Abstract
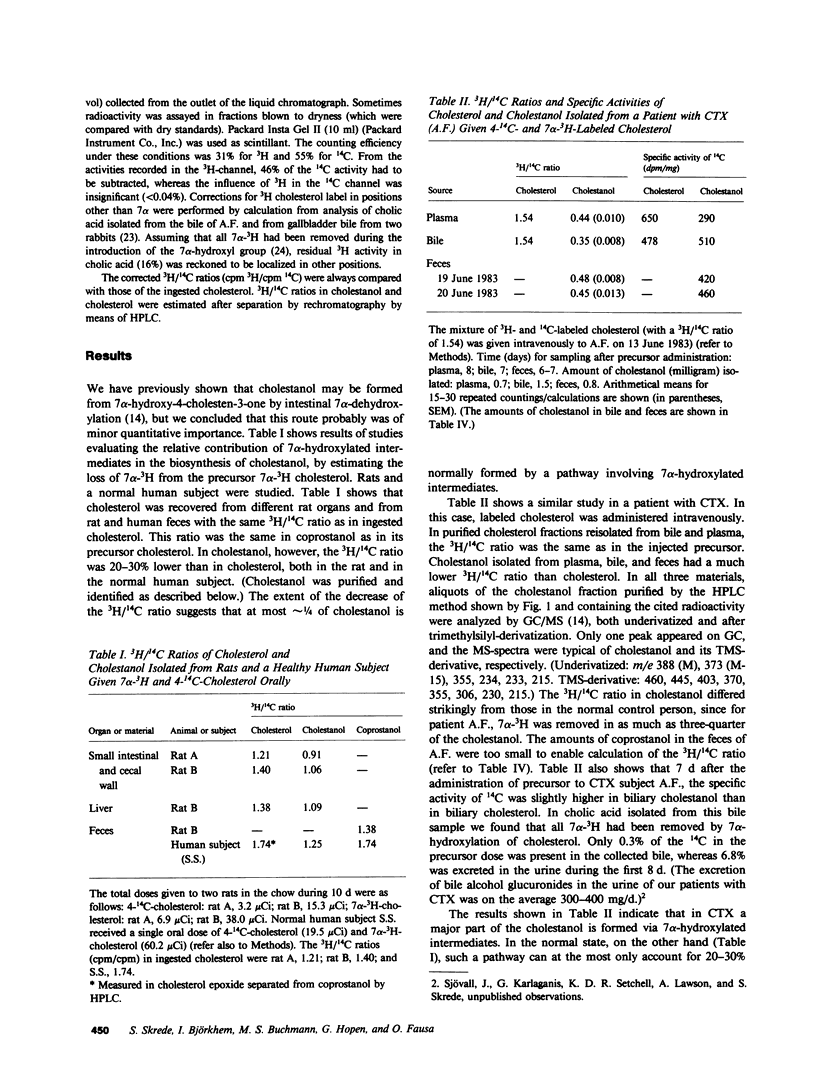
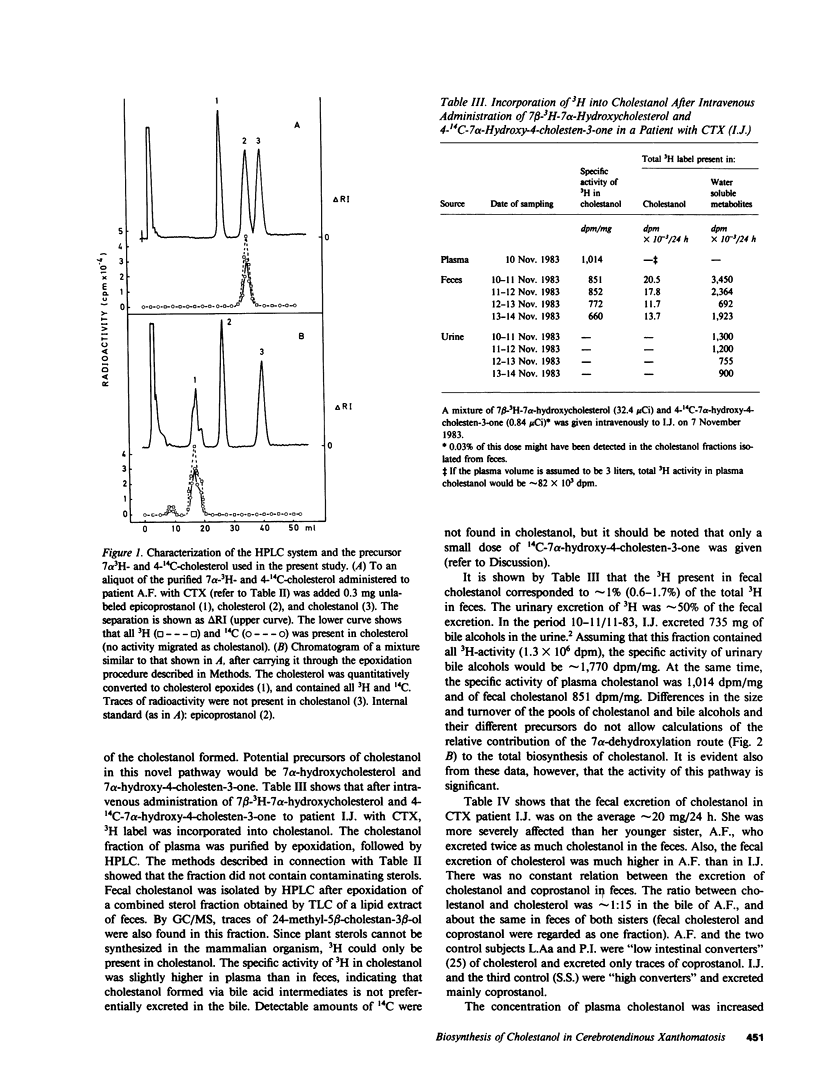
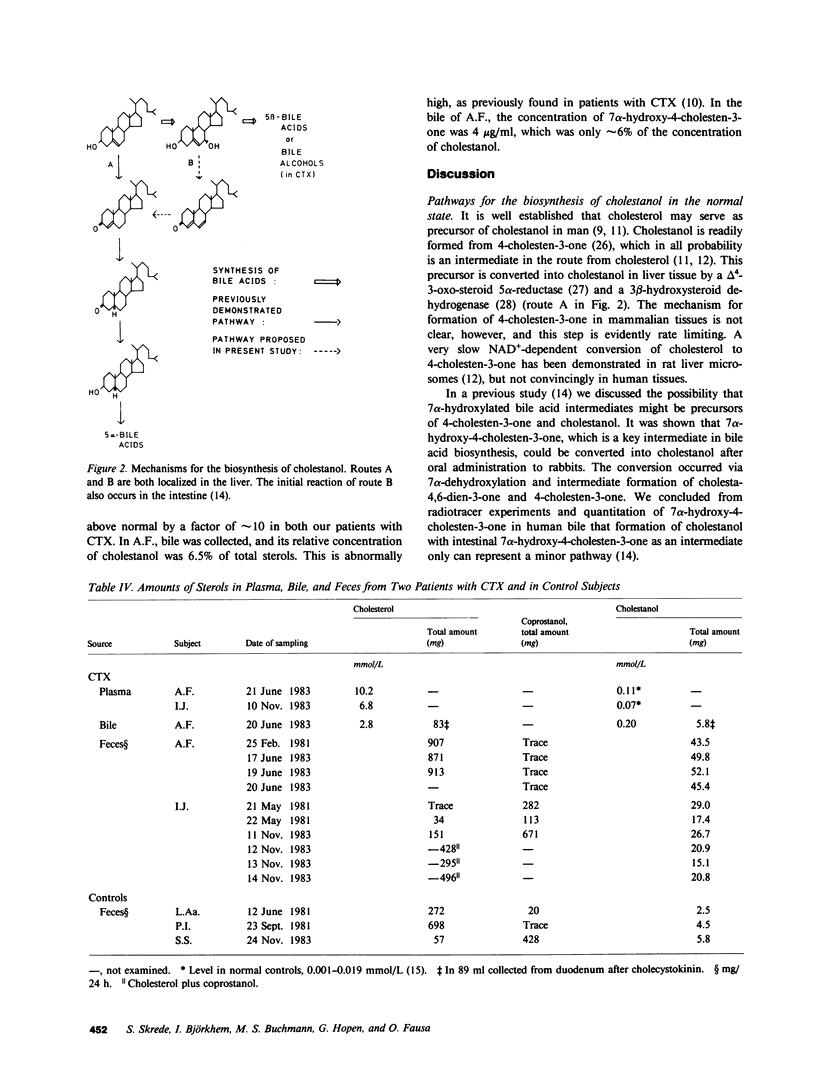
A mixture of 7 alpha-3H- and 4-14C-labeled cholesterol was administered intravenously to rats. Cholestanol with 20-30% lower ratio between 3H and 14C than in cholesterol could be isolated from different organs. In a healthy human control, cholestanol isolated from feces had a 3H/14C ratio which was 28% lower than in administered cholesterol. Cholesterol and coprostanol reisolated in these experiments had the same ratio between 3H and 14C as in the precursor. A previously unknown pathway for formation of cholestanol, involving 7 alpha-hydroxylated intermediates, may explain these results. Under normal conditions, this pathway is responsible for at most 30% of the cholestanol synthesized from cholesterol. Intravenous administration of the 7 alpha-3H- and 4-14C-labeled cholesterol to a patient with cerebrotendinous xanthomatosis (CTX) resulted in formation of cholestanol which had 70-75% lower 3H/14C ratio. It is concluded that the novel pathway involving 7 alpha-hydroxylated intermediates is accelerated in patients with CTX. This acceleration may contribute essentially to the accumulation of cholestanol, which is a predominant feature of this disease. 7 alpha-Hydroxycholesterol and 7 alpha-hydroxy-4-cholesten-3-one might be intermediates in the novel pathway to cholestanol. After intravenous administration of 7 beta-3H-labeled 7 alpha-hydroxycholesterol in a patient with CTX, significant amounts of 3H were incorporated into plasma and fecal cholestanol. Only small amounts of 7 alpha-hydroxycholesterol and 7 alpha-hydroxy-4-cholesten-3-one are excreted into the intestine, and we therefore conclude that the 7 alpha-dehydroxylation step mainly occurs in the liver. In CTX, the synthesis of cholestanol may be accelerated because the concentrations of 7 alpha-hydroxylated bile acid intermediates in the liver are increased. A possible mechanism for the conversion of a minor fraction of 7 alpha-hydroxycholesterol into cholestanol is suggested.