Abstract
Rabies is a fatal zoonotic disease for which no effective treatment measures are currently available. Rabies virus (RABV) has anti-apoptotic and anti-inflammatory properties that suppress nerve cell damage and inflammation in the CNS. These features imply that the elimination of RABV from the CNS by appropriate treatment could lead to complete recovery from rabies. Ten rabbits showing neuromuscular symptoms of rabies after subcutaneous (SC) immunization using commercially available vaccine containing inactivated whole RABV particles and subsequent fixed RABV (CVS strain) inoculation into hind limb muscles were allocated into three groups. Three rabbits received no further treatment (the SC group), three rabbits received three additional SC immunizations using the same vaccine, and four rabbits received three intrathecal (IT) immunizations, in which the vaccine was inoculated directly into the cerebrospinal fluid (the SC/IT group). An additional three naïve rabbits were inoculated intramuscularly with RABV and not vaccinated. The rabbits exhibited neuromuscular symptoms of rabies within 4–8 days post-inoculation (dpi) of RABV. All of the rabbits died within 8–12 dpi with the exception of one rabbit in the SC group and all four rabbits in SC/IT group, which recovered and started to respond to external stimuli at 11–18 dpi and survived until the end of the experimental period. RABV was eliminated from the CNS of the surviving rabbits. We report here a possible, although still incomplete, therapy for rabies using IT immunization. Our protocol may rescue the life of rabid patients and prompt the future development of novel therapies against rabies.
Keywords: animal experiment, CNS pathology, intrathecal immunization, rabies virus, treatment of rabies
Introduction
Rabies is one of the oldest zoonotic diseases and causes approximately 55 000 fatalities annually. Rabies is invariably fatal when rabies virus (RABV) invades the brain.1 High levels of viral neutralizing antibody (VNA) have been found in the cerebrospinal fluid (CSF) in rare recovered cases of rabies.2–6 Two rabid human patients survived after being put into a therapeutic coma under the so-called “Milwaukee protocol”.5,6 However, therapeutic coma was not successful in other patients, and the scientific rationale of the Milwaukee protocol remains controversial.3,7
Recent virological studies have revealed that RABV8,9 has anti-apoptotic and anti-inflammatory effects that suppress nerve cell damage and inflammatory responses in the CNS of heavily infected animals.10,11 These features imply that the elimination of RABV from the CNS by appropriate treatment could lead to recovery from rabies.
We have reported that intrathecal (IT) immunization, which involves the direct inoculation of antigens into the CSF, induced specific antibodies against RABV in the CSF.12,13 The immunization induced a protective immune response against the transneural spread of RABV12,13 and suppressed the spread of intracerebrally inoculated RABV in mice.14 Subcutaneous (SC) immunization prior to IT immunization induced a more rapid and higher antibody response in the CSF than IT immunization alone.12 We report here for the first time that repeated IT immunization of rabbits showing clinical symptoms of rabies can clear RABV from the brain and prevent fatality. Our protocol may rescue the life of rabid patients and prompt the future development of novel therapies against rabies.
Materials and Methods
Virus
The CVS strain, a neurovirulent, fixed RABV15 strain, was used in the present study since this virus does not descend from the CNS to the periphery and is safe to handle in a laboratory setting, unlike street RABV.15,16 Furthermore and more importantly, the latent period between virus inoculation and the clinical appearance of rabies is fixed at about 1 week for the CVS strain.
