Abstract
There is a high prevalence of cannabis use reported in non-affective psychosis. Early prospective longitudinal studies conclude that cannabis use is a risk factor for psychosis, and neurochemical studies on cannabis have suggested potential mechanisms for this effect. Recent advances in the field of neuroscience and genetics may have important implications for our understanding of this relationship. Importantly, we need to better understand the vulnerability × cannabis interaction to shed light on the mediators of cannabis as a risk factor for psychosis. Thus, the present study reviews recent literature on several variables relevant for understanding the relationship between cannabis and psychosis, including age of onset, cognition, brain functioning, family history, genetics, and neurological soft signs (NSS) in non-affective psychosis. Compared with non-using non-affective psychosis, the present review shows that there seem to be fewer stable cognitive deficits in patients with cannabis use and psychosis, in addition to fewer NSS and possibly more normalized brain functioning, indicating less neurobiological vulnerability for psychosis. There are, however, some familiar and genetic vulnerabilities present in the cannabis psychosis group, which may influence the cannabis pathway to psychosis by increasing sensitivity to cannabis. Furthermore, an earlier age of onset suggests a different pathway to psychosis in the cannabis-using patients. Two alternative vulnerability models are presented to integrate these seemingly paradoxical findings
Keywords: cannabis, psychosis, schizophrenia, cognition, age of onset
Introduction
Prevalence and clinical implications
Use of illicit drugs is common in non-affective psychosis (ICD-10 F20–29; (1)), usually seen in about half of the patients; 40–60%, ranging from 10 to 70% (2–9). Illicit drug use in psychosis has clinical implications and has been associated with more relapse and re-hospitalizations, poorer social functioning, medication non-adherence, heightened suicide risk, increased treatment needs, and worse clinical outcomes (10–17). However, there are few clinical differences in relation to symptoms and family loading between drug-using and non-drug-using patients (18).
KEY CONCEPT 1. Non-affective psychosis.
A broader diagnostic group than schizophrenia only; includes schizophrenia-spectrum psychosis, but not affective psychosis or drug-induced psychosis. The term reflects a more contemporary view of psychosis, e.g., as best reflected by a continuum.
Cannabis is the most widely used illicit drug in non-affective psychosis, and life-time cannabis use has typically been reported to be about 50% (2, 19–21). The rate of cannabis use disorder is somewhat lower; about every fourth patients with schizophrenia according to a recent meta-analysis by Koskinen et al. (22), with particularly high current and life-time rates in first-episode samples (28.6 and 44.4%, respectively). Cannabis is derived from the plant Cannabis sativa, and is usually used as an illicit substance in the form of dried flower buds (marijuana), resin from the trichomes (hashish), or various extracts collectively known as hashish oil. Cannabis has psychological and cognitive effects, and can have psychosis-imitating properties. These drug effects are usually attributed to cannabinoids, with delta-(9)-tetrahydrocannabinol (THC) as the main psychoactive substance influencing experience and cognition (23). There is large intra-individual variability in the psychological reactions to THC, possibly related to individual differences in the corresponding brain activation changes (24). Psychosis-prone individuals, people with psychosis, and others who are genetically vulnerable to psychosis have an increased sensitivity to the adverse effects of THC (23).
KEY CONCEPT 2. Cannabis.
The most widely used illicit drug worldwide; taken from the plant Cannabis sativa, and usually used as an illicit substance in the form of dried flower buds (marijuana), resin from the trichomes (hashish), or various extracts collectively known as hashish oil. Cannabis has psychological and cognitive effects, and can have psychosis-imitating properties.
KEY CONCEPT 3. THC.
Delta-(9)-tetrahydrocannabinol; a cannabinoid and the main psychoactive substance in cannabis, influencing experience and cognition.
Cannabis use as a risk factor for non-affective psychosis
Longitudinal studies have reported an increased likelihood for developing schizophrenia and other psychoses after cannabis use (25, 26), especially when cannabis use has been moderate to severe and/or is started in the early teens (27–29). Schizotopy has also been associated with cannabis use in a recent meta-analysis (30). In addition, several large-scale longitudinal studies have reported a relationship between cannabis use in adolescence as well as later symptoms of sub-threshold psychosis in the general population (31–37). The relationship between cannabis and psychosis seems fairly specific to schizophrenia, as compared to other mental disorders (38–40), even though there are a relationship between symptoms of anxiety and cannabis (41). The relationship cannot be explained by potentially confounding factors such as premorbid disorders, other types of drug use, intoxication effects, personality traits, sociodemographic markers, or intellectual ability (40). Accordingly, several reviews conclude with an increased risk for psychosis in individuals who have used cannabis, typically in the magnitude of an odds ratio of 1.5–2 (20, 40, 42–44).
There are opposing views on cannabis as a risk factor for psychosis, however [see in Ref. (45) for an overview]. Some authors propose that there is a causal relationship between illicit drug use and non-affective psychosis (45, 46). Others suggest that illicit drugs only precipitate non-affective psychosis in vulnerable individuals on their pathway to psychosis (45–48). A variant of this is reversed causality; cannabis is used as a form of self-medication in psychosis, although existing data do not seem to support this hypothesis (38). Cannabis debut usually precedes onset of psychosis (10, 39, 49), e.g., by 7–8 years (50). However, most individuals do not develop psychosis after cannabis use, suggesting that risk of psychosis must be modulated by other factors. In line with this, data from recent comprehensive studies suggest that cannabis is an environmental risk factor interacting with more basic genetic and biological vulnerability for psychosis (51–53).
The effect of cannabis on neurotransmitter systems
Tetrahydrocannabinol probably influences the endogenous cannabinoid and dopamine systems (23, 54, 55), via cannabinoid receptors, which are distributed with high density in the cerebral cortex, including brain regions implicated in schizophrenia, and influence dopamine synthesis and uptake (23). Most studies on the neurotoxicity of THC in general are based on animal models, suggesting that THC increases dopamine levels in several regions of the brain, including striatal and prefrontal areas (56). Animal studies have also found more irreversible residual effects in prepubertal rats after chronic exposure to THC as compared to more mature rats (57). THC is a cannabinoid receptor 1(CB1) agonist, and Casadio et al. (58) suggest that cannabis produces its effects via the influence on CB1 receptors on GABA and glutamate, which modulate the excitability of midbrain dopamine neurons and prefrontal cortical pyramidal cells; it thus appears to switch off inhibitory inputs to dopamine neurons. THC may aggravate dopaminergic imbalances by increasing the dopaminergic tone in striatal regions of the brain, which, when administered repeatedly, decreases dopamine levels in prefrontal regions of the brain via sensitization processes resulting in expressions of a psychotic disorder (56, 59). It is possible that the repeated administration of THC alters the functioning of the prefrontal cortex by acting on dopamine signaling via activation of CB1 receptors. Kuepper et al. (56) argue for interpretative caution, however, since most evidence is based on animal research and the effects of endocannabinoids are not yet fully understood. No relationship between striatal postsynaptic dopamine receptors and cannabis use has also been reported (60), and pretreatment with the dopamine receptor antagonist haloperidol did not alter the behavioral effects of delta-9-THC (23). Bloomfield et al. (61) on the other hand found that chronic cannabis use was associated with reduced presynaptic dopamine synthesis capacity in the striatum, suggesting a complex relationship between cannabis and changes of dopamine availability.
In addition, there may be psychoactive substances in cannabis not yet studied, as there are at least 85 different cannabinoids in cannabis (62). Cannabidiol (CBD) may have a buffer effect against the negative effects of THC, e.g., Schubart et al. (63) found an association between estimated CBD in cannabis and fewer web-based self-reported experiences of psychotic symptoms. The CBD/THC ratio is possibly of importance for the psychological effects of cannabis, suggesting that the increasingly popular THC-potent variants of cannabis (e.g., “skunk”) may be more psychosis-inducing. Finally, CBD has been under investigation in a few small clinical trials as a potentially novel antipsychotic agent, with equivocal results thus far and larger studies being needed (64). One promising study found CBD to be comparable in its antipsychotic effect to the antipsychotic amisulpride in a double-blind, randomized clinical trial in acute schizophrenia, and attributed the antipsychotic properties of CBD to its effect on the level of the endocannabinioid anandamide (65).
Need for translational knowledge
Thus, there is a high prevalence of cannabis use in non-affective psychosis with clinical implications. Early prospective longitudinal studies conclude that cannabis use is a risk factor for psychosis, and biochemical studies on cannabis have suggested potential mechanisms for this effect. Recent advances in the field of neuroscience and genetics may have important implications for our understanding of this relationship. Importantly, we need to better understand the vulnerability × cannabis interaction to shed light on the mediators of cannabis as a risk factor for psychosis in order to create targeted interventions; e.g., for whom is cannabis precarious? Thus, the present study reviews recent literature on vulnerability variables and/or mediating variables and cannabis, including data from our own laboratory. This includes focus on age of onset, cognition, brain functioning, family history, genetics, and neurological soft signs (NSS) in non-affective psychosis. Models will be presented to explain the findings. This review is also based on previous work by the present authors in Frontiers in Psychiatry (66); putting the work into a broader context and outlining the implications for scientific questions and issues within the field.
Age of Onset
Earlier psychosis debut – age of onset has been shown in patients who have been using cannabis (21, 67–71). However, some studies report no effect of illicit drug use on onset age (15, 72–74). The inconsistencies are possibly due to methodological differences and small sample sizes in several studies. Furthermore, some studies have used “first treatment contact” instead of first psychotic symptoms as an estimate for psychosis debut (69), which is sub-optimal since psychosis may last for several years before first treatment (75). Meta-analyses conclude, however, that there is an earlier onset age of psychosis in cannabis and polysubstance users compared with those without illicit drug use also when confounders have been controlled for (68, 76). In addition, there is an effect of THC dose – patients with a history of cannabis use had about 3 years earlier debut of psychosis, and the subjects who had been using high-potency cannabis everyday had the earliest onset (29).
KEY CONCEPT 4. Age of onset.
Age of psychosis debut, usually defined as the first psychotic breakthrough, defined by, e.g., a PANSS psychosis symptom item score of ≥4.
The earlier onset age can possibly be attributed to the illicit drug use acting on the developing brain in adolescence (29, 32, 77). This neurodevelopmental hypothesis is also supported by a stronger relationship between adolescent cannabis use and psychosis as compared to adult use (27, 28). The effect of cannabis timing is also supported by longitudinal studies. As compared to non-users, more adult psychosis was predicted by cannabis use at 15 versus 18 years (32) and cannabis use at around 15 years or younger versus older debut (78). Furthermore, Schubart et al. (79) found very early that both cannabis use (under 12 years of age) and heavy cannabis use (>25 €/week) were associated with an increased likelihood of psychiatric hospitalizations. A recent study of 410 first-episode psychosis patients reported that those who had started cannabis at age 15 years or younger had an earlier onset of psychosis for about 2 years, and that the users of high-potency cannabis everyday had the earliest psychosis onset (29). It is not possible to fully rule out, however, that the relationship between the development of psychosis and the younger age of cannabis debut is driven by a larger cumulative exposure to cannabis. Alternatively, early cannabis users may be the ones who have been using cannabis before the development of psychosis and thus cannabis could have been an actual risk factor for them. The older cannabis users, however, may have used cannabis more in parallel with the first signs of psychosis, hence clouding the dynamics of cannabis a risk factor. Still, transition to psychosis in an ultra-high-risk sample was found to be highest among those who started using cannabis before the age of 15 years and went on to frequent use (49).
