Abstract
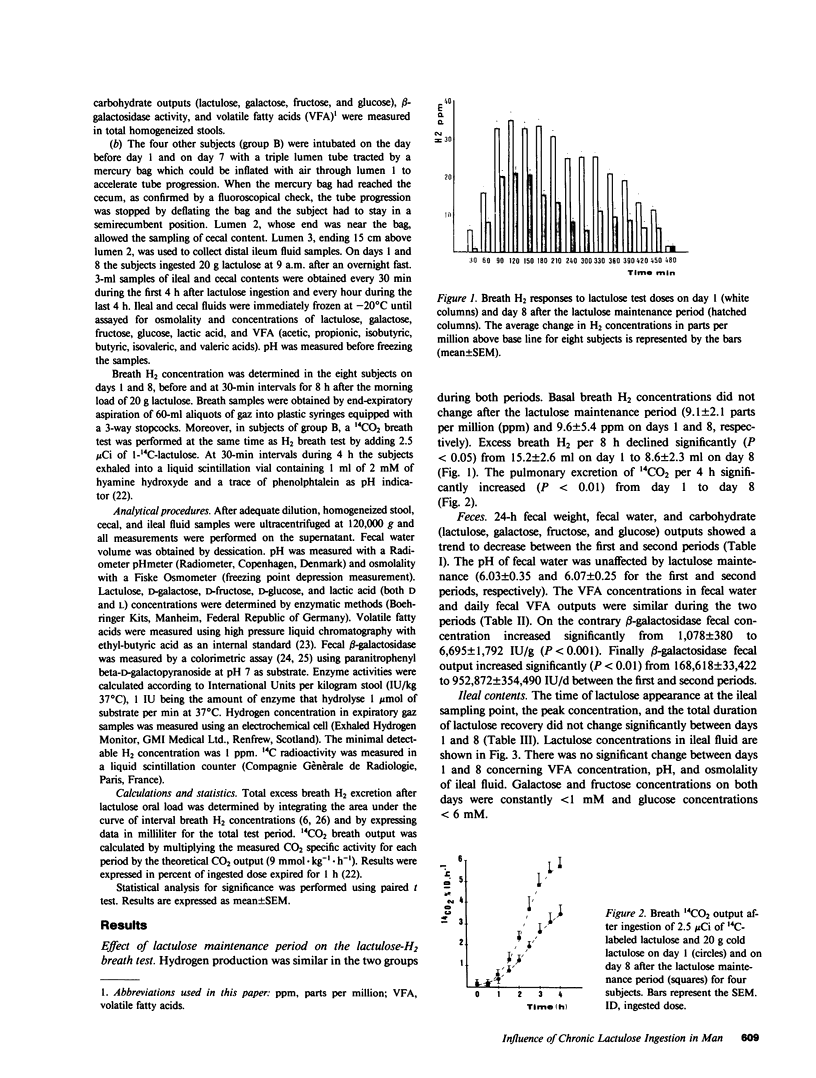
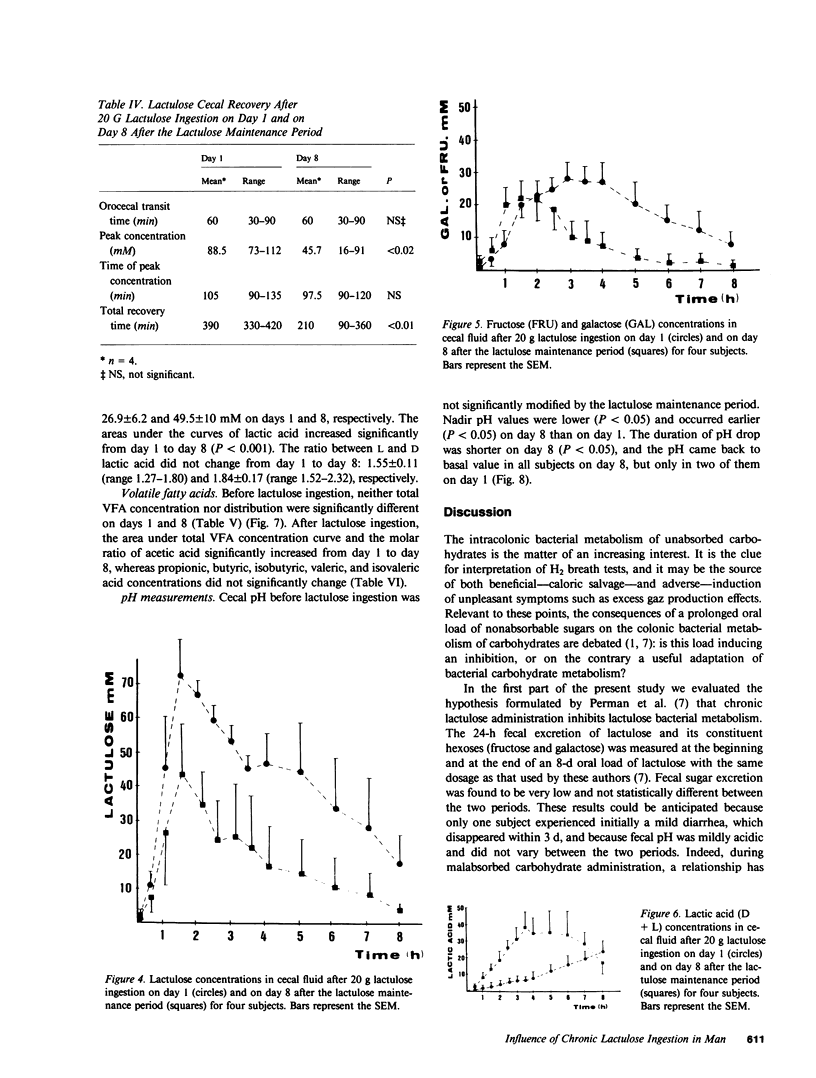
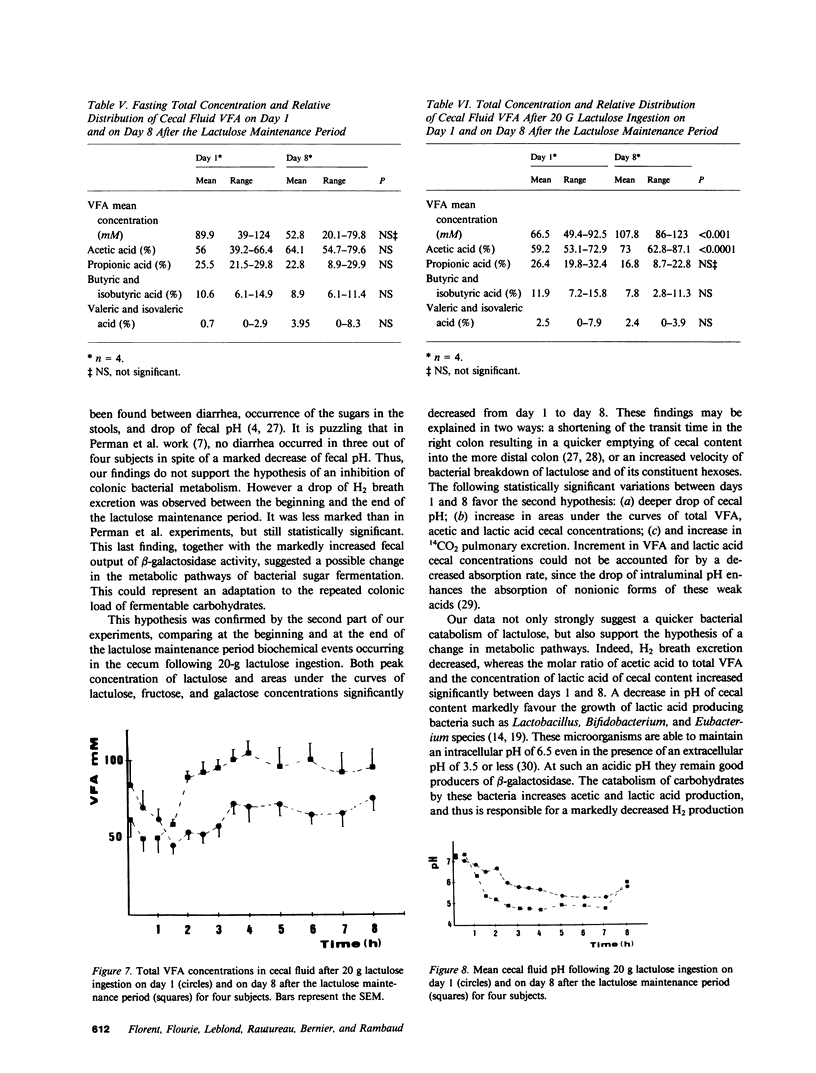
The effects of a chronic load of nonabsorbable sugars on intracolonic bacterial metabolism of carbohydrates and on H2 breath excretion are disputed. However, most of the discussion relies on indirect evidence or on results of in vitro studies. Thus, we attempted to assess directly and in vivo the effects on intracolonic metabolism of lactulose of a chronic oral load of this nonabsorbable disaccharide. 20 g of lactulose was given orally twice daily during 8 d to eight normal volunteers. In all, breath H2 concentration was measured on days 1 and 8 after ingestion of the morning lactulose dose. In four subjects, stools were collected during 2 d at the beginning and at the end of the lactulose maintenance period to measure fecal pH and daily outputs of carbohydrates and beta-galactosidase. The four other subjects were intubated on days 1 and 8 to measure the pH and the concentrations of carbohydrates, lactic acid, and volatile fatty acids (VFA) in the distal ileum and cecal contents. Moreover, 14C-lactulose was added to cold lactulose and 14CO2 breath outputs determined. Pulmonary H2 excretion fell from day 1 to day 8 (P less than 0.05), whereas 14CO2 excretion increased (P less than 0.01). Fecal water pH, lactic acid, and VFA concentrations did not vary between the two stool collection periods. 24-h fecal weight, fecal water, and carbohydrate outputs showed a trend to decrease between days 1 and 2 and days 7-8, whereas beta-galactosidase activity rose markedly (P less than 0.01). No significant variations were observed for all parameters measured in ileal fluid. In the cecum, areas under the concentration curves decreased from day 1 to day 8 for lactulose, galactose, and fructose (P less than 0.01), while an increase was found for lactic acid (P less than 0.001), acetic acid (P less than 0.0001), and total VFA (P less than 0.001). Cecal