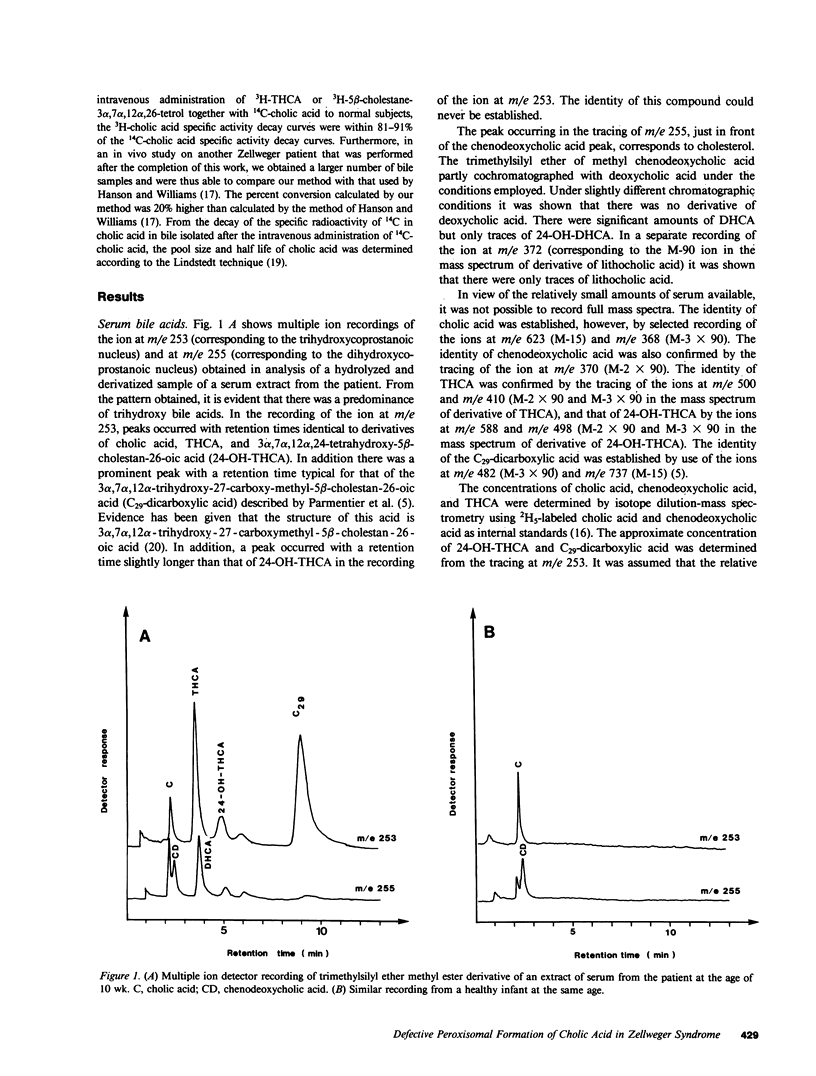
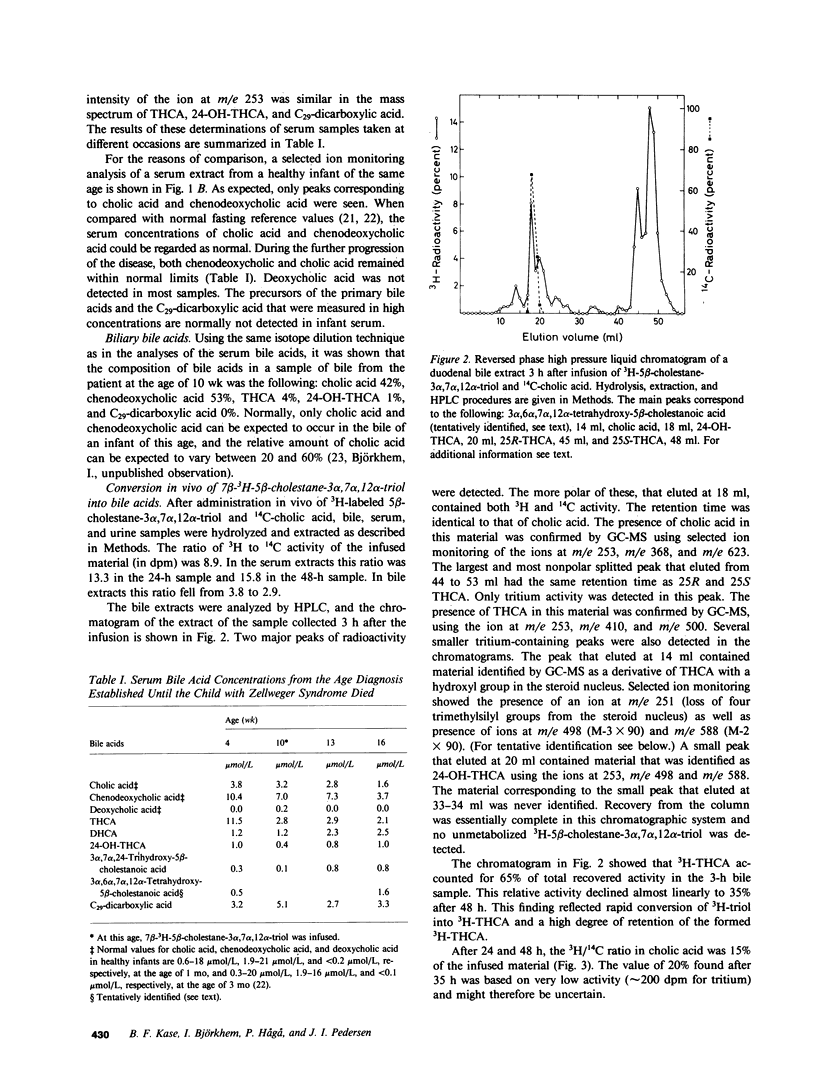
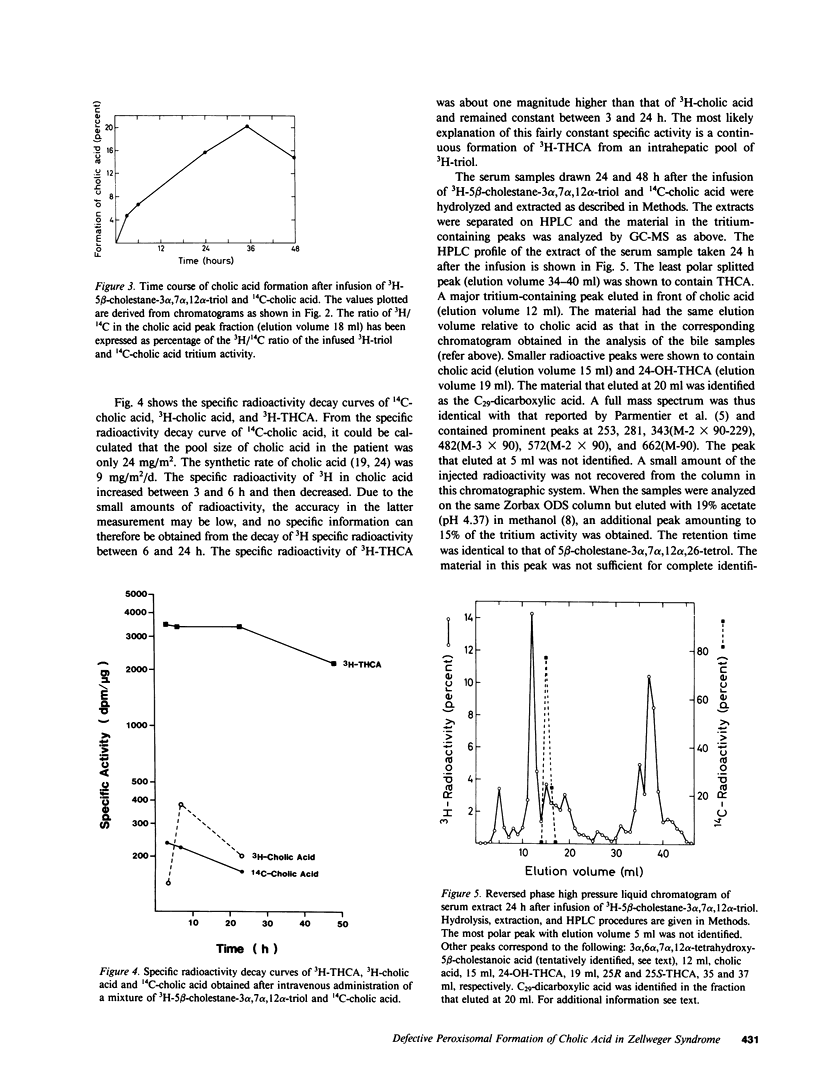
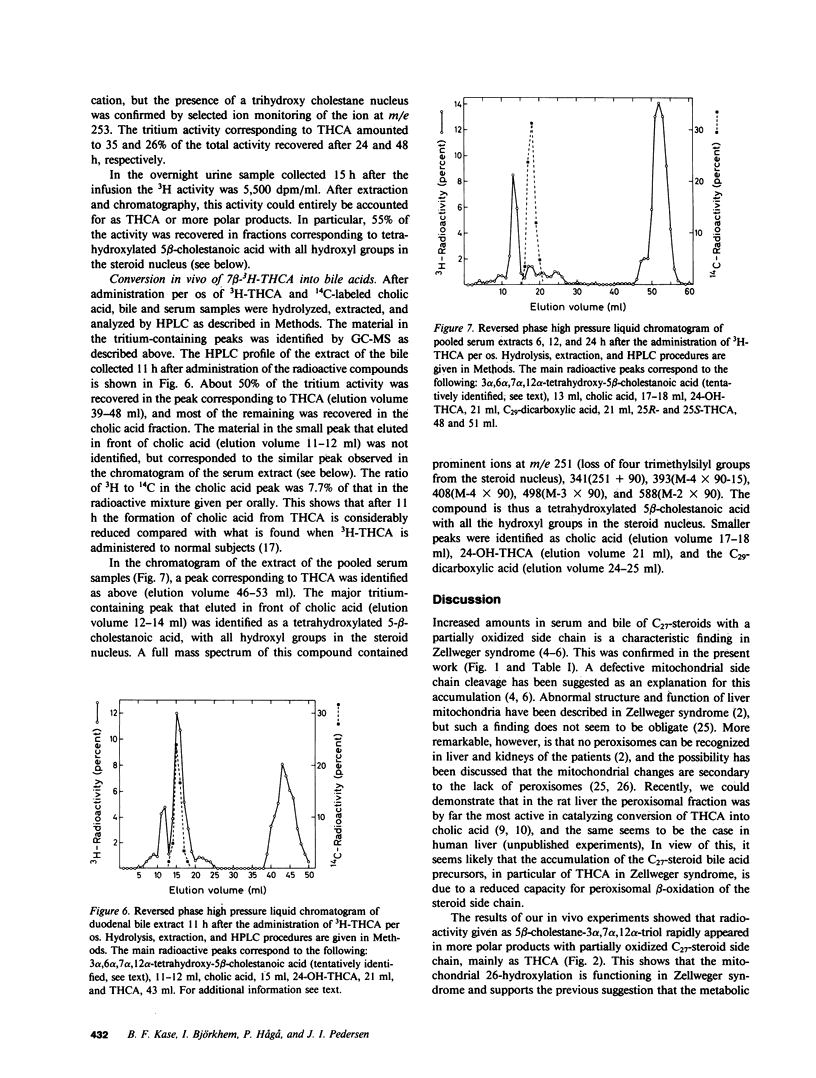
Abstract
Based on in vitro work with rat liver, we recently suggested that the peroxisomal fraction is most important for the oxidation of 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid (THCA) into cholic acid. The cerebro-hepato-renal syndrome of Zellweger is a fatal recessive autosomal disorder, the most characteristic histological feature of which is a virtual absence of peroxisomes in liver and kidneys. This disease offers a unique opportunity to evaluate the relative importance of peroxisomes in bile acid biosynthesis. A child with Zellweger syndrome was studied in the present work. In accordance with previous work, there was a considerable accumulation of THCA, 3 alpha, 7 alpha, 12 alpha, 24-tetrahydroxy-5 beta-cholestanoic acid (24-OH-THCA), 3 alpha, 7 alpha, 12 alpha-trihydroxy-27-carboxymethyl-5 beta-cholestan-26-oic acid (C29-dicarboxylic acid), and 3 alpha, 7 alpha-dihydroxy-5 beta-cholestanoic acid in serum. In addition, a tetrahydroxylated 5 beta-cholestanoic acid with all the hydroxyl groups in the steroid nucleus was found. 3H-Labeled 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol was administered intravenously together with 14C-labeled cholic acid. There was a rapid incorporation of 3H in THCA and a slow incorporation into cholic acid. The specific radioactivity of 3H in THCA was about one magnitude higher than that in cholic acid. The conversion was evaluated by following the increasing ratio between 3H and 14C in biliary cholic acid. The rate of incorporation of 3H in cholic acid was considerably less than previously reported in experiments with healthy subjects, and the maximal conversion of the triol into cholic acid was only 15-20%. About the same rate of conversion was found after oral administration of 3H-THCA. Both in the