Abstract
The dentin sialophosphoprotein (dspp) transcript is expressed during tooth development as a DSPP precursor protein, which then undergoes cleavage to form mature dentin sialoprotein (DSP) and phosphophoryn (PP) proteins. Previous studies using DSPP-knockout (KO) mice have reported that these animals have hypomineralized teeth, thin dentin, and a large dental pulp chamber, similar to those from patients with dentinogenesis imperfecta III. However, there is no information about factors that regulate dental pulp stem cell lineage fate, a critical early event in the odontoblast-dentin mineralization scheme. To reveal the role of DSPP in odontoblast lineage differentiation during tooth development, we systematically examined teeth from wild-type (wt) and DSPP-KO C57BL/6 mice between the ages of postnatal day 1 and 3 months. We found developmental abnormalities not previously reported, such as circular dentin formation within dental pulp cells and altered odontoblast differentiation in DSPP-KO mice, even as early as 1 day after birth. Surprisingly, we also identified chondrocyte-like cells in the dental pulp from KO-mice teeth. Thus, these studies that compare wt and DSPP-KO mice suggest that the expression of DSPP precursor protein is required for normal odontoblast lineage differentiation and that the absence of DSPP allows dental pulp cells to differentiate into chondrocyte-like cells, which could negatively impact pulpal wound healing and tissue regeneration.
Introduction
During tooth morphogenesis, a series of reciprocal epithelial–mesenchymal (E-M) interactions lead to odontoblast differentiation and dentin mineralization [1–3]. Transcription factors originating from mesenchymal cells, such as Runx2 and Msx1, are well known to be involved in these E-M interactions [4,5]. In addition to these transcription factors, dentin matrix protein transcripts, including collagen type I, Dmp1, and dentin sialophosphoprotein (DSPP), are also expressed early in development [6–10]. For example, the dspp transcript is first expressed in cap-stage papilla at embryonic day (ED) 13–15 [7] (and Ritchie, unpublished data). The initial product of the dspp transcript is DSPP precursor protein. DSPP processing by tolloid-related protein 1 or by bone morphogenic protein 1 yields mature dentin sialoprotein (DSP) and phosphophoryn (PP) proteins [11], which are critical for dentin mineralization [12].
Proper tooth development requires cell signaling between epithelial and mesenchymal cell layers during tooth morphogenesis that ultimately limits dental pulp stem cell differentiation to the odontoblast cell lineage pathway. While factors affecting dental pulp stem cell fate determination are unknown, it is known that these stem cells are pluripotent since they are capable of differentiating into osteoblasts, chondrocytes, adipocytes, myocytes, and neuroblasts as well as odontoblasts, both in vivo and in vitro [13–17]. Studies on the transcription factor Runx2, which is mainly expressed in bud and cap stages, but begins to diminish thereafter, and on DSPP, which begins to be expressed at cap stage, have offered some valuable insights into this process. Runx2-knockout (KO) mice show arrested embryonic tooth development at ED 11–13 (“bud stage”) [4,18]. The DSPP-KO mouse is a unique model to study the role of DSPP precursor protein in vivo during tooth development. Most interestingly, DSPP-KO mice exhibited hypomineralized teeth, thin dentin, and a large dental pulp chamber, similar to teeth from human patients with dentinogenesis imperfecta III [19]. These earlier studies demonstrated the critical roles played by Runx2 in tooth morphogenesis and DSPP in dentin formation, but did not report on odontoblast lineage differentiation during tooth development.
To reveal the role of DSPP in odontoblast lineage differentiation during tooth development, we have now systematically examined teeth from wild-type (wt) and DSPP-KO C57BL/6 mice between the ages of postnatal day 1 and 3 months. We found developmental abnormalities, such as circular dentin formation within dental pulp cells and altered odontoblast differentiation, in DSPP-KO mice, even as early as 1 day after birth. Surprisingly, we also identified chondrocyte-like cells in the dental pulp from KO-mice teeth. Thus our new studies, comparing wt and DSPP-KO mice, suggest that the expression of DSPP precursor protein is required for normal odontoblast lineage differentiation and that the absence of DSPP results in the appearance of chondrocyte-like cells.