Animals and experimental design
A total of 27 conventional clean New Zealand White rabbits (16-week-old, Japan SLC Inc., Hamamatsu, Japan) were used in this study. Twenty-four rabbits were injected subcutaneously with 1 mL of the inactivated whole RABV particles (Nisseiken Rabies TC Vaccine, Nisseiken Co., Tokyo, Japan) into the dorsal subcutis. Three days later, the rabbits were intramuscularly inoculated with RABV in both hind limbs with 2 mL of inoculums (4 × 107 FFU/mL). Ten of the 24 rabbits (41.7%) showed neuromuscular symptoms of rabies prior to 8 days post-inoculation (dpi) and the remaining 14 rabbits were excluded from the experiment. These 10 rabbits were allocated into three groups. Three rabbits received no further treatment after showing symptoms of rabies (the SC group); three rabbits received three additional SC immunizations (the SC/SC group) using the vaccine and four rabbits were treated with three additional IT immunizations (SC/IT immunization) on days 1, 2 and 4 after showing symptoms of rabies. For IT immunization, the rabbit was inoculated with 1 mL of the vaccine into the subarachnoid space via cisterna cerebellomedullaris immediately after collecting 1 mL of CSF under anesthesia using xylazine hydrochloride (2 mg/kg Selactar; Bayer Health Care, Leverkusen, Germany) and ketamine hydrochloride (35 mg/kg Ketalar; Daiichi Sankyo Co., Tokyo, Japan). An additional three naïve rabbits were inoculated intramuscularly with RABV and no vaccination was given (the non-treatment group; see Figure 1 for the treatment schema). All the recumbent rabbits were given daily injections of 100–150 mL saline containing 5% glucose and 10 mL of amino acid solution (Aminoleban, Otsuka Pharmaceutical Co., Tokyo, Japan) through the ear vein. Surviving rabbits were kept up to 28 days after showing rabies symptoms and were euthanized by exsanguination under deep anesthesia using xylazine hydrochloride and ketamine hydrochloride.
Figure 1.
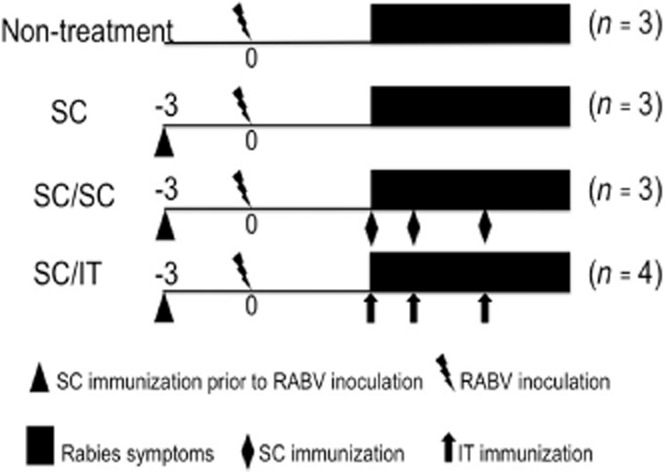
Experimental protocol. ▴, Subcutaneous (SC) immunization prior to rabies virus (RABV) inoculation; 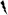 , RABV inoculation; ▪, Rabies symptoms; ♦, SC immunization;
, RABV inoculation; ▪, Rabies symptoms; ♦, SC immunization;  , intrathecal (IT) immunization.
, intrathecal (IT) immunization.
Antibody measurements
Serum and CSF were collected at each time point shown in Figures 2 and 3 and were stored at −20°C until antibody titers were assayed. The VNA assay was performed using a rapid fluorescent focus inhibition test, as previously described.2,17 ELISAs were conducted as previously described.12
Figure 2.
Viral neutralizing antibody titers in the serum and CSF. Tx: treatment.  , Surviving (Serum);
, Surviving (Serum);  , Surviving (CSF);
, Surviving (CSF);  , Non-surviving (Serum);
, Non-surviving (Serum);  , Non-surviving (CSF).
, Non-surviving (CSF).
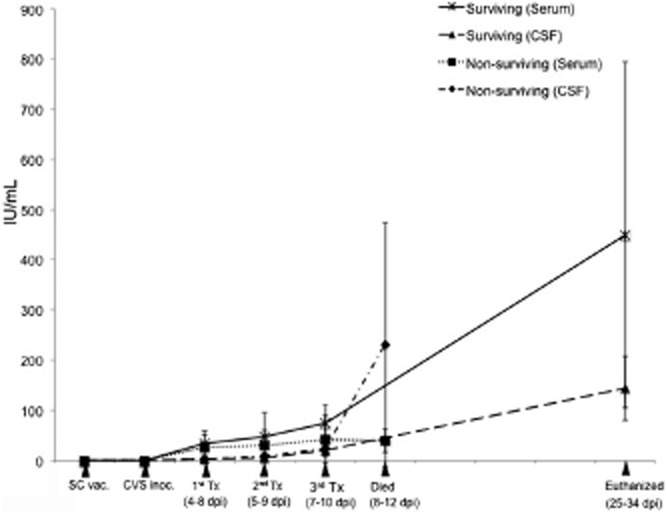
Figure 3.