experiment with 3H-5 beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol and with 3H-THCA, there was an efficient incorporation of 3H in the above unidentified tetrahydroxylated 5 beta-cholestanoic acid. There was only slow incorporation of radioactivity into 24-OH-THCA and into the C29-dicarboxylic acid. From the specific activity decay curve of 14C in cholic acid obtained after intravenous injection of 14C-cholic acid, the pool size of cholic acid was calculated to be 24 mg/m2 and the daily production rate to 9 mg/m2 per d. This corresponds to a reduction of approximately 85 and 90%, respectively, when compared with normal infants. It is concluded that liver peroxisomes are essential in the normal conversion of THCA to cholic acid. In the Zellweger syndrome this conversion is defective and as a consequence the accumulated THCA is either excreted as such or transformed into other metabolites by hydroxylation or side chain elongation. The accumulation of THCA, as well as the similar rate of conversion of 5 beta-cholestane-3 alpha,7 alpha.12 alpha-triol and THCA into cholic acid, support the contention that the 26-hydroxylase pathway with intermediate formation of THCA is the most important pathway for formation of cholic acid in man.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelin B., Björkhem I., Einarsson K., Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982 Oct;70(4):724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWEN P., LEE C. S., ZELLWEGER H., LINDENBERG R. A FAMILIAL SYNDROME OF MULTIPLE CONGENITAL DEFECTS. Bull Johns Hopkins Hosp. 1964 Jun;114:402–414. [PubMed] [Google Scholar]
- Björkhem I., Falk O. Assay of the major bile acids in serum by isotope dilution-mass spectrometry. Scand J Clin Lab Invest. 1983 Apr;43(2):163–170. doi: 10.1080/00365518309168239. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Fausa O., Hopen G., Oftebro H., Pedersen J. I., Skrede S. Role of the 26-hydroxylase in the biosynthesis of bile acids in the normal state and in cerebrotendinous xanthomatosis. An in vivo study. J Clin Invest. 