Materials and Methods
Wt and DSPP-KO mice
In this study we chose C57BL/6J mice as the experimental model. Wt mice were obtained from the Jackson Laboratory (Bar Harbor, ME). DSPP-KO mice (strain name: B6; 129-Dspptm1Kul/Mmnc) were obtained from MMRRC, UNC (Chapel Hill, NC). To minimize tooth wear while maintaining proper nutrition, both wt and DSPP-KO mice were fed with LabDietFormula 5008 (PMI Nutrition International, LLC, Brentwood, MO), which is softer than regular chow. All animal colonies were handled and maintained in accordance with the guidelines and protocols approved by the University Committee on Use and Care of Animals (UCUCA, protocol number: 10401-1).
RNA extraction from ground mandibular teeth and reverse transcription–polymerase chain reaction analysis
Twenty-one-day wt and DSPP-KO mice were euthanized and mandibles were collected. Teeth were extracted using a dissecting microscope in order to remove bone, periodontal ligaments, and muscular tissue. Both mandibular teeth from the same mouse were used for each RNA extraction. The teeth were ground by mechanical force in liquid nitrogen, and RNA was extracted using Trizol reagent kit (Invitrogen, Life Technologies, Carlsbad, CA). Thirty cycles of reverse transcription–polymerase chain reaction (RT-PCR) were performed using a Thermo Scientific Verso cDNA kit according to the manufacturer's protocol. Mouse dspp mRNA expression level was determined with the following primers: 5′-TGAAGAAGGCGACAGTACCC-3′ (forward) and 5′-TCACTTTCGTCACTTCCGTTAG-3′ (reverse), which produces a 441-bp DNA fragment. Mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression level was determined with the following primers: 5′-GGTGAAGGTCGGTGTGAACG-3′ (forward) and 5′-CTCGCTCCTGGAAGATGGTG-3′ (reverse), which yielded a 233-bp band. GAPDH served as an internal control.
RNA extraction from incisor dental pulp cell culture and RT-PCR analysis
Incisor dental pulps from 21-day wt and DSPP-KO mice were obtained as described previously and the alveolar bones were removed under a dissecting microscope. The incisors were then split sagittally into two halves and dental pulps were removed with a mini-scalpel and cultured in α-MEM containing 15% fetal bovine serum, penicillin/streptomycin (100 units/mL), 4 mM l-glutamine, and 1% nonessential amino acids for 2 days. Dental pulp cells were then washed twice with 1× phosphate-buffered saline (PBS), followed by adding Trizol to the pulp cells for RNA extraction as per company protocol (Invitrogen, Life Technologies). RNA with an OD260/OD280 ratio between 1.8 and 2.0 was used for RT-PCR analysis. AMV reverse transcriptase (Promega, Madison, WI) was used to synthesize the first-strand cDNA from wt and DSPP-KO RNA. Sox9 and col type II mRNA expression was evaluated using mouse Sox9 forward/reverse primers 5′TCGGTGAAGAACGGACAAG3′/5′TTGGGTGGCAAGTATTGG3′ to generate a 360-bp PCR fragment, and mouse collagen type II forward/reverse primers 5′CAACAGCAGGTTCACATACAC3′/5′GCCCAGTTCAGGTCTCTTAG3′ to generate a 383-bp PCR fragment. Mouse GAPDH forward/reverse primers 5′GGTGAAGGTCGGTGTGAACG3′/5′CTCGCTCCTGGAAGATGGTG3′ were used to generate a 233-bp PCR fragment as an internal control. For Sox9 and GAPDH primers, PCR was performed at 96°C for 5 min, 30 cycles of 95°C for 15 min, 57°C for 15 min, and 72°C for 30 min, and then 72°C for 5 min and stopped at 10°C. For collagen type II primers, a touchdown PCR at −1°C per cycle was used. PCR products were analyzed via electrophoresis on a 1% agarose gel.