The neurodevelopmental explanation is further substantiated by central brain development processes in adolescence that may be particularly sensitive to cannabis. Levels of endocannabinoids and cannabinoid receptors increase at this age, peaking at puberty (80). Furthermore, the endocannabinoid system is involved in key processes of brain maturation during adolescence, e.g., the control of neuronal specification and maturation (81). Thus, exposure to cannabis during critical neurodevelopmental stages may impact the maturation of the endocannabinoid system and other key neurotransmitter systems (58). Bossong and Niesink (55) suggest that cannabis use during adolescence results in the disturbance of certain local neural circuits within the prefrontal cortex, and that the disturbance occurs as an interaction between THC and the CB1 receptors involved in the control of GABA and glutamate release. In line with this, studies have found that age at first use of cannabis predicted age at first psychotic symptom in patients with recent non-affective psychosis (50, 77, 82).
Our laboratory in Bergen, Norway, together with collaborating study organizations in Oslo and Stavanger, examined the effect of illicit drug use on onset age in a large sample of 1,119 patients with non-affective psychosis (83). Patients with illicit drug use had a significantly lower onset age of about 3 years compared with the abstinent group, primarily related to cannabis use and not to alcohol or other substances. This supports the notion that the effect of cannabis on age of onset is specific. As an overall conclusion, there is now evidence for an earlier onset age of psychosis in cannabis users often reported to be about 2–4 years. There are few studies suggesting that the earlier onset is related to early initiation of cannabis use, e.g., before the age of 15 years and related to neurodevelopmental processes, but there may also be alternative explanations for this relationship.
Cognition
A majority of patients with schizophrenia and non-affective psychoses have clinically significant cognitive deficits (84–87). Cognitive deficits are vulnerability markers, present before the development of psychosis (87, 88), in high-risk populations (89, 90), and which persist after treatment of clinical symptoms (91, 92). General cognitive dysfunctions across cognitive domains are present, with additional selective deficits in working memory, executive function, attention, verbal fluency, episodic memory, and processing speed (93, 94). Typically, the impairment is between 1 and 2 SD, indicating a clinically significant loss of function (87, 95–98). Cognitive functioning is more important than positive psychotic symptoms in determining the patient’s functional outcome (93, 99–102).
KEY CONCEPT 5. Cognitive deficits.
Reduced ability or capacity for mental information processing; in non-affective psychosis often seen as problems in the following areas: attention/vigilance, working memory, learning, executive functioning, perceptual processing, visuomotor speed, and verbal fluency.
There have been difficulties in drawing firm conclusions regarding the long-term effects of cannabis on cognition, and studies should be interpreted with caution, however, due to often uncertain abstinence periods (103). Decrements in the ability to learn and remember new information in chronic users have been suggested, whereas other cognitive abilities are more unaffected, and younger cannabis users may be particularly vulnerable to these effects (104, 105). This age affect is, however, not substantiated by many studies. Studies making a distinction between adolescence and adult debut have shown some cognitive decrements in early-onset cannabis users (106, 107).
Cannabis use may affect cognition differentially in psychosis. In a preliminary study in our laboratory, we examined the effects of cannabis on cognition in 31 patients with schizophrenia (19, 108). Surprisingly, we found that patients with schizophrenia who had a history of cannabis use scored significantly better than psychosis patients without cannabis use. This was found for almost all cognitive domains investigated except learning; general intellectual ability, executive functions, attention, working memory, and psychomotor speed. These results did not change when other illegal drugs where controlled for, and there were no differences in the two groups with regard to clinical variables (19). We hypothesized that there was less cognitive vulnerability in the cannabis group. Interestingly, other authors, such as Schnell et al. (109), also suggested similar explanations at this point in time.
This finding prompted a review of the existing literature on the relationship between cannabis use and cognitive functioning in non-affective psychosis, and a systematic PubMed search resulted in 23 studies (110). Fourteen of the studies reported that the cannabis groups showed better cognitive performance than the no-cannabis groups (19, 73, 108, 109, 111–120). Eight of the studies reported no or minimal differences in cognitive performance in the two groups (21, 121–127), and one study reported better cognitive performance in the no-cannabis compared with the drug group (128).
The results showed that most studies had found better cognitive functioning in psychosis patients with cannabis use compared with psychosis alone (110). This pattern has been found by other meta-analysis (112, 119, 129) and replicated by more recent studies (130, 131) as well as two meta-analyses (132, 133). There are also studies with contradictory findings, possibly due to methodological issues such as different definitions of drug abuse (110); there are methodological differences related to ongoing versus previous drug use, frequent versus infrequent use, younger age in drug groups, and differences in relation to test batteries and cognitive domains examined. However, superior cognitive functioning in the drug-using group has been reported in first-episode psychosis patients (131, 134) and at 10-year follow-ups after onset of psychosis (116). The meta-analysis by Yücel et al. (132) found that cannabis-using patients performed moderately better than non-using patients on measures of global cognition, visual memory, processing speed, working memory, planning, and reasoning. Rabin et al. (133) reported the following effect sizes for superior cognitive functioning in cannabis-using patient compared with non-using patients for each neurocognitive domains in their meta-analysis: general cognitive ability and intelligence 0.48; selective, sustained and divided attention 0.35; executive abilities 0.14; working memory and learning 0.07; retrieval and recognition 0.12; receptive and expressive language abilities 0.06; and visuospatial and constructional abilities 0.33; these thus show effect sizes in the small to moderate range.
To explain the different cognitive profiles, we hypothesized that there was less cognitive vulnerability in the cannabis group; their psychosis breakthrough was related to aberrant information processing due to cannabis-induced transient cognitive deficits. To test this, we first performed a preliminary prospective study of 31 patients with acute psychosis, assessing cognitive function at admission to a psychiatric emergency ward, after 6 weeks and after 3 months. The patients with both cannabis history and psychosis showed a significantly larger improvement in their cognitive performance in the 3 months after admission compared with the psychotic patients without cannabis use (108).
In a continuation of this study, we improved the design by focusing on the period when the patients were in-patients to control for the illicit drug use after admission to the acute ward in a total sample of 123 patients (135, 136). The patients were examined by the Battery for the Assessment of Neuropsychological Status (RBANS) with alternative forms to minimize practice effects (95, 137, 138) at baseline and follow-up (mean time to follow-up 4 weeks). As expected, the cannabis-using group showed the largest improvement in cognition, especially among the youngest patients. This suggests that indeed cannabis use did induce transient cognitive deficits in the cannabis-using psychosis group, and that younger patients could have a larger capacity for restoring their cognitive capability. Thus, overall, there seems to be less persistent cognitive deficits in patients with psychosis and cannabis use, indicating less neurocognitive vulnerability.
Brain Functioning
Reviews of structural brain imaging studies in non-affective psychosis, usually by means of magnetic resonance imaging (MRI) paradigms, have shown several distinct alterations in gray matter and white matter, both widespread and, in some studies, progressive changes (139). Voxel-based-morphometry (VBM) has, according to a review, most consistently shown volume reduction in the superior temporal cortex in chronic patients, and in frontal brain regions in first-episode and high-risk individuals (140). Functional brain imaging studies, often by means of functional MRI (fMRI), find abnormal communication between and/or integration of brain activation in local and distributed circuits (141–143), and connectivity deficits and additional transient states of hyper- and/or hypo-connectivity related to specific tasks (142). Decreased brain activation to effort-demanding tasks is a typical finding in non-affective psychosis (144–147), and lately several studies have shown increased brain activation in the default mode network (which includes activation in the medial prefrontal and temporal lobes reflecting endogenous generated thought) in psychosis compared with normal controls (147–150).
KEY CONCEPT 6. Brain imaging.
Techniques to image the structure and function of the brain, typically by means of magnetic resonance imaging (MRI) and functional magnetic resonance imaging (fMRI). There are also other brain-imaging techniques.
Studies have mostly been inconclusive in regard to the effect of long-term exposure to cannabis on the brain, but some brain imaging studies have found effects of long-term heavy use on gray matter volume (151–154). Mata et al. (155) suggested that normal neurodevelopment was affected after observing gyrification abnormalities in the cortex after long-term use. Long-term cannabis use has also been found to affect white matter as measured by diffusion tensor imaging (DTl), e.g., in the corpus callosum (156). Bossong et al. (157) recently reviewed neuroimaging studies on the long-term and acute effects of cannabis in both adult and adolescents on brain function related to learning and memory functions. Cannabis did not affect performance, but there were subtle changes in brain activation patterns in the cannabis-using group – both acute and long-term effects. The authors concluded that there was increased activity and a higher level of deactivation in the cannabis groups, and attributed this to compensatory increased or changed neural effort or non-cognitive factors like cerebral perfusion; however, they also pointed to the methodological problems related to comparing the studies.
Structural MRI and DTI studies comparing psychoses with and without cannabis use have shown less altered (134, 158), more anomalous (159–164), and equivalent (165–167) brain anatomy in the cannabis group, thus making firm conclusions difficult. Ongoing, long-term, and heavy use may influence the results: the continuation of cannabis use was found to increase gray matter loss and lateral and third ventricle enlargement after 5 years (161), and users of more than five joints daily for more than 10 years were shown to have bilaterally reduced hippocampal and amygdala volumes (168). A systematic review of 15 MRI and 4 post-mortem studies found evidence for brain structural abnormalities after cannabis use in psychosis in CB1-rich areas of the brain-like cingulum, the dorsolateral prefrontal cortex, and the cerebellum, and the authors suggested that the effect of cannabis is actually more distinct in psychosis than in the normal controls (154). Smith et al. (169) compared healthy controls, subjects with a history of cannabis use disorder, schizophrenia only, and both schizophrenia and a history of cannabis use disorder, while subjects with recent cannabis use were excluded. Both cannabis groups showed differences in relation to WM-related subcortical morphology, and the authors attributed this to either chronic cannabis abuse or the presence of biomarkers that characterize a vulnerability to the effects of cannabis.