1983 Jan;71(1):142–148. doi: 10.1172/JCI110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Omega-hydroxylation of steriod side-chain in biosynthesis of bile acids. Eur J Biochem. 1973 Jul 2;36(1):201–212. doi: 10.1111/j.1432-1033.1973.tb02902.x. [DOI] [PubMed] [Google Scholar]
- Bremer J., Norum K. R. Metabolism of very long-chain monounsaturated fatty acids (22:1) and the adaptation to their presence in the diet. J Lipid Res. 1982 Feb;23(2):243–256. [PubMed] [Google Scholar]
- Brown F. R., 3rd, McAdams A. J., Cummins J. W., Konkol R., Singh I., Moser A. B., Moser H. W. Cerebro-hepato-renal (Zellweger) syndrome and neonatal adrenoleukodystrophy: similarities in phenotype and accumulation of very long chain fatty acids. Johns Hopkins Med J. 1982 Dec;151(6):344–351. [PubMed] [Google Scholar]
- Clayton P. T., Muller D. P., Lawson A. M. The bile acid composition of gastric contents from neonates with high intestinal obstruction. Biochem J. 1982 Sep 15;206(3):489–498. doi: 10.1042/bj2060489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard P., Caviness V. S., Jr, Prats-Vinas J., Lyon G. The mechanism of arrest of neuronal migration in the Zellweger malformation: an hypothesis bases upon cytoarchitectonic analysis. Acta Neuropathol. 1978 Feb 20;41(2):109–117. doi: 10.1007/BF00689761. [DOI] [PubMed] [Google Scholar]
- Eyssen H., Parmentier G., Compernolle F., Boon J., Eggermont E. Trihydroxycoprostanic acid in the duodenal fluid of two children with intrahepatic bile duct anomalies. Biochim Biophys Acta. 1972 Jun 26;273(1):212–221. doi: 10.1016/0304-4165(72)90209-7. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. Biosynthesis of cholic acid in rat liver: formation of cholic acid from 3 alpha, 7 alpha, 12 alpha-trihydroxy- and 3 alpha, 7 alpha, 12 alpha, 24-tetrahydroxy-5 beta-cholestanoic acids. Lipids. 