ELISA antibody titers in the serum and CSF. Tx: treatment.  , Surviving (Serum);
, Surviving (Serum);  , Surviving (CSF);
, Surviving (CSF);  , Non-surviving (Serum);
, Non-surviving (Serum);  , Non-surviving (CSF).
, Non-surviving (CSF).
Histopathology and immunohistochemistry
Selected tissues, including visceral organs and nervous tissues, were collected and fixed in 20% buffered formalin for histopathological examination. For immunohistochemistry (IHC), a streptavidin-biotin-peroxidase system (SAB-PO Kit; Nichirei Bioscience, Tokyo, Japan) was employed. Primary antibodies used for IHC were monoclonal mouse anti-rabies nucleoprotein (clone N13-27; kindly provided by Dr. Naoto Ito, Gifu University), monoclonal mouse anti-human GFAP (clone 6F2; DAKO, Carpinteria, CA, USA), monoclonal mouse anti-human CD3 (clone F7.2.38; DAKO, USA), monoclonal mouse anti-human CD79α (clone MH57; DAKO), and goat polyclonal anti-rabbit Iba-1 (code ab5076; Abcam, Cambridge, UK).
RT-PCR
Total RNA was extracted from brain tissue using the RNeasy Kit (Qiagen, Germantown, MD, USA) and 5 μg of RNA was used for reverse transcription with the Superscript First-Strand Synthesis system (Life Technologies, Carlsbad, CA, USA). The fragment of the RABV genome encoding matrix protein was amplified using Go Taq DNA polymerase (Promega, Madison, WI, USA) and the following primer pairs: F, 5′-GTC GAC ATG AAC GTT CTA CGC AAG ATA G-3′ and R, 5′-GCG GCC GCT TAT TCT AGA AGC AGA GAA G-3′. Hypoxanthine phosphoribosyltransferase (HPRT) was used as an internal control.
Statistical analysis
Statistically significant differences in antibody levels between surviving and non-surviving rabbits were evaluated by repeated measures analysis of variance (ANOVA) and significance was set at P < 0.05.
Ethics statement
All animal experiments were conducted within the BSL2 facility of Hokkaido University Research Center for Zoonosis Control after approval of the Animal Care and Use Committee of the Hokkaido University (approval number 09–0028).
Results
Clinical findings
Ten of the 24 rabbits (41.7%) showed neuromuscular symptoms of rabies prior to 8 dpi and the remaining 14 rabbits were excluded from the experiment. The clinical course of the development of rabies symptoms and rabies lethality is summarized in Table 1 and Fig. S1. All the 10 rabbits showed clinical signs of rabies, including an unstable gait, lack of coordination of the hind limbs, gradual decreases in food intake and water consumption, and increased salivation and lacrimation, within 4–8 dpi. After this period, the rabbits progressively developed tetraplegia, lateral recumbency and generalized spasms, at 8–10 dpi. All three rabbits in the non-treatment group, two of three rabbits in the SC group, and all three rabbits in the SC/SC group died within 8–12 dpi. On the other hand, one rabbit in the SC group and all four rabbits in the SC/IT group recovered from the terminal stage and resumed drinking and eating. They began responding to external stimuli again at 12–18 dpi and survived until the end of the study (Fig. S1). However, they remained recumbent, with decreased body weight (Fig. S2) and did not regain the ability to stand up or walk during the observation period.
Table 1.