There have been too few fMRI studies on long-term cannabis use and non-affective psychosis to reach a conclusion. One study, however, reported that schizophrenia patients with substance-use history showed increased cerebral activation to passive viewing of emotionally negative pictures, and concluded that the medial prefrontal cortex functioning is more preserved in dual-diagnosis schizophrenia (170). Potvin et al. (171) also found less impaired brain functioning during socio-emotional processing in patients with a dual diagnosis (mainly cannabis users) than schizophrenia alone.
To further examine brain functioning in cannabis use and psychosis, we conducted a study at our laboratory (66) to examine brain activation in 26 patients with schizophrenia with and without a history of previous cannabis use by using an fMRI paradigm comparing task-dependent (effort mode network) [cf. Ref. (172)] and task-independent (default mode network) [cf. Ref. (173)] conditions. We expected to replicate the better cognition for the cannabis users by finding less-anomalous brain activation patterns in the previous cannabis group, defined as the ability to up-regulate the effort mode network during the task-dependent condition [dichotic listening task with attention instructions, see in Ref. (174)] and down-regulate the default mode network during the task-independent condition. The present sample did all have a history of cannabis abuse as the main drug of choice, but not within the last 6 months, and patients that had been using meth-amphetamine, cocaine or opiates were excluded. The study showed different activation patterns for the task-dependent and task-independent conditions, essentially following the effort and default mode networks, respectively. Although all patients showed similarities across activation patterns, group differences emerged in the intensity and extension of the activation patterns that could not be explained by differences in clinical or demographic variables. The activation was more pronounced for the task-dependent condition in the cannabis group, while it was more pronounced for the task-independent condition in the no-cannabis group. Thus, as hypothesized, the previous cannabis group managed to up-regulate the effort mode network during the task-dependent condition and down-regulate the default mode network during the task-independent condition to a larger extent than the no-cannabis group. The no-cannabis group did show a pattern closer to the typical schizophrenia findings [see in Ref. (147)], indicating more impaired brain activation, and supporting less-anomalous brain activation in cannabis psychosis (66).
Two later studies have similar findings of better brain functioning for cannabis users. Patients with dual diagnosis of cannabis abuse and schizophrenia were found to be less impaired relative to schizophrenia only compared with healthy controls in regard to emotional memory and prefrontal lobe functioning (175), and showed a more normalized brain activation pattern during mental rotation in the left superior parietal region relative to schizophrenia only compared with healthy controls (176). It must be noted, however, that the differences between the different groups are subtle in most studies, and even group-based differences between normal controls and patients with schizophrenia and non-affective psychosis are usually subtle and of unclear clinical relevance. Still, it can be concluded that there are some data, although scarce, suggesting minor long-term effects of cannabis on brain structure in psychosis patients, but that the majority of studies show better brain functioning in this group, suggesting less neurocognitive vulnerability.
Other Variables Mediating Neurobiological Vulnerability
Neurological soft signs
Neurological soft signs are subtle sensory and motor performance anomalies that serve as markers of sub-optimal neurological development – there is an excess of NSS already evident in first-episode psychosis (177). Fewer NSS have been observed in schizophrenia with cannabis use than without cannabis use (74, 116, 160, 178). Ruiz-Veguilla et al. (178) reported an association between high NSS and not having been a heavy cannabis user and a family history of psychosis, and suggested a potentially different pathway to psychosis in relation to cannabis use. Less NSS in cannabis psychosis indicates less neurobiological vulnerability.
KEY CONCEPT 7. Neurological soft signs.
Subtle sensory and motor performance anomalies that serve as markers of a sub-optimal neurological development.
Family history and genetics
Family history of serious mental disorders is often used as a proxy for genetic vulnerability (179). Proal et al. (180) reported that those who developed schizophrenia after cannabis use in adolescence had the same family history of schizophrenia as patients without cannabis use.
A few studies have examined the effects of polymorphisms in candidate genes on cannabis use and psychosis. In a longitudinal study, an interaction between the valine versus methionine allele of the Catechol-o-methyltransferase (COMT) gene and adolescent cannabis use significantly increased the likelihood of psychosis (27). This finding has not been replicated by all studies (179), however; Zammit et al. (181) did not find an effect of cannabis use on psychosis according to variation in COMT alleles, but an interaction between the COMT alleles and sensitivity for psychosis and cognitive effects of THC has been replicated by Henquet et al. (182, 183). There are also other genes, influencing D2 receptors and the cannabinoid system, that have been found to modify the sensitivity to cannabis. Certain variant (C/C genotype) of the AKT1 gene has been shown to give an increased likelihood of psychosis after life-time cannabis use (29), and looking at schizotypy proneness in unaffected siblings, Van Winkel and Genetic Risk and Outcome of Psychosis (GROUP) Investigators (52) found that genetic variation in AKT1 mediated both short-term as well as longer-term effects associated with use of cannabis.
This indicates a gene × environment interaction (179); there is possibly a genetically based sensitivity to substances (59), consistent with the fact that most people do not develop psychosis after cannabis use. Parakh and Basu (184) reviewed evidence on the association between cannabis and psychosis and concluded that cannabis is a component cause of psychosis, increasing the risk in people with certain genetic or environment vulnerabilities. Verdoux (185) found that subjects with established vulnerability for psychoses showed a stronger risk for psychosis after cannabis use than individuals without such vulnerability. Still, a study observed that cannabis also increased the risk for schizophrenia when family history was controlled for (78). In addition, cannabis interacts with other environmental risk factors like developmental trauma and child maltreatment, minority group position, and growing up in an urban environment, increasing the risk even further with the increasing number of risk factors (51, 59, 186). Also, certain variants of the COMT gene gave an increased risk for psychotic experiences after exposure to both cannabis use and childhood maltreatment (187, 188). It has been suggested that the environmental risk factor may induce differential sensitivity to cannabis (59). Recent animal models suggest long-lasting epigenetic changes after early negative life events (189), thus epigenetic mechanisms may increase the vulnerability to cannabis in certain individuals.
KEY CONCEPT 8. Gene × environment interaction.
The interaction between genetic vulnerability and environmental risk factors for psychosis, exemplified by the notion of a genetically based sensitivity to illicit substances such as cannabis.
Summing up, there seems to be some familiar and genetic vulnerability present in non-affective psychosis with cannabis use, possibly modified by environmental risk factors, that may be acting on neurotransmitter systems and/or genetic expression that particularly sensitize the individual to cannabis, but there are also less vulnerability markers such as NSS, cognitive deficits and probably brain abnormalities than in psychosis without cannabis use.
Discussion
Critical remarks
There are inconsistencies – not all studies find better cognition or less-disturbed brain functioning in cannabis-using patients. An important variability in the methodology of the studies may explain this, e.g., the different definitions of cannabis use, current, life-time or previous use, or a cannabis use disorder. Previous use, before the development of psychosis, is most relevant for the effects of cannabis within a vulnerability framework (110). It is also important to rule out the potential confounding effects of cannabis intoxication or recent cannabis use on, e.g., brain functioning seen in some studies (190–192). Recently, a large cross-sectional study compared the effect of current cannabis use and the effect of life-time cannabis use in 956 patients. This study concluded that there was a short-term negative effect of recent cannabis use on cognition and in contrast a positive long-term effect of life-time use, suggesting that the life-time cannabis-using group formed a subgroup with a different cognitive profile (193). Furthermore, some studies exclude patients not meeting the diagnostic criteria of a substance-use disorder, biasing the drug groups to consist of quite heavy users, and there are even studies where the no-drug group includes patients with previous drug use [see Ref. (122)].
Although cannabis as a risk factor for psychosis is quite established, there are unresolved issues. Epidemiologically, it is hard to explain that there is no great increase in the prevalence of non-affective psychosis in light of the increasing use of cannabis in several countries, even though a few authors argue that this is indeed the case (194). Furthermore, most patients do start using cannabis before psychosis breakthrough (73, 195–197). But developmental processes related to the disorder itself can theoretically influence the inclination to take drugs, and this is hard to test empirically.
Theoretically, there may be alternative explanations for the better brain and cognitive functioning in cannabis psychosis, e.g., superior social skills among those with cannabis psychosis, making users “skillful” enough to get hold of illegal drugs. Superior social skills are not consistent with the finding of poorer prognosis in this group. To our knowledge, few longitudinal studies have examined this directly and the issue remains unresolved. Two studies reported poorer premorbid functioning in psychosis patients who also used illegal drugs (198), and better premorbid social functioning and poorer premorbid academic functioning in this group (199), respectively.
Understanding the path to psychosis via cannabis also needs to take into account complex psychological and motivational processes. Griffith-Lendering et al. (200) assessed self-reported thought problems, social problems, attention problems, and cannabis use at different time points during adolescence. Cannabis use predicted psychosis vulnerability and vice versa, suggesting a bidirectional relationship between these variables, even though the concept of self-medicating is misleading due to cannabis’ negative influence on symptomatology (201). Using a time-sampling technique, daily life cannabis use was shown to predict a more acute decrease in negative affect but also sub-acute increased levels of hallucinatory experiences in patients than controls, suggesting a vicious circle of deleterious use in these patients (201). In-depth interviews showed that the patients themselves reported change over time in their experience of cannabis, cannabis use preceded mental health for all; cannabis use was fun at first, but the experience changed over time to more addiction-based behavior and more frightening experiences, but also parallel positive experiences for some (202).
Vulnerability models to explain the findings
Taken together, there are now findings from studies on age of onset, cognition, brain functioning, NSS, and family history that together make a pattern. Compared with non-using non-affective psychosis, the present review shows that there seems to be less stable cognitive deficits in patients with cannabis use and psychosis, in addition to less NSS and possibly more normalized brain functioning, indicating less neurobiological vulnerability for psychosis. There is, however, some familiar and genetic vulnerability for psychosis present in the cannabis psychosis group. The familiar and genetic vulnerability may possibly influence the cannabis pathway to psychosis by acting on the neurotransmitter systems that may be particularly sensitive to cannabis, with several recent comprehensive literature reviews supporting this conclusion (45, 51, 55, 56, 58, 179, 184, 186, 203).