1980 Feb;15(2):113–121. doi: 10.1007/BF02533886. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Isenberg J. N., Williams G. C., Hachey D., Szczepanik P., Klein P. D., Sharp H. L. The metabolism of 3alpha, 7alpha, 12alpha-trihydorxy-5beta-cholestan-26-oic acid in two siblings with cholestasis due to intrahepatic bile duct anomalies. An apparent inborn error of cholic acid synthesis. J Clin Invest. 1975 Sep;56(3):577–587. doi: 10.1172/JCI108127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. F., Staples A. B., Williams G. C. Metabolism of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 26-tetrol and 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 25-tetrol into cholic acid in normal human subjects. J Lipid Res. 1979 May;20(4):489–493. [PubMed] [Google Scholar]
- Hanson R. F., Szczepanik-VanLeeuwen P., Williams G. C., Grabowski G., Sharp H. L. Defects of bile acid synthesis in Zellweger's syndrome. Science. 1979 Mar 16;203(4385):1107–1108. doi: 10.1126/science.424737. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Williams G. C. Metabolism of 3alpha, 7alpha, 12alpha-trihydroxy-5beta-cholestan-26-oic acid in normal subjects with an intact enterohepatic circulation. J Lipid Res. 1977 Sep;18(5):656–659. [PubMed] [Google Scholar]
- Heikura S., Similä S., Finni K., Mäentausta O., Jänne O. Cholic acid and chenodeoxycholic acid concentrations in serum during infancy and childhood. Acta Paediatr Scand. 1980 Sep;69(5):659–662. doi: 10.1111/j.1651-2227.1980.tb07339.x. [DOI] [PubMed] [Google Scholar]
- Janssen G., Toppet S., Parmentier G. Structure of the side chain of the C29 dicarboxylic bile acid occurring in infants with coprostanic acidemia. J Lipid Res. 1982 Mar;23(3):456–465. [PubMed] [Google Scholar]
- Kase F., Björkhem I., Pedersen J. I. Formation of cholic acid from 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid by rat liver peroxisomes. J Lipid Res. 1983 Dec;24(12):1560–1567. [PubMed] [Google Scholar]