Incisor protein extraction and polyacrylamide gel electrophoresis
Twenty-one-day wt and DSPP-KO mice were euthanized and both mandibles were collected. Incisors were extracted using a dissecting microscope to remove bone, periodontal ligament, and muscular tissue. For each mouse type, eight mandibular incisors were collected for protein extraction, and the weight of incisors was recorded. The incisors were washed in 1× PBS with 1× proteinase inhibitors (PIs; Amresco, Solon, OH) and 0.3 mM phenylmethyl sulfonyl fluoride (PMSF) protease inhibitor and then ground to powder with mechanical force in liquid nitrogen. The powdered tissues containing both enamel and dentin were transferred to solubilization buffer [150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 1% NP-40, and 5 mM EDTA] with 1× PIs and 0.3 mM PMSF, and then trichloroacetic acid (TCA) was added to the mixture to reach a final concentration of 5% for dentin protein extraction. Dentin matrix proteins included collagen type I and noncollagenous proteins, such as DSP, PP, osteocalcin, and osteopontin. Under these extraction conditions, acidic proteins were soluble in 5% TCA while other nonacidic proteins were precipitated. The extraction was carried out for 1 h at room temperature, and the mixture was centrifuged at 12,000 g for 5 min. The supernatants were then collected, neutralized, and precipitated with calcium by adding 3 M Tris-HCl (pH 8.8) and 1 M CaCl2 and then centrifuged at 12,000 g for 5 min. The supernatant was aspirated and the precipitant was resuspended in 0.1 M ethylenediaminetetraacetic acid (EDTA; pH 8.0). The extracted dentin matrix proteins were analyzed by polyacrylamide gel electrophoresis with Stains-All staining as described previously [20].
Tissue preparation and histological analyses
Tissues were collected from wt and DSPP-KO mice at various ages. For 1- and 6-day-old mice, the entire head was collected; for 21-day-old and 3-month-old mice, only mandibles were collected. Tissues were fixed with 10% formalin at room temperature for 48 h and then demineralized with 0.25 M EDTA (pH 7.4) at room temperature for 15 days. Then, tissues were dehydrated, paraffin-embedded, sectioned at 5-μm thickness, stained with hematoxylin and eosin (H&E) (Fisher Scientific, Waltham, MA and Sigma-Aldrich, St. Louis, MI), and mounted with Permount (Fisher Scientific). Images were taken using Nikon Eclipse E400 microscope (Tokyo, Japan) and SPOT RT Slider Microscope Camera (Diagnostic Instruments, Sterling Heights, MI). Scale bars for 200×, 400×, and 1,000× zooms were added to the images using the manufacturer's software. Safranin O/fast green staining (Sigma-Aldrich) was carried out according to the manufacturer's directions to detect the presence of acidic proteoglycan, a characteristic marker for chondrocyte tissues. Anti-collagen type II antibody was purchased from the University of Iowa Hybridoma Bank (Iowa City, IA). A 1:500 dilution of anti-collagen type II antibodies was used to detect collagen presence in wt and DSPP-KO molar teeth.
Dental pulp cell density
The dental pulp cell numbers were quantitated in the following four groups: (a) wt 1-day dental pulp cells, (b) wt 6-day dental pulp cells, (c) DSPP-KO 1-day dental pulp cells, and (d) DSPP-KO 6-day dental pulp cells. Each group contained three animals. Four images (100× magnification) were taken from the first molar (M1) H&E-stained sections from each animal, yielding 12 images per group. Cell density was then determined by counting the number of cells in an (100 μm)2 area from each image.
Microcomputed tomography analysis of dentin mineral density
Wt and DSPP-KO mandibles were extracted as described previously, and fixed in 10% formalin at room temperature for 48 h. Fixed mandibular samples were scanned at the University of Michigan School of Dentistry μCT Core Facility. Specimens were mounted in 1% agarose and placed in a 19-mm-diameter tube and scanned over the entire length of the left mandible using a microcomputed tomography (μCT) system (μCT100; Scanco Medical, Bassersdorf, Switzerland). Scan settings were as follows: voxel size=16 μm, 70 kVp, 114 μA, 0.5-mm AL filter, and integration time=500 ms. Three-dimensional reconstruction was created from μCT 2D images using a fixed global threshold of 22% (220 on a grayscale of 0–1,000). Analysis was performed using the manufacturer's evaluation software, and a fixed global threshold of 30% (300 on a grayscale of 0–1,000) was used to segment bone from nonbone; upper threshold of 60% was used to segment dentin from enamel. Contours were manually drawn around the first molar of each specimen (∼100 image slices/molar).
Statistical analysis
Results were presented as means±standard deviations. Two-sample t-test for mean difference with unequal variances was carried out using the program Statistical Analysis System (SAS Institute, Inc., Cary, NC) in the Center for Statistical Consultation and Research Center of The University of Michigan. Data were plotted using KaleidaGraph (Synergy Software, Reading, PA).