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Buchmann M. S., Skrede S. Isolation of 5 alpha-cholestane-3 beta, 7 alpha-diol from bile of patients with cerebrotendinous xanthomatosis. Inefficiency of this steroid as a precursor to cholestanol. Biochim Biophys Acta. 1983 Sep 20;753(2):220–226. [PubMed] [Google Scholar]
- Björkhem I., Fausa O., Hopen G., Oftebro H., Pedersen J. I., Skrede S. Role of the 26-hydroxylase in the biosynthesis of bile acids in the normal state and in cerebrotendinous xanthomatosis. An in vivo study. J Clin Invest. 1983 Jan;71(1):142–148. doi: 10.1172/JCI110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. A. Mechanism of microbial transformation of cholesterol into coprostanol. Eur J Biochem. 1971 Aug 16;21(3):428–432. doi: 10.1111/j.1432-1033.1971.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Karlmar K. E. Biosynthesis of cholestanol: conversion of cholesterol into 4-cholesten-3-one by rat liver microsomes. Biochim Biophys Acta. 1974 Jan 23;337(1):129–131. doi: 10.1016/0005-2760(74)90046-0. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Oftebro H., Skrede S., Pedersen J. I. Assay of intermediates in bile acid biosynthesis using isotope dilution--mass spectrometry: hepatic levels in the normal state and in cerebrotendinous xanthomatosis. J Lipid Res. 1981 Feb;22(2):191–200. [PubMed] [Google Scholar]
- Danielsson H., Einarsson K. On the conversion of cholesterol to 7-alpha,12-alpha-dihydroxycholest-4-en-3-one. Bile acids and steroids 168. J Biol Chem. 1966 Apr 10;241(7):1449–1454. [PubMed] [Google Scholar]
- KANDUTSCH A. A. METABOLISM OF CHOLESTA-4,7-DIEN-3-ONE AND CHOLESTA-4,6-DIEN-3-ONE BY MOUSE LIVER MICROSOMES. J Lipid Res. 1963 Apr;4:179–187. [PubMed] [Google Scholar]