fluid pH dropped faster (P less than 0.05) and to a lower level (P less than 0.05) on day 8 than on day 1. These data clearly show that a chronic load of a nonabsorbable sugar induces changes in colonic bacterial metabolic pathways resulting in a better efficiency of the flora to digest the carbohydrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. H., Jr, Levitt M. D. Use of pulmonary hydrogen (H 2 ) measurements to quantitate carbohydrate absorption. Study of partially gastrectomized patients. J Clin Invest. 1972 May;51(5):1219–1225. doi: 10.1172/JCI106916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. H., Levitt M. D. Use of breath hydrogen (H2) in the study of carbohydrate absorption. Am J Dig Dis. 1977 Apr;22(4):379–382. doi: 10.1007/BF01072197. [DOI] [PubMed] [Google Scholar]
- Bornside G. H., Cohn I., Jr Stability of normal human fecal flora during a chemically defined, low residue liquid diet. Ann Surg. 1975 Jan;181(1):58–60. doi: 10.1097/00000658-197501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornside G. H. Stability of human fecal flora. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S141–S144. doi: 10.1093/ajcn/31.10.S141. [DOI] [PubMed] [Google Scholar]
- Chauve A., Devroede G., Bastin E. Intraluminal pressures during perfusion of the human colon in situ. Gastroenterology. 1976 Mar;70(3):336–340. [PubMed] [Google Scholar]
- Finegold S. M., Sutter V. L. Fecal flora in different populations, with special reference to diet. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S116–S122. doi: 10.1093/ajcn/31.10.S116. [DOI] [PubMed] [Google Scholar]
- Fuchs H-M, Dorfman S., Floch M. H. The effect of dietary fiber supplementation in man. II. Alteration in fecal physiology and bacterial flora. Am J Clin Nutr. 1976 Dec;29(12):1443–1447. doi: 10.1093/ajcn/29.12.1443. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Nahas L., Lerner P. I., Weinstein L. Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology. 1967 Dec;53(6):845–855. [PubMed] [Google Scholar]