Results
dspp mRNA, and DSP and PP proteins are absent in DSPP-KO teeth
Postnatal 21-day teeth were obtained from C57BL/6 DSPP-KO mice and wt mice. Total RNAs were extracted from these tissues and analyzed for dspp mRNA by RT-PCR. dspp mRNA was detected in the wt teeth, but it was not detected in DSPP-KO teeth (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd). Protein extractions from wt and DSPP-KO 21-day incisors were analyzed on a polyacrylamide gel electrophoresis stained with Stains-All (Sigma-Aldrich). Supplementary Figure S1B shows PP as a major band and DSP as a minor band in wt 21-day incisors. As expected, no DSPP precursor protein was detected in wt incisors because secreted DSPP precursor protein is immediately processed into mature DSP and PP proteins in teeth [11,21]. We used 5% TCA to extract dentin acidic proteins as described in “Materials and Methods” section. The detection of less DSP protein than PP protein in wt 21-day incisors could be due to the poor solubility of DSP in 5% TCA and the actions of metalloproteinases [22]. No PP or DSP bands were detected in DSPP-KO incisors from 21-day animals (Supplementary Fig. S1). Thus, our DSPP-KO mice contain no dspp mRNA, and no DSP or PP proteins.
Dental pulp cell density was reduced in DSPP-KO mice
To follow the developmental progression of DSPP-KO mouse molar teeth characteristics, we systematically examined the postnatal day-1 and day-6 KO molar teeth and compared them with molars of similarly aged wt mice. Teeth were collected from (a) wt 1-day first molar (M1), (b) wt 6-day M1, (c) DSPP-KO 1-day M1, and (d) DSPP-KO 6-day M1; processed; sectioned; stained; and photographed (Supplementary Fig. S2A). Cells from each type of molar teeth were counted to determine dental pulp cell density (see “Materials and Methods” section). The results obtained from wt and DSPP-KO mice at postnatal 1 and 6 days are shown in Supplementary Figure S2B. Dental pulp cell density is significantly reduced (P<0.05) in DSPP-KO mice compared with those in wt mice in both postnatal 1- and 6-day animals.
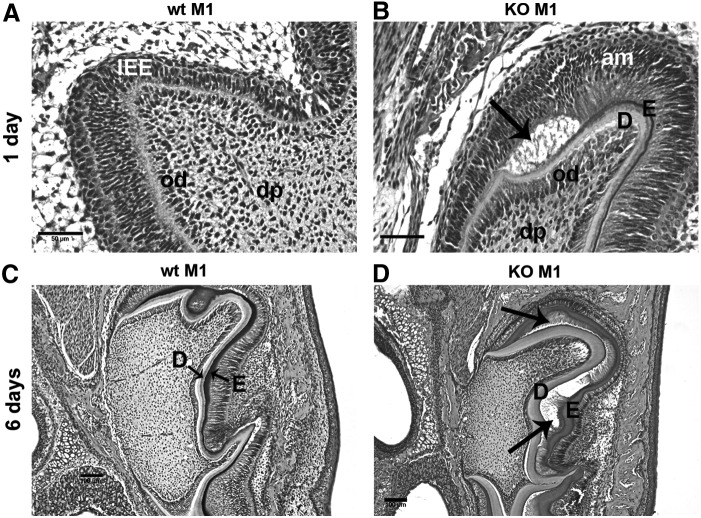
Enamel and dentin junction was abnormal in postnatal 1- and 6-day first molar (M1) DSPP-KO teeth
In first molar (M1) wt 1-day teeth, the odontoblast layer and inner enamel epithelium (IEE) layer are tightly connected. Predentin and dentin are present in half (3/6) of the animals tested (Fig. 1A). In DSPP-KO 1-day M1 tissue, the odontoblast and ameloblast layers exhibited a thicker dentin and enamel matrix than comparable wt M1 tissue, and were separated from one another (see arrow, Fig. 1B). In wt 6-day M1, the odontoblast and ameloblast layers had well-defined dentin and enamel matrix and were tightly connected (Fig. 1C). In 6-day DSPP-KO M1 teeth, a significantly thicker dentin layer was present compared with wt 6-day M1 teeth. However, there was a space (see arrows) located between the dentin and enamel layers (Fig. 1D). In 6-day DSPP-KO second molar (M2) teeth, a space located between the dentin and enamel layers was also observed (data not shown). The observed abnormal molar morphogenesis in late bell stage (ie, postnatal day 1) and early secretory stage (ie, postnatal day 6), together with the absence of DSPP in DSPP-KO mice, suggests that DSPP protein expression is required for normal odontoblast and ameloblast development.