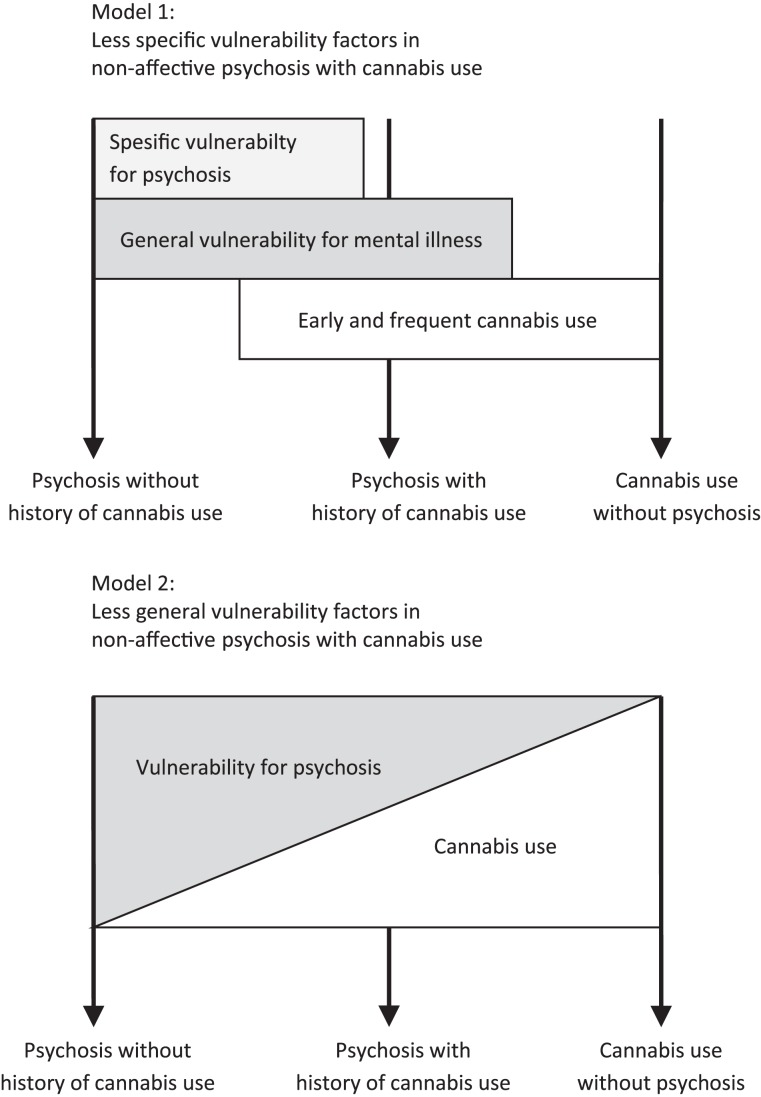
This gene–environment interaction does not, however, integrate the seemingly paradoxical findings of both less vulnerability markers and some genetic vulnerability. Bloomfield et al. (61) also suggested that cannabis increased the risk of psychosis by a different mechanism than typically seen in schizophrenia after examining dopamine synthesis capacity in the striatum. Furthermore, an earlier age of onset suggests a different pathway to psychosis in cannabis-using patients. It can be speculated that there are different levels of vulnerability in cannabis psychosis and no-cannabis psychosis, both groups having more general genetic vulnerability, while the cannabis pathway to psychosis has less neurobiological vulnerability. This can reflect two alternative models; (1) less specific vulnerability factors for psychosis, or (2) less general vulnerability.
The first model (see model 1 in Figure 1) suggests that cannabis-using psychosis patients have an unspecific vulnerability for mental disorder in general, explaining the family loading results. There are, e.g., several findings on the unspecific family loading in mental disorders; almost any psychiatric disorder in first-degree relatives is associated with an increased risk of schizophrenia (59), and there seems to be a partly shared genetic underpinning behind several mental disorders (204). These could be related to specific domains of risk phenotypes suggested by Van Os et al. (59) such as affective dysregulation. Childhood trauma and maltreatment may also be unspecific to psychosis [see in Ref. (205)]. Cognitive dysfunction, sub-optimal brain functioning, and NSS are more specific to non-affective psychosis, and may constitute a more illness-specific vulnerability marker, seen in the more typical non-cannabis-using psychotic patients.
Figure 1.
Two alternative models for differential pathways to non-affective psychosis as a result of cannabis use and vulnerability profile.
Still, a breakdown of information processing is central to the development of psychosis (87). Cannabis use of sufficient magnitude, or in individuals particularly vulnerable to the effects of cannabis, may lead to transient compromised brain functioning, causing a breakdown of reality testing (135, 136). These changes can cause psychosis for some individuals, but will normally not cause the characteristic persistent cognitive impairments seen in psychosis. Thus, cannabis is viewed as an environmental factor imitating the effect of the typical neurobiological vulnerability (54). In line with this, cognitive improvement was shown in those who stopped using cannabis (206).
An alternative model (see model 2 in Figure 1) could be illustrated by using the classical stress–vulnerability framework (207, 208); the tendency to develop psychosis is a function of vulnerability × stress. When cannabis is entered into the equation as a stress factor, the need for a high vulnerability load is decreased. More cannabis x less vulnerability generates a tendency to develop psychosis that is similar to less cannabis × high vulnerability. The less vulnerability load is primarily of neurobiological origin and is shown by fewer lasting cognitive deficits, fewer brain abnormalities, and fewer NSS.
Concluding Remarks
Cannabis is a risk factor for non-affective psychosis, interacting with genetic and environmental vulnerability. Patients with cannabis use have fewer cognitive deficits, probably fewer brain abnormalities, and fewer NSS, but at the same time genetic loading and family history of mental illness comparable to other patients with non-affective psychosis. It is suggested that the pathway to psychosis via cannabis is less influenced by neurobiological vulnerability factors. Better knowledge of vulnerability profiles in those sensitive to cannabis could help us detect individuals for whom cannabis is more precarious. At this point, it can be speculated that young people with family loading of mental illness, and sub-threshold positive symptoms indicating a more general psychosis risk, should be careful with cannabis – the last point is especially relevant for early intervention services. Thus, the presence of such markers may suggest to the clinician that these patients may be particularly sensitive to cannabis. At the same time, the patients that develop psychosis after a period of cannabis use may have less neurobiological vulnerability. They may to a larger extent be able to recover their cognitive abilities if they are abstinent from illicit substances, with positive implications for the rehabilitation process and work capacity. In addition, it can also be argued that a general public warning about the age effect of cannabis use and the effect of high THC content could be put forward, but existing research does not directly test this.
Conflict of Interest Statement
Else-Marie Løberg has received honoria in relation to the development of the Norwegian version of the RBANS by Pearson Assessment. Jan øystein Berle has consulted for Eli Lilly & Co. Norway and received honoraria from BioPhausia AB/Medivir AB, Eli Lilly & Co., H. Lundbeck AS, and Otsuka Pharmaceutical Europe Ltd. Erik Johnsen and Rune A. Kroken declare no conflicts of interests in the last 3 years. All other authors declare that they have no conflicts of interest.
Acknowledgments
The authors thank the patients and clinical staff at the Division of Psychiatry, Haukeland University Hospital for their participation in the study and support of this project. The present research was funded by grants from the Research Council of Norway and Helse-Vest Health Authorities to Else-Marie Løberg, and from the Research Council of Norway, Helse-Vest Health Authorities, and the Research Council of Norway and Helse-Vest Health Authorities to Erik Johnsen.
Biography
 Else-Marie Løberg is an associate professor at the Inst. of Clinical Psychology, University of Bergen, Norway; chief adviser for the Division of Psychiatry, Haukeland University Hospital; and the leader of an early psychosis detection service. She is also a member of the development group for the national psychosis guideline for the Norwegian Directorate of Health. Her main research areas are non-affective psychoses and schizophrenia in relation to neurocognition, basic neuroscience, illicit drug use, pharmacological treatments, inflammation, early intervention and childhood trauma.
Else-Marie Løberg is an associate professor at the Inst. of Clinical Psychology, University of Bergen, Norway; chief adviser for the Division of Psychiatry, Haukeland University Hospital; and the leader of an early psychosis detection service. She is also a member of the development group for the national psychosis guideline for the Norwegian Directorate of Health. Her main research areas are non-affective psychoses and schizophrenia in relation to neurocognition, basic neuroscience, illicit drug use, pharmacological treatments, inflammation, early intervention and childhood trauma.
References
- 1.WHO. The ICD-10 Classification of Mental And Behavioral Disorders. Clinical Descriptions Diagnostic Guidelines. Geneva: World Health Organization; (1992). [Google Scholar]
- 2.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA (1990) 264:2511–8. 10.1001/jama.1990.03450190043026 [DOI] [PubMed] [Google Scholar]
- 3.Kovasznay B, Fleischer J, Tanenberg-Karant M, Jandorf L, Miller AD, Bromet E. Substance use disorder and the early course of illness in schizophrenia and affective psychosis. Schizophr Bull (1997) 23:195–201. 10.1093/schbul/23.2.195 [DOI] [PubMed] [Google Scholar]
- 4.Cantor-Graae E, Nordstrom LG, Mcneil TF. Substance abuse in schizophrenia: a review of the literature and a study of correlates in Sweden. Schizophr Res (2001) 48:69–82. 10.1016/S0920-9964(00)00114-6 [DOI] [PubMed] [Google Scholar]
- 5.Stroup TS, Mcevoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, et al. The National Institute of Mental Health clinical antipsychotic trials of intervention effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull (2003) 29:15–31. 10.1093/oxfordjournals.schbul.a006986 [DOI] [PubMed] [Google Scholar]
- 6.Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr Res (2004) 67:157–66. 10.1016/S0920-9964(02)00523-6 [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Birnbaum H, Demler O, Falloon IR, Gagnon E, Guyer M, et al. The prevalence and correlates of nonaffective psychosis in the national comorbidity survey replication (NCS-R). Biol Psychiatry (2005) 58:668–76. 10.1016/j.biopsych.2005.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiménez-Castro L, Raventós-Vorst H, Escamilla M. Substance use disorder and schizophrenia: prevalence and sociodemographic characteristics in the latin American population. Actas Esp Psiquiatr (2011) 39:123–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Hartz SM, Pato CN, Medeiros H, Cavazos-Rehg P, Sobell JL, Knowles JA, et al. Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry (2014) 71(3):248–54. 10.1001/jamapsychiatry.2013.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry (1994) 51:273–9. 10.1001/archpsyc.1994.03950040017002 [DOI] [PubMed] [Google Scholar]
- 11.Caspari D. Cannabis schizophrenia: results of a follow-up study. Eur Arch Psychiatry Clin Neurosci (1999) 249:45–9. 10.1007/s004060050064 [DOI] [PubMed] [Google Scholar]
- 12.Grech A, Van Os J, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. Eur Psychiatry (2005) 20:349–53. 10.1016/j.eurpsy.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 13.Talamo A, Centorrino F, Tondo L, Dimitri A, Hennen J, Baldessarini RJ. Comorbid substance-use in schizophrenia: relation to positive and negative symptoms. Schizophr Res (2006) 86:251–5. 10.1016/j.schres.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Effects of Cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry (2008) 193:357–63. 10.1192/bjp.bp.107.046375 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt LM, Hesse M, Lykke J. The impact of substance use disorders on the course of schizophrenia – a 15-year follow-up study: dual diagnosis over 15 years. Schizophr Res (2011) 130:228–33. 10.1016/j.schres.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 16.Large M, Mullin K, Gupta P, Harris A, Nielssen O. Systematic meta-analysis of outcomes associated with psychosis and co-morbid substance use. Aust N Z J Psychiatry (2014) 48:418. Available from: http://anp.sagepub.com/content/48/5/418 10.1177/0004867414525838 [DOI] [PubMed] [Google Scholar]