- Haines A., Metz G., Dilawari J., Blendis L., Wiggins H. Breath-methane in patients with cancer of the large bowel. Lancet. 1977 Sep 3;2(8036):481–483. doi: 10.1016/s0140-6736(77)91605-1. [DOI] [PubMed] [Google Scholar]
- Hentges D. J. Fecal flora of volunteers on controlled diets. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S123–S124. doi: 10.1093/ajcn/31.10.S123. [DOI] [PubMed] [Google Scholar]
- Hill M. J. Diet and the human intestinal bacterial flora. Cancer Res. 1981 Sep;41(9 Pt 2):3778–3780. [PubMed] [Google Scholar]
- Lifschitz C. H., Irving C. S., Gopalakrishna G. S., Evans K., Nichols B. L. Carbohydrate malabsorption in infants with diarrhea studied with the breath hydrogen test. J Pediatr. 1983 Mar;102(3):371–375. doi: 10.1016/s0022-3476(83)80651-9. [DOI] [PubMed] [Google Scholar]
- MacLean W. C., Jr, Fink B. B. Lactose malabsorption by premature infants: magnitude and clinical significance. J Pediatr. 1980 Sep;97(3):383–388. doi: 10.1016/s0022-3476(80)80186-7. [DOI] [PubMed] [Google Scholar]
- Maruhn D. Rapid colorimetric assay of beta-galactosidase and N-acetyl-beta-glucosaminidase in human urine. Clin Chim Acta. 1976 Dec;73(3):453–461. doi: 10.1016/0009-8981(76)90147-9. [DOI] [PubMed] [Google Scholar]
- Newcomer A. D., McGill D. B., Thomas P. J., Hofmann A. F. Prospective comparison of indirect methods for detecting lactase deficiency. N Engl J Med. 1975 Dec 11;293(24):1232–1236. doi: 10.1056/NEJM197512112932405. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Perman J. A., Modler S., Olson A. C. Role of pH in production of hydrogen from carbohydrates by colonic bacterial flora. Studies in vivo and in vitro. J Clin Invest. 1981 Mar;67(3):643–650. doi: 10.1172/JCI110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt P., de Bruijn K. M., Beeching M. F., Goldberg E., Blendis L. M. Studies on breath methane: the effect of ethnic origins and lactulose. Gut. 1980 Nov;21(11):951–954. doi: 10.1136/gut.21.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppin H., Bar-Meir S., Soergel K. H., Wood C. M., Schmitt M. G., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980 Jun;78(6):1500–1507. [PubMed] [Google Scholar]