FIG. 1.
Enamel and dentin junctions in 1- and 6-day wt and DSPP-KO mice. All samples were derived from six 1-day and six 6-day wt and KO mice. The sections were stained with H&E. (A) One-day wt mouse M1. Note that the odontoblast layer and IEE layer were tightly connected. (B) One-day DSPP-KO M1. Note that odontoblast layer and ameloblast layer were separated as indicated by arrow. (C) Six-day wt M1. Note that dentin and enamel layers were tightly connected. (D) Six-day DSPP-KO M1. Note that the dentin and enamel layers were separated as indicated by arrows. The dentin layer is thickened relative to wt M1. am, ameloblast; D, dentin; dp, dental pulp; E, enamel; IEE, innerenamel epithelium; M1, first molar; od, odontoblast. Scale bar=50 μm (400× magnification) for (A, B). Scale bar=100 μm (100× magnification) for (C, D). DSPP, dentin sialophosphoprotein; H&E, hematoxylin and eosin; KO, knockout; wt, wild type.
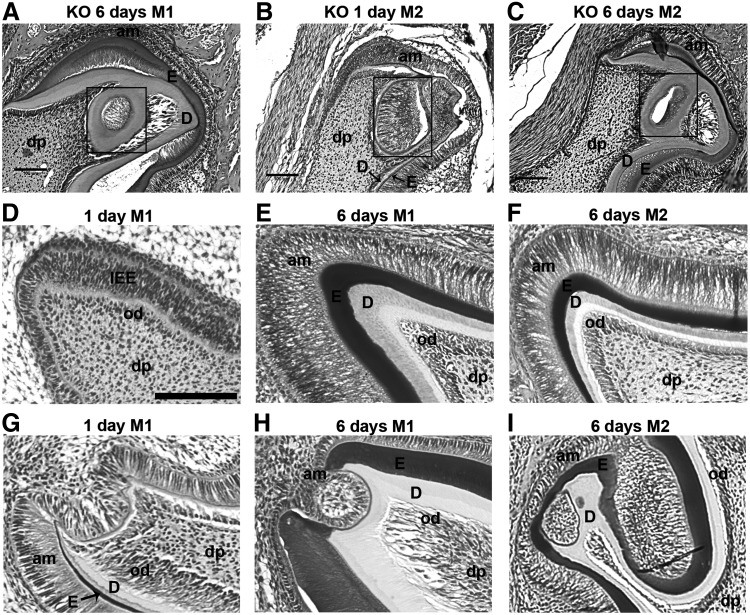
A circular odontoblast layer was present within dental pulp in postnatal 1- and 6-day DSPP-KO mice
In addition to the abnormal molar morphologies outlined earlier, we also observed that the dentin layer in 6-day DSPP-KO M1 teeth can form a circular structure within the dental pulp (Fig. 2A). In 1- and 6-day DSPP-KO second molar (M2) teeth, dentin also forms a circular structure within the dental pulp (Fig. 2B, C). In these examples as well, it appears that DSPP is required for normal odontoblast development. These circular structures are not found in wt molars.
FIG. 2.
Presence of a circular odontoblast layer within dental pulp in 1- and 6-day DSPP-KO mice (A–C) and abnormal cusp formation in 1- and 6-day DSPP-KO mice (G–I). Representative samples were derived from six 1-day and six 6-day DSPP-KO mice. The sections were stained with H&E. Panels (A–C) show circular odontoblast layer. (A) Six-day DSPP-KO M1 (100× magnification). (B) One-day DSPP-KO M2 (200× magnification). (C) Six-day DSPP-KO M2 (200× magnification). Note that dentin formed a circular structure, indicated by the box, within the dental pulp in each of these samples and the enamel and dentin layers were abnormally separated. M2, second molar. Scale bar=100 μm. (D–F) Represent normal cusp formation. (D) One-day wt M1. Note intact cusp. (E) Six-day wt M1. Note intact cusp and normal dentin/enamel formation. (F) Six-day wt M2. Note intact cusp and normal dentin/enamel formation. (G–I) Represent abnormal cusp formation. (G) One-day DSPP-KO M1. Note cusp with thin dentin without enamel. (H) Six-day DSPP-KO M1. Note abnormal cusp. The dentin layer in the cusp is thinner than that of wt M1. (I) Six-day DSPP-KO M2. Note abnormal cusp. Scale bar=100 μm for all frames.
