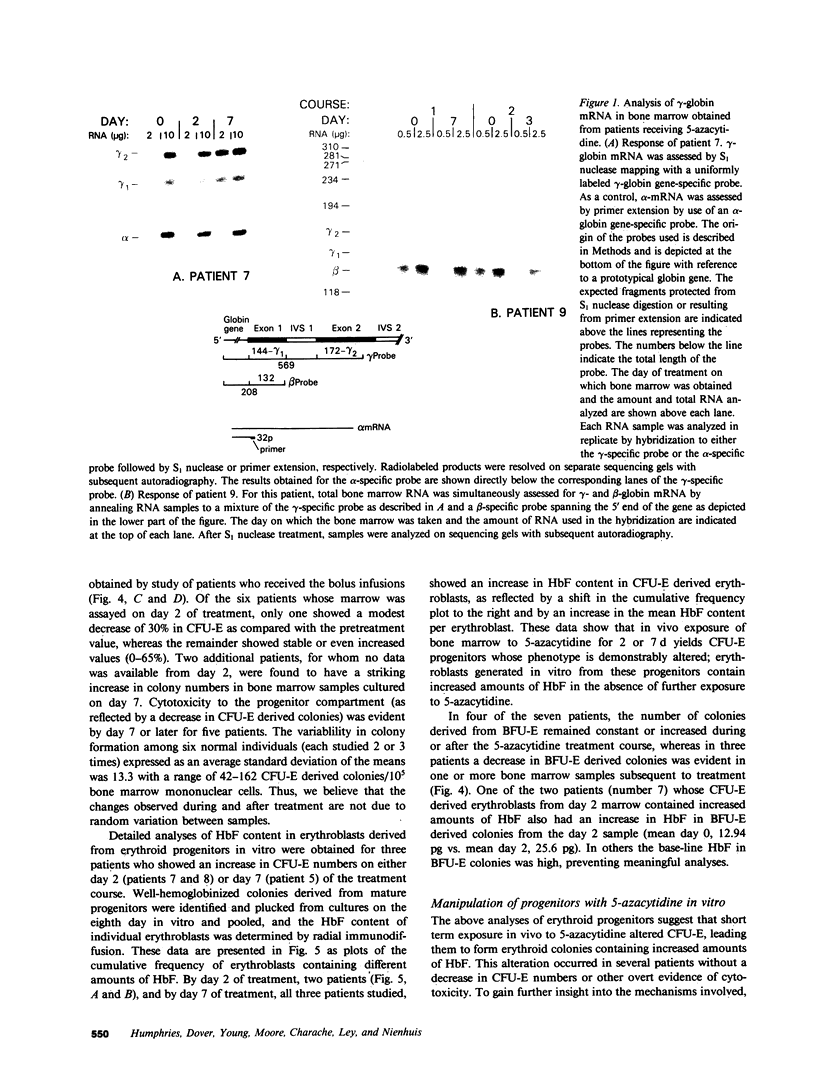
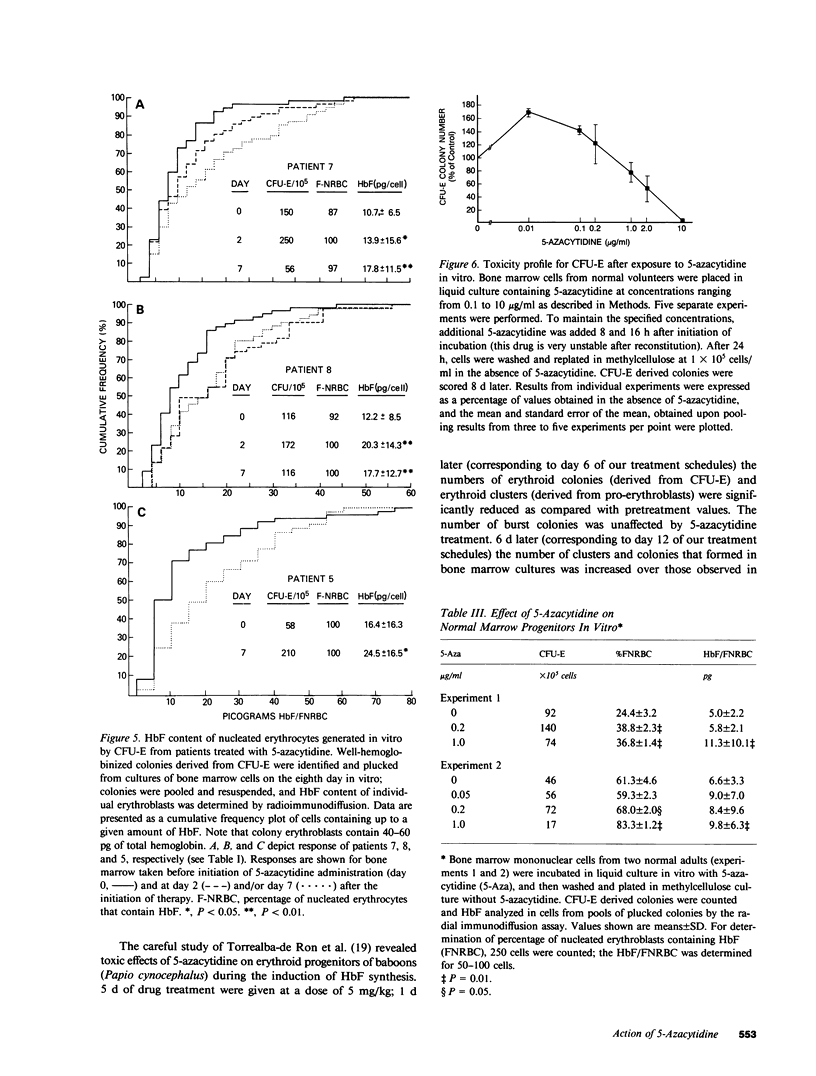
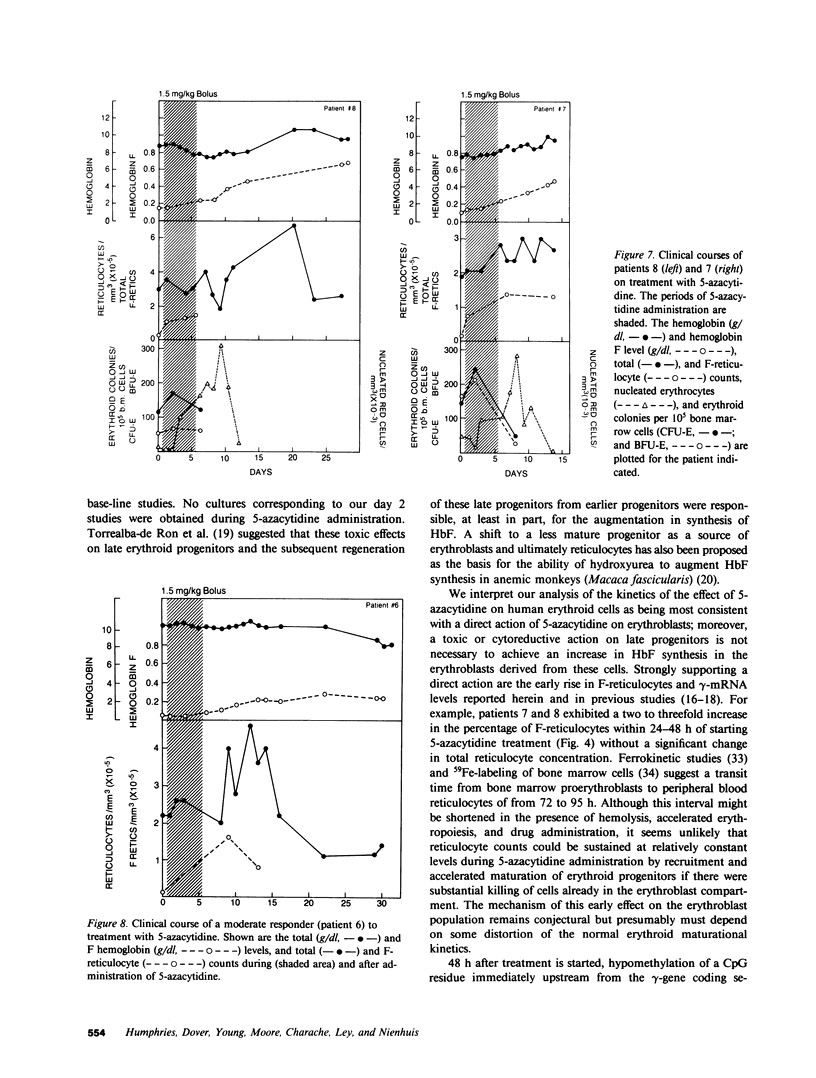
Abstract
The effect of 5-azacytidine on erythroid precursors and progenitors was studied in nine patients with sickle cell anemia or severe thalassemia. Each patient received the drug intravenously for 5 or 7 d. 5-Azacytidine caused a four- to sixfold increase in gamma-messenger RNA concentration in bone marrow cells of eight of the nine patients and decreased the methylation frequency of a specific cytosine residue in the gamma-globin gene promoter in all nine patients. Within 2 d of the start of drug treatment there was a rise in the percentage of reticulocytes containing fetal hemoglobin (HbF; F-reticulocytes) without a significant change in the total number of reticulocytes, which suggested that there was a direct action of 5-azacytidine on erythroid precursors. Late erythroid progenitors (CFU-E), present in bone marrow after 2 d of drug administration, formed colonies containing an increased amount of HbF as compared with control colonies. Moreover, the number of CFU-E derived colonies was not decreased at these early times, which suggested that there was a direct action of 5-azacytidine on erythroid progenitors in the absence of cytotoxicity. Exposure of normal bone marrow cells in tissue culture to 5-azacytidine for 24 h reproduced both of these effects as judged during subsequent colony formation. The combined direct effects of 5-azacytidine on both the erythroid precursor and progenitor compartments resulted in an increase in HbF synthesis that was sustained for 2-3 wk. Toxicity to bone marrow as reflected by cytoreduction was evident after treatment in some patients but was not accompanied by an increase in HbF production. A correlation was found between the effects of 5-azacytidine on bone marrow, as assessed by in vitro measurements, and the hematological response of the individual patients to drug treatment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPEN E. L., CRANMORE D. Cellular kinetics and iron utilization in bone marrow as observed by Fe59 radioautography. Ann N Y Acad Sci. 1959 Jun 25;77:753–765. doi: 10.1111/j.1749-6632.1959.tb36938.x. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Belding T. K., Margolet L., Noyes A. N. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975 Apr 25;188(4186):361–363. doi: 10.1126/science.804182. [DOI] [PubMed] [Google Scholar]