- Saunders D. R., Wiggins H. S. Conservation of mannitol, lactulose, and raffinose by the human colon. Am J Physiol. 1981 Nov;241(5):G397–G402. doi: 10.1152/ajpgi.1981.241.5.G397. [DOI] [PubMed] [Google Scholar]
- Simon G. L., Gorbach S. L. Intestinal flora in health and disease. Gastroenterology. 1984 Jan;86(1):174–193. [PubMed] [Google Scholar]
- Smith C. J., Bryant M. P. Introduction to metabolic activities of intestinal bacteria. Am J Clin Nutr. 1979 Jan;32(1):149–157. doi: 10.1093/ajcn/32.1.149. [DOI] [PubMed] [Google Scholar]
- Solomons N. W., Viteri F. E., Hamilton L. H. Application of a simple gas chromatographic technique for measuring breath hydrogen. J Lab Clin Med. 1977 Nov;90(5):856–862. [PubMed] [Google Scholar]
- Solomons N. W., Viteri F., Rosenberg I. H. Development of an interval sampling hydrogen (H2) breath test for carbohydrate malabsorption in children: evidence for a circadian pattern of breath H2 concentration. Pediatr Res. 1978 Aug;12(8):816–823. doi: 10.1203/00006450-197808000-00002. [DOI] [PubMed] [Google Scholar]
- Vince A., Dawson A. M., Park N., O'Grady F. Ammonia production by intestinal bacteria. Gut. 1973 Mar;14(3):171–177. doi: 10.1136/gut.14.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F. L., Jr, Fresard K. M., Lally B. R. Effects of lactulose and neomycin on urea metabolism in cirrhotic subjects. Gastroenterology. 1982 Feb;82(2):213–217. [PubMed] [Google Scholar]
- Yuen C. T., Price R. G., Chattagoon L., Richardson A. C., Praill P. F. Colorimetric assays for N-acetyl-beta-D-glucosaminidase and beta-D-galactosidase in human urine using newly-developed omega-nitrostyryl substrates. Clin Chim Acta. 1982 Sep 15;124(2):195–204. doi: 10.1016/0009-8981(82)90387-4. [DOI] [PubMed] [Google Scholar]