- Kawamura N., Moser A. B., Moser H. W., Ogino T., Suzuki K., Schaumburg H., Milunsky A., Murphy J., Kishimoto Y. High concentration of hexacosanoate in cultured skin fibroblast lipids from adrenoleukodystrophy patients. Biochem Biophys Res Commun. 1978 May 15;82(1):114–120. doi: 10.1016/0006-291x(78)90584-3. [DOI] [PubMed] [Google Scholar]
- LINDSTEDT S. The turnover of cholic acid in man: bile acids and steroids. Acta Physiol Scand. 1957 Sep 17;40(1):1–9. doi: 10.1111/j.1748-1716.1957.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Mathis R. K., Watkins J. B., Szczepanik-Van Leeuwen P., Lott I. T. Liver in the cerebro-hepato-renal syndrome: defective bile acid synthesis and abnormal mitochondria. Gastroenterology. 1980 Dec;79(6):1311–1317. [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Skrede S., Schreiner A., Pederson J. I. Cerebrotendinous xanthomatosis: a defect in mitochondrial 26-hydroxylation required for normal biosynthesis of cholic acid. J Clin Invest. 1980 Jun;65(6):1418–1430. doi: 10.1172/JCI109806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier G. G., Janssen G. A., Eggermont E. A., Eyssen H. J. C27 bile acids in infants with coprostanic acidemia and occurrence of a 3 alpha,7 alpha,12 alpha-tridhydroxy-5 beta-C29 dicarboxylic bile acid as a major component in their serum. Eur J Biochem. 1979 Dec;102(1):173–183. doi: 10.1111/j.1432-1033.1979.tb06278.x. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I., Gustafsson J. Conversion of 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid into cholic acid by rat liver peroxisomes. FEBS Lett. 1980 Dec 1;121(2):345–348. doi: 10.1016/0014-5793(80)80377-2. [DOI] [PubMed] [Google Scholar]
- Salen G., Shefer S. Bile acid synthesis. Annu Rev Physiol. 1983;45:679–685. doi: 10.1146/annurev.ph.45.030183.003335. [DOI] [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandvik B., Wikström S. A. Tetrahydroxylated bile acids in healthy human newborns. Eur J Clin Invest. 1982 Aug;12(4):301–305. doi: 10.1111/j.1365-2362.1982.tb02236.x. [DOI] [PubMed] [Google Scholar]