- Charache S., Dover G., Smith K., Talbot C. C., Jr, Moyer M., Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J., Heller P., Hall L., Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Bell W. R. Microscopic method for assaying F cell production: illustrative changes during infancy and in aplastic anemia. Blood. 1978 Oct;52(4):664–672. [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Charache S., Heintzelman K. Individual variation in the production and survival of F cells in sickle-cell disease. N Engl J Med. 1978 Dec 28;299(26):1428–1435. doi: 10.1056/NEJM197812282992603. [DOI] [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H. Quantitation of hemoglobins within individual red cells: asynchronous biosynthesis of fetal and adult hemoglobin during erythroid maturation in normal subjects. Blood. 1980 Dec;56(6):1082–1091. [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Zinkham W. H. Production of erythrocytes that contain fetal hemoglobin in anemia. Transient in vivo changes. J Clin Invest. 1979 Feb;63(2):173–176. doi: 10.1172/JCI109286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. J., Ogawa M. Cellular mechanisms for increased fetal hemoglobin production in culture. Evidence for continuous commitment to fetal hemoglobin production during burst formation. J Clin Invest. 1980 Nov;66(5):1175–1178. doi: 10.1172/JCI109949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Finch C. A., Deubelbeiss K., Cook J. D., Eschbach J. W., Harker L. A., Funk D. D., Marsaglia G., Hillman R. S., Slichter S., Adamson J. W. Ferrokinetics in man. Medicine (Baltimore) 1970 Jan;49(1):17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Human marrow cells capable of erythropoietic differentiation in vitro: definition of three erythroid colony responses. Blood. 1977 Jun;49(6):855–864. [PubMed] [Google Scholar]