- 17.Tarricone I, Boydell J, Panigada S, Allegri F, Marcacci T, Minenna MG, et al. The impact of substance use at psychosis onset on First Episode Psychosis course: results from a 1 year follow-up study in Bologna. Schizophr Res (2014) 153(1–3):60–3. 10.1016/j.schres.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 18.Boydell J, Dean K, Dutta R, Giouroukou E, Fearon P, Murray R. A comparison of symptoms and family history in schizophrenia with and without prior Cannabis use: implications for the concept of Cannabis psychosis. Schizophr Res (2007) 93:203–10. 10.1016/j.schres.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 19.Løberg EM, Jørgensen HA, Hugdahl K. The effects of previous drug abuse on neurocognition in schizophrenia. J Int Neuropsychol Soc (2003) 9:172. [Google Scholar]
- 20.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between Cannabis and psychosis: examination of the evidence. Br J Psychiatry (2004) 184:110–7. 10.1192/bjp.184.2.110 [DOI] [PubMed] [Google Scholar]
- 21.Barnes TR, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM. Comorbid substance use and age at onset of schizophrenia. Br J Psychiatry (2006) 188:237–42. 10.1192/bjp.bp.104.007237 [DOI] [PubMed] [Google Scholar]
- 22.Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of Cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull (2010) 36:1115–30. 10.1093/schbul/sbp031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry (2005) 57:594–608. 10.1016/j.biopsych.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 24.Atakan Z, Bhattacharyya S, Allen P, Martin-Santos R, Crippa JA, Borgwardt SJ, et al. Cannabis affects people differently: inter-subject variation in the psychotogenic effects of Delta9-tetrahydrocannabinol: a functional magnetic resonance imaging study with healthy volunteers. Psychol Med (2013) 43:1255–67. 10.1017/S0033291712001924 [DOI] [PubMed] [Google Scholar]
- 25.Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet (1987) 2:1483–6. 10.1016/S0140-6736(87)92620-1 [DOI] [PubMed] [Google Scholar]
- 26.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported Cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ (2002) 325:1199. 10.1136/bmj.325.7374.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caspi A, Moffitt TE, Cannon M, Mcclay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset Cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry (2005) 57:1117–27. 10.1016/j.biopsych.2005.01.026 [DOI] [PubMed] [Google Scholar]
- 28.Konings M, Henquet C, Maharajh HD, Hutchinson G, Van Os J. Early exposure to Cannabis and risk for psychosis in young adolescents in Trinidad. Acta Psychiatr Scand (2008) 118:209–13. 10.1111/j.1600-0447.2008.01202.x [DOI] [PubMed] [Google Scholar]
- 29.Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency Cannabis, drives the earlier onset of psychosis in Cannabis users. Schizophr Bull (2014) 40(6):1509–17. 10.1093/schbul/sbt181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szoke A, Galliot AM, Richard JR, Ferchiou A, Baudin G, Leboyer M, et al. Association between Cannabis use and schizotypal dimensions - a meta-analysis of cross-sectional studies. Psychiatry Res (2014) 219:58–66. 10.1016/j.psychres.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 31.Tien AY, Anthony JC. Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. J Nerv Ment Dis (1990) 178:473–80. 10.1097/00005053-199017880-00001 [DOI] [PubMed] [Google Scholar]
- 32.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ (2002) 325:1212–3. 10.1136/bmj.325.7374.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Os J, Bak M, Hanssen M, Bijl RV, De Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol (2002) 156:319–27. 10.1093/aje/kwf043 [DOI] [PubMed] [Google Scholar]
- 34.Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med (2003) 33:15–21. 10.1017/S0033291702006402 [DOI] [PubMed] [Google Scholar]
- 35.Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent Cannabis exposure and positive and negative dimensions of psychosis. Addiction (2004) 99:1333–41. 10.1111/j.1360-0443.2004.00806.x [DOI] [PubMed] [Google Scholar]
- 36.Ferdinand RF, Sondeijker F, Van Der Ende J, Selten JP, Huizink A, Verhulst FC. Cannabis use predicts future psychotic symptoms, and vice versa. Addiction (2005) 100:612–8. 10.1111/j.1360-0443.2005.01070.x [DOI] [PubMed] [Google Scholar]
- 37.Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, et al. Prospective cohort study of Cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ (2005) 330:11. 10.1136/bmj.38267.664086.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry (2001) 50:71–83. 10.1016/S0006-3223(01)01134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degenhardt L, Tennant C, Gilmour S, Schofield D, Nash L, Hall W, et al. The temporal dynamics of relationships between Cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol Med (2007) 37:927–34. 10.1017/S0033291707009956 [DOI] [PubMed] [Google Scholar]
- 40.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet (2007) 370:319–28. 10.1016/S0140-6736(07)61162-3 [DOI] [PubMed] [Google Scholar]
- 41.Kedzior KK, Laeber LT. A positive association between anxiety disorders and Cannabis use or Cannabis use disorders in the general population – a meta-analysis of 31 studies. BMC Psychiatry (2014) 14:136. 10.1186/1471-244X-14-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macleod J, Oakes R, Copello A, Crome I, Egger M, Hickman M, et al. Psychological and social sequelae of Cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet (2004) 363:1579–88. 10.1016/S0140-6736(04)16200-4 [DOI] [PubMed] [Google Scholar]
- 43.Henquet C, Murray R, Linszen D, Van Os J. The environment and schizophrenia: the role of Cannabis use. Schizophr Bull (2005) 31:608–12. 10.1093/schbul/sbi027 [DOI] [PubMed] [Google Scholar]
- 44.Semple DM, Mcintosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol (2005) 19:187–94. 10.1177/0269881105049040 [DOI] [PubMed] [Google Scholar]
- 45.Van Winkel R, Kuepper R. Epidemiological, neurobiological, and genetic clues to the mechanisms linking Cannabis use to risk for nonaffective psychosis. Annu Rev Clin Psychol (2014) 10:767–91. 10.1146/annurev-clinpsy-032813-153631 [DOI] [PubMed] [Google Scholar]
- 46.Hall W. Cannabis use psychosis. Drug Alcohol Rev (1998) 17:433–44. 10.1080/09595239800187271 [DOI] [PubMed] [Google Scholar]
- 47.Degenhardt L, Hall W, Lynskey M. Testing hypotheses about the relationship between Cannabis use and psychosis. Drug Alcohol Depend (2003) 71:37–48. 10.1016/S0376-8716(03)00064-4 [DOI] [PubMed] [Google Scholar]
- 48.Martin AK, Robinson G, Reutens D, Mowry B. Cannabis abuse and age at onset in schizophrenia patients with large, rare copy number variants. Schizophr Res (2014) 155(1–3):21–5. 10.1016/j.schres.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 49.Valmaggia LR, Day FL, Jones C, Bissoli S, Pugh C, Hall D, et al. Cannabis use and transition to psychosis in people at ultra-high risk. Psychol Med (2014) 44:2503–12. 10.1017/S0033291714000117 [DOI] [PubMed] [Google Scholar]
- 50.Stefanis NC, Dragovic M, Power BD, Jablensky A, Castle D, Morgan VA. Age at initiation of Cannabis use predicts age at onset of psychosis: the 7- to 8-year trend. Schizophr Bull (2013) 39:251–4. 10.1093/schbul/sbs188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull (2008) 34:1066–82. 10.1093/schbul/sbn117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Winkel R, Genetic Risk and Outcome of Psychosis (GROUP) Investigators . Family-based analysis of genetic variation underlying psychosis-inducing effects of Cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry (2011) 68:148–57. 10.1001/archgenpsychiatry.2010.152 [DOI] [PubMed] [Google Scholar]
- 53.Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in Cannabis users. Biol Psychiatry (2012) 72:811–6. 10.1016/j.biopsych.2012.06.020 [DOI] [PubMed] [Google Scholar]
- 54.Solowij N, Michie PT. Cannabis and cognitive dysfunction: parallels with endophenotypes of schizophrenia? J Psychiatry Neurosci (2007) 32:30–52. [PMC free article] [PubMed] [Google Scholar]
- 55.Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of Cannabis-induced schizophrenia. Prog Neurobiol (2010) 92:370–85. 10.1016/j.pneurobio.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 56.Kuepper R, Morrison PD, Van Os J, Murray RM, Kenis G, Henquet C. Does dopamine mediate the psychosis-inducing effects of Cannabis? A review and integration of findings across disciplines. Schizophr Res (2010) 121:107–17. 10.1016/j.schres.2010.05.031 [DOI] [PubMed] [Google Scholar]
- 57.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology (2003) 28:1760–9. 10.1038/sj.npp.1300225 [DOI] [PubMed] [Google Scholar]
- 58.Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev (2011) 35:1779–87. 10.1016/j.neubiorev.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 59.Van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature (2010) 468:203–12. 10.1038/nature09563 [DOI] [PubMed] [Google Scholar]
- 60.Safont G, Corripio I, Escarti MJ, Portella MJ, Perez V, Ferrer M, et al. Cannabis use and striatal D2 receptor density in untreated first-episode psychosis: an in vivo SPECT study. Schizophr Res (2011) 129:169–71. 10.1016/j.schres.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 61.Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in Cannabis users and its relationship to Cannabis-induced psychotic symptoms. Biol Psychiatry (2014) 75:470–8. 10.1016/j.biopsych.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 62.El-Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D, et al. Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav (2010) 95:434–42. 10.1016/j.pbb.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schubart CD, Sommer IE, Van Gastel WA, Goetgebuer RL, Kahn RS, Boks MP. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res (2011) 130:216–21. 10.1016/j.schres.2011.04.017 [DOI] [PubMed] [Google Scholar]
- 64.Miyamoto S, Jarskog LF, Fleischhacker WW. Alternative pharmacologic targets for the treatment of schizophrenia: results from phase I and II trials. Curr Opin Psychiatry (2013) 26:158–65. 10.1097/YCO.0b013e32835d8296 [DOI] [PubMed] [Google Scholar]