Summary of the clinical course of the rabbits of four groups
| Groups | ID | Neuromuscular symptom | Terminal point (dpi) | Euthanized (dpi) | Lethality (%) | ||
|---|---|---|---|---|---|---|---|
| Appearance (dpi) | Peak (dpi) | Recovery (dpi) | |||||
| Non-treatment | 1 | 6 | 9 | – | 12 | – | 100 |
| 2 | 8 | 10 | – | 12 | – | ||
| 3 | 7 | 9 | – | 10 | – | ||
| SC | 1 | 6 | 9 | – | 10 | – | 66.7 |
| 2 | 5 | 8 | – | 8 | – | ||
| 3 | 4 | 9 | 16 | – | 32 | ||
| SC/SC | 1 | 6 | 8 | – | 10 | – | 100 |
| 2 | 6 | 10 | – | 10 | – | ||
| 3 | 6 | 9 | – | 9 | – | ||
| SC/IT | 1 | 4 | 7 | 11 | – | 25 | 0 |
| 2 | 6 | 8 | 18 | – | 34 | ||
| 3 | 5 | 7 | 12 | – | 33 | ||
| 4 | 4 | 9 | 16 | – | 32 | ||
dpi, days post inoculation; SC, subcutaneous; IT, intrathecal.
Antibody response
VNA and ELISA antibody titers in the serum and CSF increased gradually after RABV inoculation and peaked at the end of the experiment in surviving rabbits (Figs 3). At the time of the third treatment (7–10 dpi), antibody titers were not significantly different between the rabbits that ultimately survived and those that did not.
Pathological findings
Macroscopically, the surviving rabbits showed muscular atrophy and a decreased amount of subcutaneous and abdominal adipose tissues.
Microscopically, the eight rabbits that died (three from the non-treatment group, two from the SC group and three from the SC/SC group) had neuronal necrosis with occasional neuronophagia and deposition of a large amount of RABV antigen in the nerve cell bodies/projections throughout the CNS. These changes were most prominent in the cerebral cortex of the parietal lobe, the thalamus, the hypothalamus, the nuclei of ascending pathways and reticular formation of the brain stem, the vermis of the cerebellum (Fig. 4a), the dorsal horn and intermediate substance of gray matter of the spinal cord, and the lumbar and sacral dorsal ganglia. Proliferation and hypertrophy of Iba-1+ microglia and diffuse perivascular and meningeal lymphocytic infiltrates consisting mainly of CD3+ T lymphocytes were also observed in these tissues. A small number of CD79+ B lymphocytes infiltrated in the meninges, perivascular spaces of the CNS and dorsal ganglia, and T lymphocytes were dominant over B lymphocytes in number at all of the areas. In contrast, descending motor and pyramidal nerve routes such as the cerebral basal nuclei, the hippocampus, the cerebellar hemisphere, and the ventral horn of the spinal cord were relatively spared from severe pathological changes.
Figure 4.
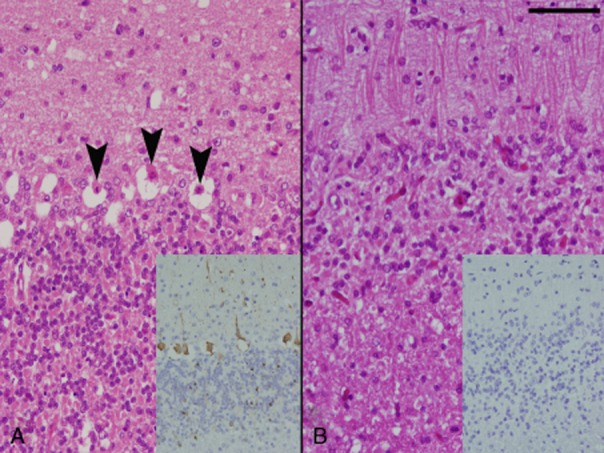
The cerebellar cortex of a non-surviving rabbit from the non-treatment group showing necrosis of Purkinje cells (arrowheads). Immunohistochemistry of the cerebellum reveals abundant rabies virus (RABV) antigen in Purkinje and granular cells (inset, A). The cerebellar cortex of a surviving rabbit from the subcutaneous (SC)/intrathecal (IT) group showed the loss of Purkinje and granular cells together with gliosis of the molecular layer. RABV antigen is not found in the cerebellum (inset, B). Scale bar represents 50 μm .