- Groudine M., Kohwi-Shigematsu T., Gelinas R., Stamatoyannopoulos G., Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Kantor J. A., Turner P. H., Nienhuis A. W. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. doi: 10.1016/0092-8674(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Kidoguchi K., Ogawa M., Karam J. D. Hemoglobin biosynthesis in individual erythropoietic bursts in culture. Studies of adult peripheral blood. J Clin Invest. 1979 Apr;63(4):804–806. doi: 10.1172/JCI109366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., Linch D. C., Beardsley G. P., McIntyre K. W., Nathan D. G. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984 Apr 5;310(14):869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Anagnou N. P., Pepe G., Nienhuis A. W. RNA processing errors in patients with beta-thalassemia. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4775–4779. doi: 10.1073/pnas.79.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T. J., Chiang Y. L., Haidaris D., Anagnou N. P., Wilson V. L., Anderson W. F. DNA methylation and regulation of the human beta-globin-like genes in mouse erythroleukemia cells containing human chromosome 11. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6618–6622. doi: 10.1073/pnas.81.21.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Anagnou N. P., Keller G. H., Humphries R. K., Turner P. H., Young N. S., Keller P., Nienhuis A. W. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Noguchi C. T., Turner P. H., Schechter A. N., Heller P., Nienhuis A. W. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983 Aug;62(2):370–380. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T. H., Brice M., Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2033–2037. doi: 10.1073/pnas.73.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kalmantis T., Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Torrealba de Ron A., Veith R., Knitter G., Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984 May 11;224(4649):617–619. doi: 10.1126/science.6200940. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Appella E., Jay G. Developmental activation of the H-2K gene is correlated with an increase in DNA methylation. Cell. 1983 Dec;35(2 Pt 1):457–465. doi: 10.1016/0092-8674(83)90179-4. [DOI] [PubMed] [Google Scholar]
- Torrealba-de Ron A. T., Papayannopoulou T., Knapp M. S., Fu M. F., Knitter G., Stamatoyannopoulos G. Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of HbF by 5-azacytidine. Blood. 1984 Jan;63(1):201–210. [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]