Abnormal cusp formation seen in 1- and 6-day DSPP-KO mice
Normal cusp development in wt 1-day M1, wt 6-day M1, and wt 6-day M2 teeth is shown in Figure 2D–F. These three wt examples share a common characteristic: tight cellular connections between the odontoblast layer, dentin layer, enamel layer, and ameloblast layer. However, first-molar teeth obtained from 1- and 6-day DSPP-KO mice display abnormal morphogenesis and structures at the cusps of the teeth (Fig. 2G, H). For example, Figure 2H shows a disrupted enamel layer and a thinner dentin layer in the 6-day M1 cusp. Abnormal enamel and dentin formation was also observed in 6-day DSPP-KO second molar (M2) in the cusp region (Fig. 2I). These results suggest that the absence of DSPP in these DSPP-KO animals disrupted normal E-M interactions required for the establishment of normal teeth.
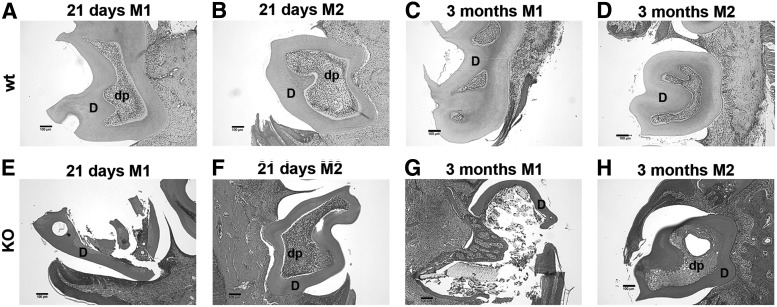
Comparison of dental pulp and dentin structure in postnatal 21-day and 3-month wt and DSPP-KO mice
At later developmental stages (ie, 21 days and 3 months), wt first and second molars (M1 and M2) displayed normal dental pulp with a polarized odontoblast layer and healthy pulp cells, as well as a thick, intact dentin structure (Fig. 3A–D). In contrast, 21-day M1 teeth from DSPP-KO mice (at the weaning stage) displayed broken dentin structure and severely damaged dental pulp (Fig. 3E). Tightly packed cells were present in M2 dental pulp, which was likely caused by inflammation (Fig. 3F). Upon further sectioning of this particular tooth, we also observed that the dental pulp was exposed (data not shown). The dentin in M1 and M2 teeth from 21-day KO mice is much thinner than dentin from 21-day wt M1 and M2 teeth. In general, most M1 and M2 teeth we examined from postnatal 21-day DSPP-KO mice exhibited fragmented dentin, exposed dental pulp, and inflammatory cells. Moreover, cells adjacent to the dentin did not show any polarization or odontoblast-like cells as would be expected for normal wt teeth. Three-month DSPP-KO mice displayed severely damaged first-molar teeth (Fig. 3G), while M2 teeth showed thin dentin and an enlarged dental pulp chamber (Fig. 3H).
FIG. 3.
Comparison of dental pulp and dentin structure in 21-day and 3-month wt and DSPP-KO mice. All samples were stained with H&E. (A) Twenty-one-day wt M1 and (B) 21-day wt M2. Note the intact dental pulp and dentin structures. Three-month wt M1 (C) and M2 (D). Note the intact dental pulp and dentin structures. (E) Twenty-one-day DSPP-KO M1. Note the fractured dentin layer and remnant dental pulp. (F) Twenty-one-day DSPP-KO M2. Note the tightly packed cells. (G) Three-month DSPP-KO M1. Note the fractured dentin and miscellaneous structures within the dental pulp. (H) Three-month DSPP-KO M2. Note the intact dentin layer with fibrocartilage-like matrix within the dental pulp. All 21-day and 3-month DSPP-KO M1 and M2 teeth showed thin dentin relative to wt M1 and M2. Scale bar=100 μm for all frames.