- 65.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry (2012) 2:e94. 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Løberg EM, Nygard M, Berle JO, Johnsen E, Kroken RA, Jørgensen HA, et al. An fMRI study of neuronal activation in schizophrenia patients with and without previous Cannabis use. Front Psychiatry (2012) 3:94. 10.3389/fpsyt.2012.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruz-Cordova A, Rocha-Ramirez LM, Ochoa SA, Gonzalez-Pedrajo B, Espinosa N, Eslava C, et al. Flagella from five Cronobacter species induce pro-inflammatory cytokines in macrophage derivatives from human monocytes. PLoS One (2012) 7:e52091. 10.1371/journal.pone.0052091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Myles N, Newall H, Nielssen O, Large M. The association between Cannabis use and earlier age at onset of schizophrenia and other psychoses: meta-analysis of possible confounding factors. Curr Pharm Des (2012) 18:5055–69. 10.2174/138161212802884816 [DOI] [PubMed] [Google Scholar]
- 69.Power BD, Dragovic M, Jablensky A, Stefanis NC. Does accumulating exposure to illicit drugs bring forward the age at onset in schizophrenia? Aust N Z J Psychiatry (2013) 47:51–8. 10.1177/0004867412461957 [DOI] [PubMed] [Google Scholar]
- 70.Tosato S, Lasalvia A, Bonetto C, Mazzoncini R, Cristofalo D, De Santi K, et al. The impact of Cannabis use on age of onset and clinical characteristics in first-episode psychotic patients. Data from the psychosis incident cohort outcome study (PICOS). J Psychiatr Res (2013) 47:438–44. 10.1016/j.jpsychires.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 71.Donoghue K, Doody GA, Murray RM, Jones PB, Morgan C, Dazzan P, et al. Cannabis use, gender and age of onset of schizophrenia: data from the AESOP study. Psychiatry Res (2014) 215:528–32. 10.1016/j.psychres.2013.12.038 [DOI] [PubMed] [Google Scholar]
- 72.Kovasznay B, Bromet E, Schwartz JE, Ram R, Lavelle J, Brandon L. Substance abuse and onset of psychotic illness. Hosp Community Psychiatry (1993) 44:567–71. [DOI] [PubMed] [Google Scholar]
- 73.Sevy S, Robinson DG, Holloway S, Alvir JM, Woerner MG, Bilder R, et al. Correlates of substance misuse in patients with first-episode schizophrenia and schizoaffective disorder. Acta Psychiatr Scand (2001) 104:367–74. 10.1034/j.1600-0447.2001.00452.x [DOI] [PubMed] [Google Scholar]
- 74.Bersani G, Orlandi V, Gherardelli S, Pancheri P. Cannabis and neurological soft signs in schizophrenia: absence of relationship and influence on psychopathology. Psychopathology (2002) 35:289–95. 10.1159/000067064 [DOI] [PubMed] [Google Scholar]
- 75.Breitborde NJ, Srihari VH, Woods SW. Review of the operational definition for first-episode psychosis. Early Interv Psychiatry (2009) 3:259–65. 10.1111/j.1751-7893.2009.00148.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry (2011) 68:555–61. 10.1001/archgenpsychiatry.2011.5 [DOI] [PubMed] [Google Scholar]
- 77.Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM. The effect of Cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophr Bull (2012) 38:873–80. 10.1093/schbul/sbq153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mcgrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakhsh MR, et al. Association between Cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry (2010) 67:440–7. 10.1001/archgenpsychiatry.2010.6 [DOI] [PubMed] [Google Scholar]
- 79.Schubart CD, Boks MP, Breetvelt EJ, Van Gastel WA, Groenwold RH, Ophoff RA, et al. Association between Cannabis and psychiatric hospitalization. Acta Psychiatr Scand (2011) 123:368–75. 10.1111/j.1600-0447.2010.01640.x [DOI] [PubMed] [Google Scholar]
- 80.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of Cannabis exposure. Addict Biol (2008) 13:253–63. 10.1111/j.1369-1600.2008.00110.x [DOI] [PubMed] [Google Scholar]
- 81.Galve-Roperh I, Palazuelos J, Aguado T, Guzman M. The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci (2009) 259:371–82. 10.1007/s00406-009-0028-y [DOI] [PubMed] [Google Scholar]
- 82.Galvez-Buccollini JA, Proal AC, Tomaselli V, Trachtenberg M, Coconcea C, Chun J, et al. Association between age at onset of psychosis and age at onset of Cannabis use in non-affective psychosis. Schizophr Res (2012) 139:157–60. 10.1016/j.schres.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helle S, Ringen PA, Melle I, Larsen TK, Gjestad R, Johnsen E, et al. Cannabis use is associated with 3 years earlier onset of non-affective psychosis in a large, naturalistic, multi-site sample. Schizophr Bull (Forthcoming). [DOI] [PubMed] [Google Scholar]
- 84.Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull (2007) 33:912–20. 10.1093/schbul/sbm046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev (2009) 19:365–84. 10.1007/s11065-009-9109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med (2011) 41:225–41. 10.1017/S0033291710001042 [DOI] [PubMed] [Google Scholar]
- 87.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA psychiatry (2013) 70:1107–12. 10.1001/jamapsychiatry.2013.155 [DOI] [PubMed] [Google Scholar]
- 88.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry (2008) 165:579–87. 10.1176/appi.ajp.2008.07081242 [DOI] [PubMed] [Google Scholar]
- 89.Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry (2005) 162:71–8. 10.1176/appi.ajp.162.1.71 [DOI] [PubMed] [Google Scholar]
- 90.Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, Mcfarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res (2010) 123:188–98. 10.1016/j.schres.2010.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caspi A, Reichenberg A, Weiser M, Rabinowitz J, Kaplan Z, Knobler H, et al. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr Res (2003) 65:87–94. 10.1016/S0920-9964(03)00056-2 [DOI] [PubMed] [Google Scholar]
- 92.Gschwandtner U, Aston J, Borgwardt S, Drewe M, Feinendegen C, Lacher D, et al. Neuropsychological and neurophysiological findings in individuals suspected to be at risk for schizophrenia: preliminary results from the basel early detection of psychosis study - Fruherkennung von Psychosen (FEPSY). Acta Psychiatr Scand (2003) 108:152–5. 10.1034/j.1600-0447.2003.00157.x [DOI] [PubMed] [Google Scholar]
- 93.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry (1996) 153:321–30. [DOI] [PubMed] [Google Scholar]
- 94.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res (2004) 72:29–39. 10.1016/j.schres.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 95.Gold MJ, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry (1999) 156:1944–50. [DOI] [PubMed] [Google Scholar]
- 96.Wilk CM, Gold JM, Bartko JJ, Dickerson F, Fenton WS, Knable M, et al. Test-retest stability of the repeatable battery for the assessment of neuropsychological status in schizophrenia. Am J Psychiatry (2002) 159:838–44. 10.1176/appi.ajp.159.5.838 [DOI] [PubMed] [Google Scholar]
- 97.Wilk CM, Gold JM, Humber K, Dickerson F, Fenton WS, Buchanan RW. Brief cognitive assessment in schizophrenia: normative data for the repeatable battery for the assessment of neuropsychological status. Schizophr Res (2004) 70:175–86. 10.1016/j.schres.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 98.Keefe RS. Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry (2008) 7:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull (2000) 26:119–36. 10.1093/oxfordjournals.schbul.a033430 [DOI] [PubMed] [Google Scholar]
- 100.Laes JR, Sponheim SR. Does cognition predict community function only in schizophrenia? A study of schizophrenia patients, bipolar affective disorder patients, and community control subjects. Schizophr Res (2006) 84:121–31. 10.1016/j.schres.2005.11.023 [DOI] [PubMed] [Google Scholar]
- 101.Kaneda Y, Jayathilak K, Meltzer HY. Determinants of work outcome in schizophrenia and schizoaffective disorder: role of cognitive function. Psychiatry Res (2009) 169:178–9. 10.1016/j.psychres.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 102.Tsang HWH, Leung AY, Chung RCK, Bell M, Cheung WM. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry (2010) 44:495–504. 10.3109/00048671003785716 [DOI] [PubMed] [Google Scholar]
- 103.Brust JC. Cognition Cannabis: from anecdote to advanced technology. Brain (2012) 135:2004–5. 10.1093/brain/aws165 [DOI] [PubMed] [Google Scholar]
- 104.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of Cannabis use: a meta-analytic study. J Int Neuropsychol Soc (2003) 9:679–89. 10.1017/S1355617703950016 [DOI] [PubMed] [Google Scholar]
- 105.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev (2008) 1:99–111. 10.2174/1874473710801010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of Cannabis use. Psychopharmacology (Berl) (1999) 142:295–301. 10.1007/s002130050892 [DOI] [PubMed] [Google Scholar]
- 107.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset Cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend (2003) 69:303–10. 10.1016/S0376-8716(02)00334-4 [DOI] [PubMed] [Google Scholar]
- 108.Løberg EM, Hugdahl K, Jørgensen HA. Lower neurocognitive vulnerability in schizophrenia with a history of Cannabis abuse? Abstract. Schizophr Res (2008) 98(Suppl S):73. 10.1016/j.schres.2007.12.164 [DOI] [Google Scholar]
- 109.Schnell T, Koethe D, Daumann J, Gouzoulis-Mayfrank E. The role of Cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology (Berl) (2009) 205(1):45–52. 10.1007/s00213-009-1512-9 [DOI] [PubMed] [Google Scholar]
- 110.Løberg EM, Hugdahl K. Cannabis use and cognition in schizophrenia. Front Hum Neurosci (2009) 3:53. 10.3389/neuro.09.053.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carey KB, Carey MP, Simons JS. Correlates of substance use disorder among psychiatric outpatients: focus on cognition, social role functioning, and psychiatric status. J Nerv Ment Dis (2003) 191:300–8. 10.1097/00005053-200304000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joyal CC, Halle P, Lapierre D, Hodgins S. Drug abuse and/or dependence and better neuropsychological performance in patients with schizophrenia. Schizophr Res (2003) 63:297–9. 10.1016/S0920-9964(02)00387-0 [DOI] [PubMed] [Google Scholar]
- 113.Herman M. Neurocognitive functioning quality of life among dually diagnosed non-substance abusing schizophrenia inpatients. Int J Ment Health Nurs (2004) 13:282–91. 10.1111/j.1440-0979.2004.00346.x [DOI] [PubMed] [Google Scholar]
