Abstract
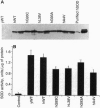
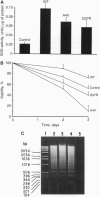
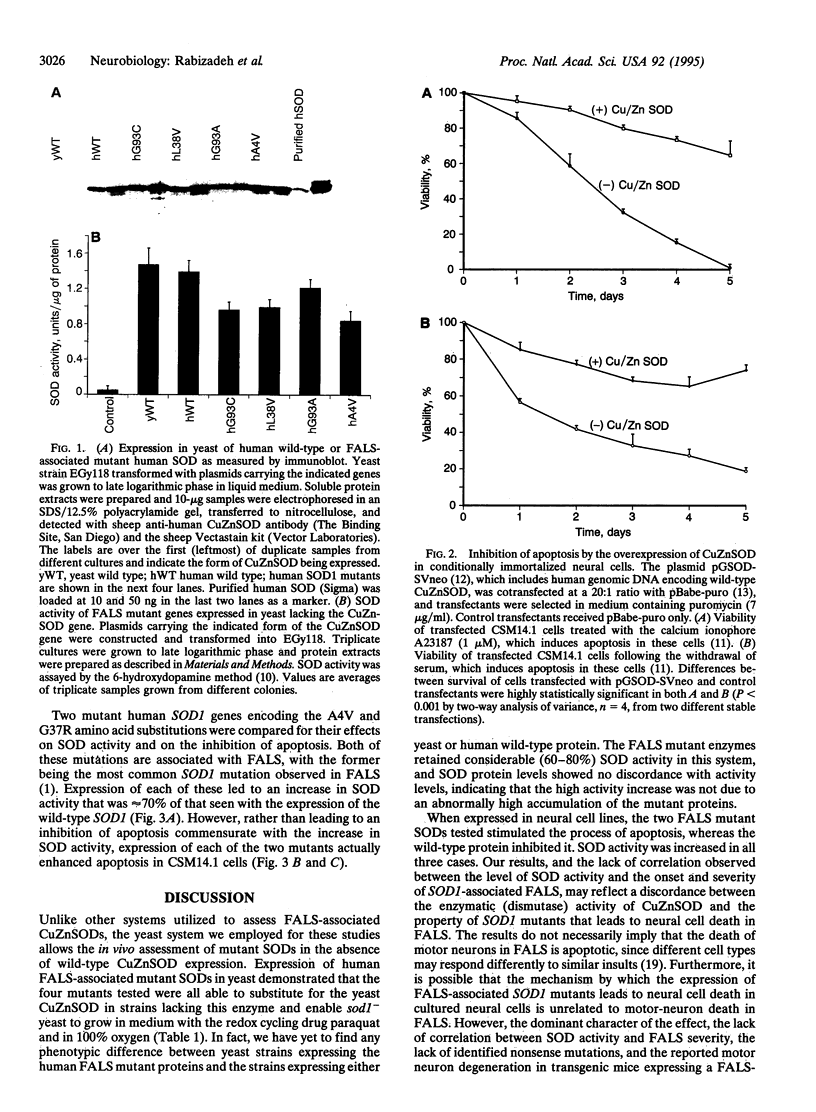
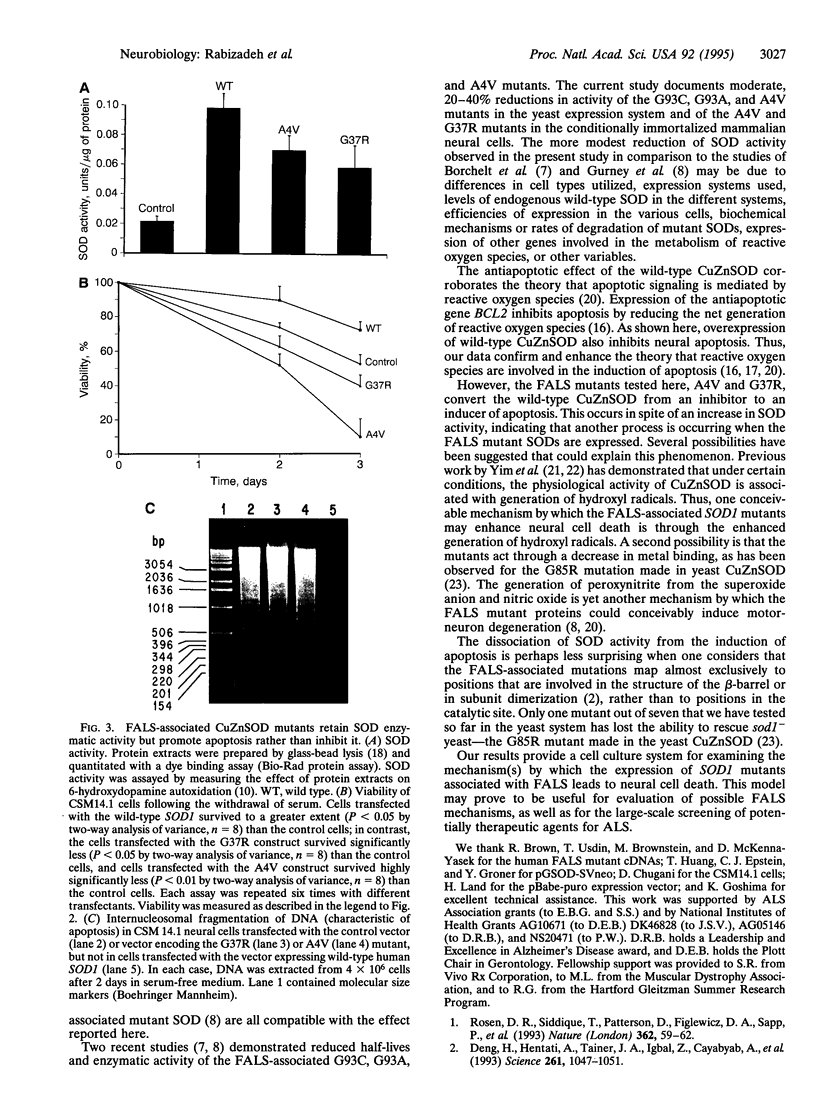
Familial amyotrophic lateral sclerosis (FALS) is associated with mutations in SOD1, the gene encoding copper/zinc superoxide dismutase (CuZnSOD). However, the mechanism by which these mutations lead to amyotrophic lateral sclerosis is unknown. We report that FALS mutant SODs expressed in yeast lacking CuZnSOD are enzymatically active and restore the yeast to the wild-type phenotype. In mammalian neural cells, the overexpression of wild-type SOD1 inhibits apoptosis induced by serum and growth factor withdrawal or calcium ionophore. In contrast, FALS-associated SOD1 mutants promote, rather than inhibit, neural apoptosis, in a dominant fashion, despite the fact that these mutants retain enzymatic SOD activity both in yeast and in mammalian neural cells. The results dissociate the SOD activity of FALS-associated mutants from the induction of neural cell death, suggesting that FALS associated with mutations in SOD1 may not be simply the result of a decrease in the enzymatic function of CuZnSOD. Furthermore, the results provide an in vitro model that may help to define the mechanism by which FALS-associated SOD1 mutations lead to neural cell death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki M., Ogasawara M., Matsubara Y., Narisawa K., Nakamura S., Itoyama Y., Abe K. Mild ALS in Japan associated with novel SOD mutation. Nat Genet. 1993 Dec;5(4):323–324. doi: 10.1038/ng1293-323. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Gralla E. B., Valentine J. S. The copper, zinc-superoxide dismutase gene of Saccharomyces cerevisiae: cloning, sequencing, and biological activity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4789–4793. doi: 10.1073/pnas.85.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt D. R., Lee M. K., Slunt H. S., Guarnieri M., Xu Z. S., Wong P. C., Brown R. H., Jr, Price D. L., Sisodia S. S., Cleveland D. W. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A. C., Schulz J. B., Brown R. H., Jr, Beal M. F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993 Dec;61(6):2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. A sensitive assay for superoxide dismutase based on the autoxidation of 6-hydroxydopamine. Anal Biochem. 1976 Oct;75(2):356–362. doi: 10.1016/0003-2697(76)90089-0. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Levanon D., Lieman-Hurwitz J., Dafni N., Wigderson M., Sherman L., Bernstein Y., Laver-Rudich Z., Danciger E., Stein O., Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985 Jan;4(1):77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida C. R., Gralla E. B., Valentine J. S. Characterization of three yeast copper-zinc superoxide dismutase mutants analogous to those coded for in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9906–9910. doi: 10.1073/pnas.91.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. C., Horvitz H. R. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics. 1986 Aug;113(4):821–852. doi: 10.1093/genetics/113.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabizadeh S., LaCount D. J., Friesen P. D., Bredesen D. E. Expression of the baculovirus p35 gene inhibits mammalian neural cell death. J Neurochem. 1993 Dec;61(6):2318–2321. doi: 10.1111/j.1471-4159.1993.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Rabizadeh S., Oh J., Zhong L. T., Yang J., Bitler C. M., Butcher L. L., Bredesen D. E. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993 Jul 16;261(5119):345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Robberecht W., Sapp P., Viaene M. K., Rosen D., McKenna-Yasek D., Haines J., Horvitz R., Theys P., Brown R., Jr Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1994 Jan;62(1):384–387. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Sarafian T. A., Bredesen D. E. Is apoptosis mediated by reactive oxygen species? Free Radic Res. 1994 Jul;21(1):1–8. doi: 10.3109/10715769409056549. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Enzyme function of copper, zinc superoxide dismutase as a free radical generator. J Biol Chem. 1993 Feb 25;268(6):4099–4105. [PubMed] [Google Scholar]
- Zhong L. T., Sarafian T., Kane D. J., Charles A. C., Mah S. P., Edwards R. H., Bredesen D. E. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]