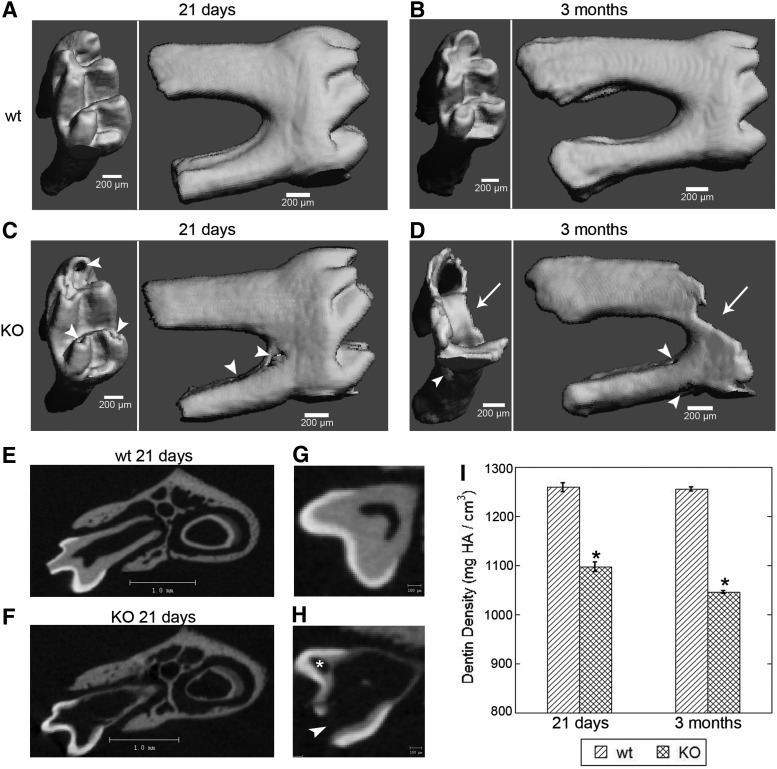
Microcomputed tomography images
First-molar teeth (M1) from 21-day wt mice displayed an intact crown and root as shown in occlusal and buccal μCT images (Fig. 4A). Three-month wt M1 teeth also had intact crown and root structures (Fig. 4B). In contrast, 21-day DSPP-KO M1 teeth showed a defective crown with pulp exposure and a defective root as indicated by arrowheads (Fig. 4C). Three-month M1 teeth from DSPP-KO mice showed complete destruction of the crown as indicated by arrows as well as defective roots as indicated by arrowheads (Fig. 4D). In μCT 2D gray-scale images, the distal root view from 21-day wt M1 teeth displayed normal enamel, dentin, and pulp chamber (Fig. 4E). An enamel-free area (EFA) was noticed in Figure 4E. EFA is reported as a region of dentin without any enamel covering on the occlusal surface of rodent molars [23]. The distal root view from 21-day DSPP-KO M1 teeth displayed enamel, much thinner dentin, and an enlarged pulp chamber (Fig. 4F). Dentin thickness was substantially reduced in 21-day DSPP-KO M1 teeth compared with control wt M1 teeth.
FIG. 4.
μCT images of mandibular first molar (M1) from wt and DSPP-KO mice. Panels (A–D) represent crown in occlusal view and root in buccal view. (A) Twenty-one-day wt M1. Note the intact crown and root. (B) Three-month wt M1. Note the intact crown and root. (C) Twenty-one-day DSPP-KO M1. Note the defective crown and root as indicated by arrowheads. (D) Three-month DSPP-KO M1. Note the defective roots and complete destruction of crown as indicated by arrow. Scale bar=200 μm. μCT 2D gray-scale sections from (E) 21-day wt M1 at distal root view, note the enamel-free area, and (F) 21-day DSPP-KO M1 at distal root view. Dentin thickness is substantially reduced in DSPP-KO mice compared with wt mice. Scale bar=1.0 mm. (G) μCT 2D gray-scale sections of 21-day wt M1. (H) μCT 2D gray-scale sections of 21-day DSPP-KO M1. Note dental pulp exposure (arrowhead) and circular dentin (indicated by *). Note the enlarged pulp chamber and the presence of enamel. Scale bar=100 μm. (I) Bar graphs of wt and KO mice M1 dentin density at postnatal 21-day and 3-month animals. Each bar represents dentin density as mg of HA/cm3 (see “Materials and Methods” section). Dentin density was significantly reduced in KO mice as compared with that of wt mice in both 21-day and 3-month animals. *P<0.05, n=6. HA, hydroxyapatite; μCT, microcomputed tomography.
