi: 10.1007/BF02346248. [DOI] [PubMed] [Google Scholar]
- Israelsson B., Berglund A., Ljungqvist U., Malmquist J. Adenylate cyclase activity in human liver membranes and its inhibition by adenosine and adenine nucleotides. Scand J Clin Lab Invest. 1978 Jun;38(4):289–293. doi: 10.3109/00365517809108426. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Herberg J. T. Structural analysis of the hepatic glucagon receptor. Identification of a guanine nucleotide-sensitive hormone-binding region. J Biol Chem. 1984 Apr 25;259(8):5222–5229. [PubMed] [Google Scholar]
- Jaspan J. B., Ruddick J., Rayfield E. Transhepatic glucagon gradients in man: evidence for glucagon extraction by human liver. J Clin Endocrinol Metab. 1984 Feb;58(2):287–292. doi: 10.1210/jcem-58-2-287. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., MacAndrew V. I., Jr, Pilch P. F. Identification of the glucagon receptor in rat liver membranes by photoaffinity crosslinking. Proc Natl Acad Sci U S A. 1981 Feb;78(2):875–878. doi: 10.1073/pnas.78.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesko L., Donlon M., Marinetti G. V., Hare J. D. A rapid method for the isolation of rat liver plasma membranes using an aqueous two-phase polymer system. Biochim Biophys Acta. 1973 Jun 22;311(2):173–179. doi: 10.1016/0005-2736(73)90264-2. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Cuatrecasas P., Lockwood D. H. Studies of glucagon resistance in large rat adipocytes: 125I-labeled glucagon binding and lipolytic capacity. J Lipid Res. 1974 Jan;15(1):26–32. [PubMed] [Google Scholar]
- Pecker F., Duvaldestin P., Berthelot P., Hanoune J. The adenylate cyclase system in human liver: characterization, subcellular distribution and hormonal sensitivity in normal or cirrhotic adult, and in foetal liver. Clin Sci (Lond) 1979 Oct;57(4):313–325. doi: 10.1042/cs0570313. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Rojas F. J., Swartz T. L., Iyengar R., Garber A. J., Birnbaumer L. Monoiodoglucagon: synthesis, purification by high pressure liquid chromatography, and characteristics as a receptor probe. Endocrinology. 1983 Aug;113(2):711–719. doi: 10.1210/endo-113-2-711. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Santos A., Blazquez E. Direct evidence of a glucagon-dependent regulation of the concentration of glucagon receptors in the liver. Eur J Biochem. 1982 Jan;121(3):671–677. doi: 10.1111/j.1432-1033.1982.tb05838.x. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Sonne O. A simple, rapid, and sensitive method for measuring protein concentration in subcellular membrane fractions prepared by sucrose density ultracentrifugation. Anal Biochem. 1982 Jan 15;119(2):424–427. doi: 10.1016/0003-2697(82)90608-x. [DOI] [PubMed] [Google Scholar]
- Sperling M. A. Glucagon: secretion and actions. Adv Exp Med Biol. 1979;124:29–61. doi: 10.1007/978-1-4684-8508-0_3. [DOI] [PubMed] [Google Scholar]
- Srikant C. B., Freeman D., McCorkle K., Unger R. H. Binding and biologic activity of glucagon in liver cell membranes of chronically hyperglucagonemic rats. J Biol Chem. 1977 Nov 10;252(21):7434–7438. [PubMed] [Google Scholar]
- Starke A. A., Erhardt G., Berger M., Zimmermann H. Elevated pancreatic glucagon in obesity. Diabetes. 1984 Mar;33(3):277–280. doi: 10.2337/diab.33.3.277. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med. 1981 Jun 18;304(25):1518–1524. doi: 10.1056/NEJM198106183042504. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Glucagon and the A cell: physiology and pathophysiology (second of two parts). N Engl J Med. 1981 Jun 25;304(26):1575–1580. doi: 10.1056/NEJM198106253042604. [DOI] [PubMed] [Google Scholar]
- Von Schenck H. Production and characterization of an antiserum against pancreatic glucagon. Clin Chim Acta. 1977 Nov 1;80(3):455–463. doi: 10.1016/0009-8981(77)90138-3. [DOI] [PubMed] [Google Scholar]
- Walter R. M., Jr, Gold E. M., Michas C. A., Ensinck J. W. Portal and peripheral vein concentrations of insulin and glucagon after arginine infusion in morbidly obese subjects. Metabolism. 1980 Oct;29(11):1037–1040. doi: 10.1016/0026-0495(80)90213-9. [DOI] [PubMed] [Google Scholar]