- Swell L., Gustafsson J., Danielsson H., Schwartz C. C., Vlahcevic Z. R. Biosynthesis of bile acids in man. An in vivo evaluation of the conversion of R and S 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic and 3 alpha, 7 alpha, 12 alpha-24 xi-tetrahydroxy-5 beta-cholestanoic acids to cholic acid. J Biol Chem. 1981 Jan 25;256(2):912–916. [PubMed] [Google Scholar]
- Swell L., Gustafsson J., Schwartz C. C., Halloran L. G., Danielsson H., Vlahcevic Z. R. An in vivo evaluation of the quantitative significance of several potential pathways to cholic and chenodeoxycholic acids from cholesterol in man. J Lipid Res. 1980 May;21(4):455–466. [PubMed] [Google Scholar]
- Trijbels J. M., Berden J. A., Monnens L. A., Willems J. L., Janssen A. J., Schutgens R. B., van den Broek-Van Essen M. Biochemical studies in the liver and muscle of patients with Zellweger syndrome. Pediatr Res. 1983 Jun;17(6):514–517. doi: 10.1203/00006450-198306000-00018. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Schwartz C. C., Gustafsson J., Halloran L. G., Danielsson H., Swell L. Biosynthesis of bile acids in man. Multiple pathways to cholic acid and chenodeoxycholic acid. J Biol Chem. 1980 Apr 10;255(7):2925–2933. [PubMed] [Google Scholar]
- Watkins J. B., Ingall D., Szczepanik P., Klein P. D., Lester R. Bile-salt metabolism in the newborn. Measurement of pool size and synthesis by stable isotope technic. N Engl J Med. 1973 Mar 1;288(9):431–434. doi: 10.1056/NEJM197303012880902. [DOI] [PubMed] [Google Scholar]
- Watkins J. B., Szczepanik P., Gould J. B., Klein P., Lester R. Bile salt metabolism in the human premature infant. Preliminary observations of pool size and synthesis rate following prenatal administration of dexamethasone and phenobarbital. Gastroenterology. 1975 Sep;69(3):706–713. [PubMed] [Google Scholar]