- 114.Kumra S, Thaden E, Dethomas C, Kranzler H. Correlates of substance abuse in adolescents with treatment-refractory schizophrenia and schizoaffective disorder. Schizophr Res (2005) 73:369–71. 10.1016/j.schres.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 115.Potvin S, Briand C, Prouteau A, Bouchard RH, Lipp O, Lalonde P, et al. CANTAB explicit memory is less impaired in addicted schizophrenia patients. Brain Cogn (2005) 59:38–42. 10.1016/j.bandc.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 116.Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res (2005) 75:135–7. 10.1016/j.schres.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 117.Mccleery A, Addington J, Addington D. Substance misuse and cognitive functioning in early psychosis: a 2 year follow-up. Schizophr Res (2006) 88:187–91. 10.1016/j.schres.2006.06.040 [DOI] [PubMed] [Google Scholar]
- 118.Coulston CM, Perdices M, Tennant CC. The neuropsychological correlates of Cannabis use in schizophrenia: lifetime abuse/dependence, frequency of use, and recency of use. Schizophr Res (2007) 96:169–84. 10.1016/j.schres.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 119.Jockers-Scherubl MC, Wolf T, Radzei N, Schlattmann P, Rentzsch J, De Castro A, et al. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31:1054–63. 10.1016/j.pnpbp.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 120.Thoma P, Wiebel B, Daum I. Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophr Res (2007) 92:168–80. 10.1016/j.schres.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 121.Cleghorn JM, Kaplan RD, Szechtman B, Szechtman H, Brown GM, Franco S. Substance abuse and schizophrenia: effect on symptoms but not on neurocognitive function. J Clin Psychiatry (1991) 52:26–30. [PubMed] [Google Scholar]
- 122.Addington J, Addington D. Substance abuse and cognitive functioning in schizophrenia. J Psychiatry Neurosci (1997) 22:99–104. [PMC free article] [PubMed] [Google Scholar]
- 123.Liraud F, Verdoux H. [Effect of comorbid substance use on neuropsychological performance in subjects with psychotic or mood disorders]. Encephale (2002) 28:160–8. [PubMed] [Google Scholar]
- 124.Pencer A, Addington J. Substance use and cognition in early psychosis. J Psychiatry Neurosci (2003) 28:48–54. [PMC free article] [PubMed] [Google Scholar]
- 125.Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring Cannabis use disorders. Schizophr Res (2007) 92:74–84. 10.1016/j.schres.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wobrock T, Sittinger H, Behrendt B, D’amelio R, Falkai P, Caspari D. Comorbid substance abuse and neurocognitive function in recent-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci (2007) 257:203–10. 10.1007/s00406-006-0707-x [DOI] [PubMed] [Google Scholar]
- 127.Thoma P, Daum I. Working memory and multi-tasking in paranoid schizophrenia with and without comorbid substance use disorder. Addiction (2008) 103:774–86. 10.1111/j.1360-0443.2008.02156.x [DOI] [PubMed] [Google Scholar]
- 128.Mata I, Rodriguez-Sanchez JM, Pelayo-Teran JM, Perez-Iglesias R, Gonzalez-Blanch C, Ramirez-Bonilla M, et al. Cannabis abuse is associated with decision-making impairment among first-episode patients with schizophrenia-spectrum psychosis. Psychol Med (2008) 38:1257–66. 10.1017/S0033291707002218 [DOI] [PubMed] [Google Scholar]
- 129.Potvin S, Joyal CC, Pelletier J, Stip E. Contradictory cognitive capacities among substance-abusing patients with schizophrenia: a meta-analysis. Schizophr Res (2008) 100:242–51. 10.1016/j.schres.2007.04.022 [DOI] [PubMed] [Google Scholar]
- 130.Derosse P, Kaplan A, Burdick KE, Lencz T, Malhotra AK. Cannabis use disorders in schizophrenia: effects on cognition and symptoms. Schizophr Res (2010) 120:95–100. 10.1016/j.schres.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rodriguez-Sanchez JM, Ayesa-Arriola R, Mata I, Moreno-Calle T, Perez-Iglesias R, Gonzalez-Blanch C, et al. Cannabis use and cognitive functioning in first-episode schizophrenia patients. Schizophr Res (2010) 124:142–51. 10.1016/j.schres.2010.08.017 [DOI] [PubMed] [Google Scholar]
- 132.Yücel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, et al. The impact of Cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull (2010). 10.1093/schbul/sbq079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rabin RA, Zakzanis KK, George TP. The effects of Cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res (2011) 128:111–6. 10.1016/j.schres.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 134.Cunha PJ, Rosa PG, Ayres Ade M, Duran FL, Santos LC, Scazufca M, et al. Cannabis use, cognition and brain structure in first-episode psychosis. Schizophr Res (2013) 147:209–15. 10.1016/j.schres.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 135.Helle S, Gjestad R, Johnsen E, Kroken RA, Jørgensen HA, Løberg E-M. Cognitive changes in non-affective psychosis the first 4-6 weeks after admission to a psychiatric acute ward: effects of substance use. Schizophr Bull (2013) 39:262. [Google Scholar]
- 136.Helle S, Gjestad R, Johnsen E, Kroken RA, Jørgensen HA, Løberg EM. Cognitive changes in patients with acute phase psychosis-effects of illicit drug use. Psychiatry Res (2014). 10.1016/j.psychres.2014.08.062 [DOI] [PubMed] [Google Scholar]
- 137.Randolph C. RBANS Repeatable Battery for the Assessment of Neuropsychological Status. Manual. San Antonio, TX: The Psychological Corporation; (1998). [Google Scholar]
- 138.Beglinger LJ, Gaydos B, Tangphao-Daniels O, Duff K, Kareken DA, Crawford J, et al. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol (2005) 20:517–29. 10.1016/j.acn.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 139.Puri BK. Progressive structural brain changes in schizophrenia. Expert Rev Neurother (2010) 10:33–42. 10.1586/ern.09.142 [DOI] [PubMed] [Google Scholar]
- 140.Williams LM. Voxel-based morphometry in schizophrenia: implications for neurodevelopmental connectivity models, cognition and affect. Expert Rev Neurother (2008) 8:1049–65. 10.1586/14737175.8.7.1049 [DOI] [PubMed] [Google Scholar]
- 141.Pettersson-Yeo W, Allen P, Benetti S, Mcguire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev (2011) 35:1110–24. 10.1016/j.neubiorev.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 142.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage (2012) 62:2296–314. 10.1016/j.neuroimage.2011.12.090 [DOI] [PubMed] [Google Scholar]
- 143.Ruiz S, Birbaumer N, Sitaram R. Abnormal neural connectivity in schizophrenia and fMRI-brain-computer interface as a potential therapeutic approach. Front Psychiatry (2013) 4:17. 10.3389/fpsyt.2013.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hugdahl K, Rund BR, Lund A, Asbjørnsen A, Egeland J, Ersland L, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry (2004) 161:286–93. 10.1176/appi.ajp.161.2.286 [DOI] [PubMed] [Google Scholar]
- 145.Karlsgodt KH, Glahn DC, Van Erp TG, Therman S, Huttunen M, Manninen M, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res (2007) 89:191–7. 10.1016/j.schres.2006.08.016 [DOI] [PubMed] [Google Scholar]
- 146.Koch K, Wagner G, Nenadic I, Schachtzabel C, Schultz C, Roebel M, et al. Fronto-striatal hypoactivation during correct information retrieval in patients with schizophrenia: an fMRI study. Neuroscience (2008) 153:54–62. 10.1016/j.neuroscience.2008.01.063 [DOI] [PubMed] [Google Scholar]
- 147.Nygård M, Eichele T, Løberg EM, Jørgensen HA, Johnsen E, Kroken RA, et al. Patients with schizophrenia fail to up-regulate task-positive and down-regulate task-negative brain networks: an fmri study using an ica analysis approach. Front Hum Neurosci (2012) 6:149. 10.3389/fnhum.2012.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med (2008) 38:1185–93. 10.1017/S0033291708003565 [DOI] [PubMed] [Google Scholar]
- 149.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, Mccarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A (2009) 106:1279–84. 10.1073/pnas.0809141106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp (2010) 31:424–37. 10.1002/hbm.20876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent Cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) (2006) 185:358–68. 10.1007/s00213-005-0298-7 [DOI] [PubMed] [Google Scholar]
- 152.Delisi LE. The effect of Cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? Curr Opin Psychiatry (2008) 21:140–50. 10.1097/YCO.0b013e3282f51266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, et al. Neuroimaging in Cannabis use: a systematic review of the literature. Psychol Med (2010) 40:383–98. 10.1017/S0033291709990729 [DOI] [PubMed] [Google Scholar]
- 154.Rapp C, Bugra H, Riecher-Rossler A, Tamagni C, Borgwardt S. Effects of Cannabis use on human brain structure in psychosis: a systematic review combining in vivo structural neuroimaging and post mortem studies. Curr Pharm Des (2012) 18:5070–80. 10.2174/138161212802884861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, et al. Gyrification brain abnormalities associated with adolescence and early-adulthood Cannabis use. Brain Res (2010) 1317:297–304. 10.1016/j.brainres.2009.12.069 [DOI] [PubMed] [Google Scholar]
- 156.Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage (2008) 41:1067–74. 10.1016/j.neuroimage.2008.02.064 [DOI] [PubMed] [Google Scholar]
- 157.Bossong MG, Jager G, Bhattacharyya S, Allen P. Acute and non-acute effects of Cannabis on human memory function: a critical review of neuroimaging studies. Curr Pharm Des (2014) 20:2114–25. 10.2174/13816128113199990436 [DOI] [PubMed] [Google Scholar]
- 158.Dekker N, Schmitz N, Peters BD, Van Amelsvoort TA, Linszen DH, De Haan L. Cannabis use and callosal white matter structure and integrity in recent-onset schizophrenia. Psychiatry Res (2010) 181:51–6. 10.1016/j.pscychresns.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 159.Szeszko PR, Robinson DG, Sevy S, Kumra S, Rupp CI, Betensky JD, et al. Anterior cingulate grey-matter deficits and Cannabis use in first-episode schizophrenia. Br J Psychiatry (2007) 190:230–6. 10.1192/bjp.bp.106.024521 [DOI] [PubMed] [Google Scholar]
- 160.Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia – a region of interest, voxel based morphometric study. Schizophr Res (2008) 99:1–6. 10.1016/j.schres.2007.11.029 [DOI] [PubMed] [Google Scholar]
- 161.Rais M, Cahn W, Van Haren N, Schnack H, Caspers E, Hulshoff Pol H, et al. Excessive brain volume loss over time in Cannabis-using first-episode schizophrenia patients. Am J Psychiatry (2008) 165:490–6. 10.1176/appi.ajp.2007.07071110 [DOI] [PubMed] [Google Scholar]