In the five rabbits (one in the SC group and four in the SC/IT group) that survived the virus challenge, nerve cell loss was prominent for Purkinje and granular cells of the cerebellar vermis, the pontine reticular nuclei and tegmental areas of the brain stem, and the dorsal horn and intermediate substance of the spinal cord. Small malacic foci were also sometimes observed in these areas. These changes were accompanied by mild hyperplasia of Iba-1+ microglia, astrocyte swelling, and low levels of lymphoplasmacytic infiltration in the meninges, perivascular space and nerve tissues. The lymphoplasmacytic infiltrate was composed mainly of T lymphocytes and B lymphocytes were present in small numbers. RABV antigen was rarely detected either in the CNS (Fig. 4b) or in the peripheral nervous tissues of surviving rabbits. The RABV matrix gene was detected by RT-PCR in the brain tissue of non-surviving rabbits but was not found in the brains of surviving rabbits (Fig. 5).
Figure 5.
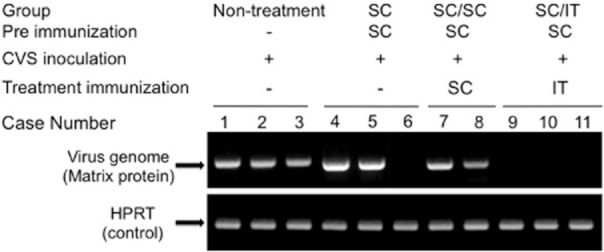
RT-PCR of the brain. A positive signal for the rabies virus (RABV) matrix gene in the brain samples from all rabbits except one surviving rabbit in the subcutaneous (SC) group (lane 6) and three surviving rabbits in the SC/intrathecal (IT) group (lanes 9–11). HPRT, phosphoribosyltransferase.
Discussion
The significance of high VNA titers in the CSF has been emphasized in humans and animals after recovery from rabies2,6,18 and VNA is considered as a crucial factor for recovery.18–22 We have reported that IT immunization induced specific antibodies against RABV in the CSF and a protective immune response against the transneural spread of RABV.12,13 The immunization could suppress the spread of intracerebrally inoculated RABV in mice.14 SC immunization prior to IT immunization induced a more rapid and higher antibody response in the CSF than IT immunization alone.12 Based on these previous findings, we tried a treatment of rabid rabbit using SC/IT immunizations. In the present study, VNA and ELISA antibody titers in the serum and CSF were markedly elevated in surviving rabbits at the end of the study. However, the antibody responses of rabbits that ultimately survived and those that did not were not significantly different at the peak of clinical symptom (8–12 dpi) (i.e., the time at which the non-surviving rabbits died), and the lymphocytic infiltrate in the CNS of rabbits that did not survive consisted predominantly of T lymphocytes. These findings indicate that the antibody titers in the serum and CSF are not the sole factors mediating the clearance of RABV from the CNS.
RABV antigen directly injected into the CSF of the brain drains into the deep cervical lymph nodes and stimulates the production of RABV-specific antibodies and cytotoxic T lymphocytes in the spleen.23 IT immunization also increases the permeability of the blood-brain barrier (BBB) and allows for the migration of effector cells into the CNS.17,24 The B lymphocytes infiltrating the CNS via up-regulation of the chemokine CXCL1225 are the source of locally produced antibodies important in the clearance RABV from the CNS.2,12,20,26 In addition, effector T lymphocytes infiltrating the CNS permit the clearance of RABV by inducing apoptosis of infected neurons in the presence of antibodies.27 These previous reports suggest that a combination of both humoral and cellular immunities17,19,24,28 contributed to the clearance of RABV from the CNS in the present study.