- Menkes J. H., Schimschock J. R., Swanson P. D. Cerebrotendinous xanthomatosis. The storage of cholestanol within the nervous system. Arch Neurol. 1968 Jul;19(1):47–53. doi: 10.1001/archneur.1968.00480010065004. [DOI] [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Skrede S., Schreiner A., Pederson J. I. Cerebrotendinous xanthomatosis: a defect in mitochondrial 26-hydroxylation required for normal biosynthesis of cholic acid. J Clin Invest. 1980 Jun;65(6):1418–1430. doi: 10.1172/JCI109806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld R. S., Zumoff B., Hellman L. Conversion of cholesterol injected into man to cholestanol via a 3-ketonic intermediate. J Lipid Res. 1967 Jan;8(1):16–23. [PubMed] [Google Scholar]
- Ross P. E., Pennington C. R., Bouchier I. A. Gas-liquid chromatographic assay of serum bile acids. Anal Biochem. 1977 Jun;80(2):458–465. doi: 10.1016/0003-2697(77)90668-6. [DOI] [PubMed] [Google Scholar]
- Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann Intern Med. 1971 Dec;75(6):843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- Salen G., Grundy S. M. The metabolism of cholestanol, cholesterol, and bile acids in cerebrotendinous xanthomatosis. J Clin Invest. 1973 Nov;52(11):2822–2835. doi: 10.1172/JCI107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Polito A. Biosynthesis of 5 -cholestan-3 -ol in cerebrotendinous xanthomatosis. J Clin Invest. 1972 Jan;51(1):134–140. doi: 10.1172/JCI106783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Cheng F. W., Dayal B., Batta A. K., Tint G. S. Cholic acid biosynthesis: the enzymatic defect in cerebrotendinous xanthomatosis. J Clin Invest. 1979 Jan;63(1):38–44. doi: 10.1172/JCI109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F. J., McDonald F. J. Determination of hepatic cholesterol 7alpha-hydroxylase activity in man. J Lipid Res. 1974 Mar;15(2):146–151. [PubMed] [Google Scholar]