- 162.Ho BC, Wassink TH, Ziebell S, Andreasen NC. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr Res (2011) 128:66–75. 10.1016/j.schres.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.James A, Hough M, James S, Winmill L, Burge L, Nijhawan S, et al. Greater white and grey matter changes associated with early Cannabis use in adolescent-onset schizophrenia (AOS). Schizophr Res (2011) 128:91–7. 10.1016/j.schres.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 164.Solowij N, Yucel M, Respondek C, Whittle S, Lindsay E, Pantelis C, et al. Cerebellar white-matter changes in Cannabis users with and without schizophrenia. Psychol Med (2011) 41:2349–59. 10.1017/S003329171100050X [DOI] [PubMed] [Google Scholar]
- 165.Cahn W, Hulshoff Pol HE, Caspers E, Van Haren NE, Schnack HG, Kahn RS. Cannabis and brain morphology in recent-onset schizophrenia. Schizophr Res (2004) 67:305–7. 10.1016/S0920-9964(03)00003-3 [DOI] [PubMed] [Google Scholar]
- 166.Wobrock T, Sittinger H, Behrendt B, D’amelio R, Falkai P. Comorbid substance abuse and brain morphology in recent-onset psychosis. Eur Arch Psychiatry Clin Neurosci (2009) 259:28–36. 10.1007/s00406-008-0831-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cohen M, Rasser PE, Peck G, Carr VJ, Ward PB, Thompson PM, et al. Cerebellar grey-matter deficits, Cannabis use and first-episode schizophrenia in adolescents and young adults. Int J Neuropsychopharmacol (2012) 15(3):297–307. 10.1017/S146114571100068X [DOI] [PubMed] [Google Scholar]
- 168.Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with long-term heavy Cannabis use. Arch Gen Psychiatry (2008) 65:694–701. 10.1001/archpsyc.65.6.694 [DOI] [PubMed] [Google Scholar]
- 169.Smith MJ, Cobia DJ, Wang L, Alpert KI, Cronenwett WJ, Goldman MB, et al. Cannabis-related working memory deficits and associated subcortical morphological differences in healthy individuals and schizophrenia subjects. Schizophr Bull (2014) 40:287–99. 10.1093/schbul/sbt176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mancini-Marie A, Potvin S, Fahim C, Beauregard M, Mensour B, Stip E. Neural correlates of the affect regulation model in schizophrenia patients with substance use history: a functional magnetic resonance imaging study. J Clin Psychiatry (2006) 67:342–50. 10.4088/JCP.v67n0302 [DOI] [PubMed] [Google Scholar]
- 171.Potvin S, Mancini-Marie A, Fahim C, Mensour B, Stip E. Processing of social emotion in patients with schizophrenia and substance use disorder: an fMRI study. Soc Neurosci (2007) 2:106–16. 10.1080/17470910701376787 [DOI] [PubMed] [Google Scholar]
- 172.Hugdahl K, Løberg EM, Nygård M. Left temporal lobe structural and functional abnormality underlying auditory hallucinations in schizophrenia. Front Neurosci (2009) 3:34–45. 10.3389/neuro.01.001.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A (2001) 98:676–82. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Løberg EM, Hugdahl K, Green MF. Hemispheric asymmetry in schizophrenia: a “dual deficits” model. Biol Psychiatry (1999) 45:76–81. 10.1016/S0006-3223(98)00219-4 [DOI] [PubMed] [Google Scholar]
- 175.Bourque J, Mendrek A, Durand M, Lakis N, Lipp O, Stip E, et al. Cannabis abuse is associated with better emotional memory in schizophrenia: a functional magnetic resonance imaging study. Psychiatry Res (2013) 214:24–32. 10.1016/j.pscychresns.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 176.Potvin S, Bourque J, Durand M, Lipp O, Lalonde P, Stip E, et al. The neural correlates of mental rotation abilities in Cannabis-abusing patients with schizophrenia: an FMRI study. Schizophr Res Treatment (2013) 2013:543842. 10.1155/2013/543842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dazzan P, Murray RM. Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry Suppl (2002) 43:s50–7 10.1192/bjp.181.43.s50 [DOI] [PubMed] [Google Scholar]
- 178.Ruiz-Veguilla M, Gurpegui M, Barrigon ML, Ferrin M, Marin E, Rubio JL, et al. Fewer neurological soft signs among first episode psychosis patients with heavy Cannabis use. Schizophr Res (2009) 107:158–64. 10.1016/j.schres.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 179.Uher R. Gene-environment interactions in severe mental illness. Front Psychiatry (2014) 5:48. 10.3389/fpsyt.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Proal AC, Fleming J, Galvez-Buccollini JA, Delisi LE. A controlled family study of Cannabis users with and without psychosis. Schizophr Res (2014) 152:283–8. 10.1016/j.schres.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zammit S, Spurlock G, Williams H, Norton N, Williams N, O’donovan MC, et al. Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and Cannabis use. Br J Psychiatry (2007) 191:402–7. 10.1192/bjp.bp.107.036129 [DOI] [PubMed] [Google Scholar]
- 182.Henquet C, Rosa A, Krabbendam L, Papiol S, Fananas L, Drukker M, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology (2006) 31:2748–57. 10.1038/sj.npp.1301197 [DOI] [PubMed] [Google Scholar]
- 183.Henquet C, Rosa A, Delespaul P, Papiol S, Fananas L, Van Os J, et al. COMT ValMet moderation of Cannabis-induced psychosis: a momentary assessment study of ‘switching on’ hallucinations in the flow of daily life. Acta Psychiatr Scand (2009) 119:156–60. 10.1111/j.1600-0447.2008.01265.x [DOI] [PubMed] [Google Scholar]
- 184.Parakh P, Basu D. Cannabis and psychosis: have we found the missing links? Asian J Psychiatr (2013) 6:281–7. 10.1016/j.ajp.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 185.Verdoux H. Cannabis and psychosis proneness. In: Castle D, Murray R. editors. Marijuana and Madness. Cambridge: University Press; (2004). p. 75–88. [Google Scholar]
- 186.Radhakrishnan R, Wilkinson ST, D’souza DC. Gone to pot - a review of the association between Cannabis and psychosis. Front Psychiatry (2014) 5:54. 10.3389/fpsyt.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vinkers CH, Van Gastel WA, Schubart CD, Van Eijk KR, Luykx JJ, Van Winkel R, et al. The effect of childhood maltreatment and Cannabis use on adult psychotic symptoms is modified by the COMT Val(1)(5)(8)Met polymorphism. Schizophr Res (2013) 150:303–11. 10.1016/j.schres.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 188.Alemany S, Arias B, Fatjo-Vilas M, Villa H, Moya J, Ibanez MI, et al. Psychosis-inducing effects of Cannabis are related to both childhood abuse and COMT genotypes. Acta Psychiatr Scand (2014) 129:54–62. 10.1111/acps.12108 [DOI] [PubMed] [Google Scholar]
- 189.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry (2009) 65:760–9. 10.1016/j.biopsych.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, et al. Induction of psychosis by Delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry (2012) 69:27–36. 10.1001/archgenpsychiatry.2011.161 [DOI] [PubMed] [Google Scholar]
- 191.Bossong MG, Jansma JM, Van Hell HH, Jager G, Oudman E, Saliasi E, et al. Effects of delta9-tetrahydrocannabinol on human working memory function. Biol Psychiatry (2012) 71:693–9. 10.1016/j.biopsych.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 192.Skosnik PD, Ranganathan M, D’souza DC. Cannabinoids, working memory, and schizophrenia. Biol Psychiatry (2012) 71:662–3. 10.1016/j.biopsych.2012.02.028 [DOI] [PubMed] [Google Scholar]
- 193.Meijer JH, Dekker N, Koeter MW, Quee PJ, Van Beveren NJ, Meijer CJ. Cannabis and cognitive performance in psychosis: a cross-sectional study in patients with non-affective psychotic illness and their unaffected siblings. Psychol Med (2012) 42:705–16. 10.1017/S0033291711001656 [DOI] [PubMed] [Google Scholar]
- 194.Boydell J, Van Os J, Lambri M, Castle D, Allardyce J, Mccreadie RG, et al. Incidence of schizophrenia in south-east London between 1965 and 1997. Br J Psychiatry (2003) 182:45–9. 10.1192/bjp.182.1.45 [DOI] [PubMed] [Google Scholar]
- 195.Rabinowitz J, Bromet EJ, Lavelle J, Carlson G, Kovasznay B, Schwartz JE. Prevalence and severity of substance use disorders and onset of psychosis in first-admission psychotic patients. Psychol Med (1998) 28:1411–9. 10.1017/S0033291798007399 [DOI] [PubMed] [Google Scholar]
- 196.Goldberger C, Dervaux A, Gourion D, Bourdel MC, Loo H, Laqueille X, et al. Variable individual sensitivity to Cannabis in patients with schizophrenia. Int J Neuropsychopharmacol (2010) 13:1145–54. 10.1017/S1461145710000647 [DOI] [PubMed] [Google Scholar]
- 197.Sevy S, Robinson DG, Napolitano B, Patel RC, Gunduz-Bruce H, Miller R, et al. Are Cannabis use disorders associated with an earlier age at onset of psychosis? A study in first episode schizophrenia. Schizophr Res (2010) 120:101–7. 10.1016/j.schres.2010.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ringen PA, Melle I, Birkenaes AB, Engh JA, Faerden A, Vaskinn A, et al. The level of illicit drug use is related to symptoms and premorbid functioning in severe mental illness. Acta Psychiatr Scand (2008) 118:297–304. 10.1111/j.1600-0447.2008.01244.x [DOI] [PubMed] [Google Scholar]
- 199.Larsen TK, Melle I, Auestad B, Friis S, Haahr U, Johannessen JO, et al. Substance abuse in first-episode non-affective psychosis. Schizophr Res (2006) 88:55–62. 10.1016/j.schres.2006.07.018 [DOI] [PubMed] [Google Scholar]
- 200.Griffith-Lendering MF, Wigman JT, Leeuwen A, Huijbregts SC, Huizink AC, Ormel J, et al. Cannabis use and vulnerability for psychosis in early adolescence – a trails study. Addiction (2013) 108:733–40. 10.1111/add.12050 [DOI] [PubMed] [Google Scholar]
- 201.Henquet C, Van Os J, Kuepper R, Delespaul P, Smits M, Campo JA, et al. Psychosis reactivity to Cannabis use in daily life: an experience sampling study. Br J Psychiatry (2010) 196:447–53. 10.1192/bjp.bp.109.072249 [DOI] [PubMed] [Google Scholar]
- 202.Childs HE, Mccarthy-Jones S, Rowse G, Turpin G. The journey through Cannabis use: a qualitative study of the experiences of young adults with psychosis. J Nerv Ment Dis (2011) 199:703–8. 10.1097/NMD.0b013e318229d6bd [DOI] [PubMed] [Google Scholar]
- 203.Shrivastava A, Johnston M, Terpstra K, Bureau Y. Pathways to psychosis in Cannabis abuse. Indian J Psychiatry (2014) 56(1):8–16. 10.4103/0019-5545.124708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet (2013) 381:1371–9. 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis (2013) 201:1007–20. 10.1097/NMD.0000000000000049 [DOI] [PubMed] [Google Scholar]
- 206.Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction (2011) 106:2195–203. 10.1111/j.1360-0443.2011.03574.x [DOI] [PubMed] [Google Scholar]
- 207.Insel TR. Rethinking schizophrenia. Nature (2010) 468:187–93. 10.1038/nature09552 [DOI] [PubMed] [Google Scholar]
- 208.Zubin J, Spring B. Vulnerability: A new view of schizophrenia. J. Abnorm. Psy. (1977) 86(2):103–26 10.1037/0021-843X.86.2.103 [DOI] [PubMed] [Google Scholar]