Our results clearly showed that the rabid rabbits treated by repeated IT immunization after SC immunization could tolerate the peak of the rabies symptoms and recover incompletely thereafter. For human patients who ever received pre- or post-exposure vaccination, it might be a possible therapeutic strategy to encourage the effective immune response in the CNS by IT immunization combined with intensive care or coma6 to secure efficient time. However, the success of this therapy was limited because: (i) the damage to the CNS tissue of surviving rabbits was too severe to allow complete recovery; and (ii) a neurovirulent, fixed RABV was used instead of street RABV. Most humans who survived rabies showed severe neural complications after recovery4 and prompt initiation of IT immunizations may benefit recovery from rabies with mild neurological complications. The CVS strain causes G protein to be expressed on the surface of infected neurons,16,29 which induces apoptosis of the infected neurons and neighboring cells, as well as inflammation in the infected brain.12,15,16,27,29 On the other hand, street RABV induces low levels of G protein on the surface of infected neurons due to post-transcriptional modification.11 The anti-apoptotic, anti-inflammatory and immunosuppressive effects of street RABV infection12,13 allow the virus to infect neurons without cellular destruction or inflammation.8,30 These findings indicate that the elimination of street RABV from the CNS by IT immunization may lead to a more complete recovery from RABV-induced rabies than from that caused by the CVS strain.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) (23380171 to T.U and 23780306 to Y.S.) and by the Global Center of Excellence Program and the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, Japan. The authors declare no competing interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Fig. S1 Clinical symptom scoring in surviving and non-surviving rabbits. The scores were determined based on the severity of the symptoms in each case with score 0-5 including 0, no symptoms; 1, unstable gait and lack of coordination of the hind limbs; 2, paralysis of the hind limbs; 3, lateral recumbency with ability to drink and eat; 4, lateral recumbency without ability to drink and eat; 5, systemic convulsion with spasm.
Fig. S2 Body weight changes in surviving and non-surviving rabbits. The weight of surviving rabbits gradually decreased even after the peak of rabies symptom to the end of experiment.
References
- Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2010;59:1–9. [PubMed] [Google Scholar]
- Hamir AN, Niezgoda M, Rupprecht CE. Recovery from and clearance of rabies virus in a domestic ferret. J Am Assoc Lab Anim Sci. 2011;50:248–251. [PMC free article] [PubMed] [Google Scholar]
- Jackson AC. Rabies: new insights into pathogenesis and treatment. Curr Opin Neurol. 2006;19:267–270. doi: 10.1097/01.wco.0000227036.93199.3b. [DOI] [PubMed] [Google Scholar]
- Tillotson JR, Axelerod D, Lyman DO. Rabies in a laboratory worker-New York. MMWR Morb Mortal Wkly Rep. 1977;26:183–184. [Google Scholar]
- Wiedeman J, Plant J, Glaser C, et al. Recovery of a patient from clinical rabies-California, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:61–65. [PubMed] [Google Scholar]
- Willoughby RE, Jr, Tieves KS, Hoffman GM, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352:2508–2514. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- Hemachudha T, Sunsaneewitayakul B, Desudchit T, et al. Failure of therapeutic coma and ketamine for therapy of human rabies. J Neurovirol. 2006;12:407–409. doi: 10.1080/13550280600902295. [DOI] [PubMed] [Google Scholar]
- Fernandes ER, de Andrade HF, Jr, Lancellotti CL, et al. In situ apoptosis of adaptive immune cells and the cellular escape of rabies virus in CNS from patients with human rabies transmitted by Desmodus rotundus. Virus Res. 2011;156:121–126. doi: 10.1016/j.virusres.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang S, Sun C, et al. Proteomic profiles of mouse neuro N2a cells infected with variant virulence of rabies viruses. J Microbiol Biotechnol. 2011;21:366–373. [PubMed] [Google Scholar]
- Suja MS, Mahadevan A, Madhusudhana SN, Vijayasarathi SK, Shankar SK. Neuroanatomical mapping of rabies nucleocapsid viral antigen distribution and apoptosis in pathogenesis in street dog rabies– an immunohistochemical study. Clin Neuropathol. 2009;28:113–124. doi: 10.5414/npp28113. [DOI] [PubMed] [Google Scholar]
- Yan X, Prosniak M, Curtis MT, et al. Silver-haired bat rabies virus variant does not induce apoptosis in the brain of experimentally infected mice. J Neurovirol. 2001;7:518–527. doi: 10.1080/135502801753248105. [DOI] [PubMed] [Google Scholar]