- Schreiner A., Hopen G., Skrede S. Cerebrotendinous xanthomatosis (cholestanolosis). Investigations on two sisters and their family. Acta Neurol Scand. 1975 May;51(5):405–416. [PubMed] [Google Scholar]
- Setchell K. D., Worthington J. A rapid method for the quantitative extraction of bile acids and their conjugates from serum using commercially available reverse-phase octadecylsilane bonded silica cartridges. Clin Chim Acta. 1982 Oct 27;125(2):135–144. doi: 10.1016/0009-8981(82)90190-5. [DOI] [PubMed] [Google Scholar]
- Setoguchi T., Salen G., Tint G. S., Mosbach E. H. A biochemical abnormality in cerebrotendinous xanthomatosis. Impairment of bile acid biosynthesis associated with incomplete degradation of the cholesterol side chain. J Clin Invest. 1974 May;53(5):1393–1401. doi: 10.1172/JCI107688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Hauser S., Mosbach E. H. Biosynthesis of cholestanol: 5-alpha-cholestan-3-one reductase of rat liver. J Lipid Res. 1966 Nov;7(6):763–771. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Mosbach E. H. Studies on the biosynthesis of 5-alpha-cholestan-3-beta-ol. I. Cholestenone 5-alpha-reductase of rat liver. J Biol Chem. 1966 Feb 25;241(4):946–952. [PubMed] [Google Scholar]
- Skrede S., Björkhem I. Biosynthesis of cholestanol from intestinal 7 alpha-hydroxy-4-cholesten-3-one. J Biol Chem. 1982 Jul 25;257(14):8363–8367. [PubMed] [Google Scholar]
- Wilkins T. D., Hackman A. S. Two patterns of neutral steroid conversion in the feces of normal North Americans. Cancer Res. 1974 Sep;34(9):2250–2254. [PubMed] [Google Scholar]
- Wolthers B. G., Volmer M., van der Molen J., Koopman B. J., de Jager A. E., Waterreus R. J. Diagnosis of cerebrotendinous xanthomatosis (CTX) and effect of chenodeoxycholic acid therapy by analysis of urine using capillary gas chromatography. Clin Chim Acta. 1983 Jun 30;131(1-2):53–65. doi: 10.1016/0009-8981(83)90352-2. [DOI] [PubMed] [Google Scholar]