- Aoshima K, Sunden Y, Ishida S, Ochiai K, Umemura T. Possible origin of CSF antibodies induced by intrathecal immunization and prophylactic effects against intracerebral rabies virus infection. J Vet Med Sci. 2011;73:1303–1308. doi: 10.1292/jvms.11-0183. [DOI] [PubMed] [Google Scholar]
- Shin JH, Sakoda Y, Yano S, Ochiai K, Kida H, Umemura T. Effective prevention against rabies by intracerebral immunization in mice. J Vet Med Sci. 2009;71:1331–1336. doi: 10.1292/jvms.001331. [DOI] [PubMed] [Google Scholar]
- Sunden Y, Yano S, Ishida S, Ochiai K, Umemura T. Intracerebral vaccination suppresses the spread of rabies virus in the mouse brain. Microbes Infect. 2010;12:1163–1169. doi: 10.1016/j.micinf.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura N, Uda A, Inoue S, et al. Gene expression analysis of host innate immune responses in the central nervous system following lethal CVS-11 infection in mice. Jpn J Infect Dis. 2011;64:463–472. [PubMed] [Google Scholar]
- Miyamoto K, Matsumoto S. Comparative studies between pathogenesis of street and fixed rabies infection. J Exp Med. 1967;125:447–456. doi: 10.1084/jem.125.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang G, Wen Y, Yang S, Xia X, Fu ZF. Intracerebral administration of recombinant rabies virus expressing GM-CSF prevents the development of rabies after infection with street virus. PLoS One. 6:e25414. doi: 10.1371/journal.pone.0025414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Morse HC, 3rd, Winkelstein J, Nathanson N. The role of antibody in recovery from experimental rabies, I. effect of depletion of B and T cells. J Immunol. 1978;121:321–326. [PubMed] [Google Scholar]
- Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72:3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Phares TW, Fabis MJ, Roy A. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl Trop Dis. 3:e535. doi: 10.1371/journal.pntd.0000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao PH, Yang HH, Chou PT, et al. Sufficient virus-neutralizing antibody in the central nerve system improves the survival of rabid rats. J Biomed Sci. 2012;19:61. doi: 10.1186/1423-0127-19-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry LL, Lodmell DL. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol. 1991;65:3429–3434. doi: 10.1128/jvi.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PG, Hawke S, Sloan DJ, Bangham CRM. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71:145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- Lee H, Sunden Y, Ochiai K, Umemura T. CXCL12 improves immune responses to neurotropic virus propagation in the CNS by attracting antibody secreting cells. Vet Immunol Immunopathol. 2012;150:19–26. doi: 10.1016/j.vetimm.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Lee H, Sunden Y, Ochiai K, Umemura T. Experimental intracerebral vaccination protects mouse from a neurotropic virus by attracting antibody secreting cells to the CNS. Immunol Lett. 2011;139:102–109. doi: 10.1016/j.imlet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Galelli A, Baloul L, Lafon M. Abortive rabies virus central nervous infection is controlled by T lymphocyte local recruiment and induction of apoptosis. J Neurovirol. 2000;6:359–372. doi: 10.3109/13550280009018300. [DOI] [PubMed] [Google Scholar]
- Dietzschold B, Kao M, Zheng YM, et al. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc Natl Acad Sci U S A. 1992;89:7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M, Pulmanausahakul R, Hodawadekar SS, et al. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J Virol. 76:3374–3381. doi: 10.1128/JVI.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suja MS, Mahadevan A, Madhusudana SN, Shankar SK. Role of apoptosis in rabies viral encephalitis: a comparative study in mice, canine, and human brain with a review of literature. Pathol Res Int. 2011 doi: 10.4061/2011/374286. doi: 10.4061/2011/374286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Clinical symptom scoring in surviving and non-surviving rabbits. The scores were determined based on the severity of the symptoms in each case with score 0-5 including 0, no symptoms; 1, unstable gait and lack of coordination of the hind limbs; 2, paralysis of the hind limbs; 3, lateral recumbency with ability to drink and eat; 4, lateral recumbency without ability to drink and eat; 5, systemic convulsion with spasm.
Fig. S2 Body weight changes in surviving and non-surviving rabbits. The weight of surviving rabbits gradually decreased even after the peak of rabies symptom to the end of experiment.