Pulp exposure and circular dentin were detected in 21-day DSPP-KO first molar (M1) via μCT analysis
The μCT 2D gray-scale images of the crown from 21-day wt M1 teeth showed very thick dentin and a small pulp chamber (Fig. 4G). In contrast, the μCT 2D gray-scale images of the crown from 21-day DSPP-KO M1 teeth showed extremely thin dentin with pulp exposure in the cusp (Fig. 4H; indicated by arrowhead) and a large pulp chamber. Encircled dentin was also observed in this tissue section (indicated by *), which is in agreement with circular dentin structures observed in Figures 2 and 3E.
μCT analysis of dentin density in 21-day and 3-month wt and DSPP-KO first molar (M1) teeth
We used μCT to measure the dentin densities of 21-day wt M1 and DSPP-KO M1 teeth and of 3-month wt M1 and DSPP-KO M1 teeth. These data are presented in Figure 4I. We found that dentin mineral density was significantly reduced in 21-day DSPP-KO mice as compared with that of 21-day wt mice (P<0.05). The dentin mineral density in 3-month DSPP-KO M1 teeth was also significantly reduced as compared with that found in 3-month wt mice (P<0.05).
Developmental differences in dentin thickness between wt and DSPP-KO mice
Because dentin thickness is highly correlated to odontoblast secretory activity, we sought to investigate whether the lack of DSPP in DSPP-KO mice might affect dentin thickness during early tooth development. Our initial findings were that dentin in 1-day DSPP-KO M1 teeth (Fig. 1B) appeared to be well formed. However, no predentin or dentin was present in wt 1-day M1 teeth cusps from 50% of the animals tested (3/6) (Fig. 1A). The dentin in 6-day DSPP-KO M1 teeth (Fig. 1D) appeared thicker than that in 6-day wt M1 teeth (Fig. 1C). To better gauge dentin thickness during early developmental stages, we compared postnatal 1-, 6-, 13-, and 21-day M1 teeth from wt and DSPP-KO mice. The results are shown in Table 1. First molars obtained from postnatal 1-day DSPP-KO mice had well-formed thick dentin (ie, 27.4 μm) while no dentin was observed in 1-day wt M1 teeth from 50% of the animals tested (3/6). At postnatal 6 days, there was significantly more dentin formation in DSPP-KO MI teeth (ie, 46.8 μm) than in wt M1 teeth (ie, 30 μm; P<0.05). However, in postnatal 13-day teeth we found no significant difference in dentin thickness between DSPP-KO and wt M1 teeth. Surprisingly, dentin thickness in first-molar teeth at postnatal 21 days was significantly greater in wt M1 teeth (ie, 210 μm) as compared with 21-day DSPP-KO M1 teeth (ie, 110 μm; P<0.05). These results suggest that the rate of dentin formation is accelerated during early (ie, 1 and 6 days) postnatal molar tooth development in DSPP-KO animals and then levels off by 13 days and drops between postnatal 13 and 21 days.
Table 1.
Comparison of Dentin Thickness in wt and DSPP-KO First Molar from Postnatal 1 to 21 Days
| Postnatal day | wt Dentin thickness | DSPP-KO dentin thickness |
|---|---|---|
| 1 | No dentin in 3/6 animals | 27.44±2.83 μma |
| 6 | 30.11±4.48 μm | 46.78±2.39 μma |
| 13 | 85.22±4.76 μm | 93.78±20.4 μm N.S. |
| 21 | 210±40.57 μm | 110±22.1 μma |
Dentin thickness was measured from histological sections stained with hematoxylin and eosin from wt and DSPP KO from postnatal 1–21-day first molars. The dentin thickness from each time point was derived from six samples.
P<0.05 compared with wt dentin thickness.
DSPP, dentin sialophosphoprotein; KO, knockout; N.S., no significant difference; wt, wild-type.
Appearance of chondrocyte-like cells in 1- and 6-day DSPP-KO M1 and M2 dental pulp
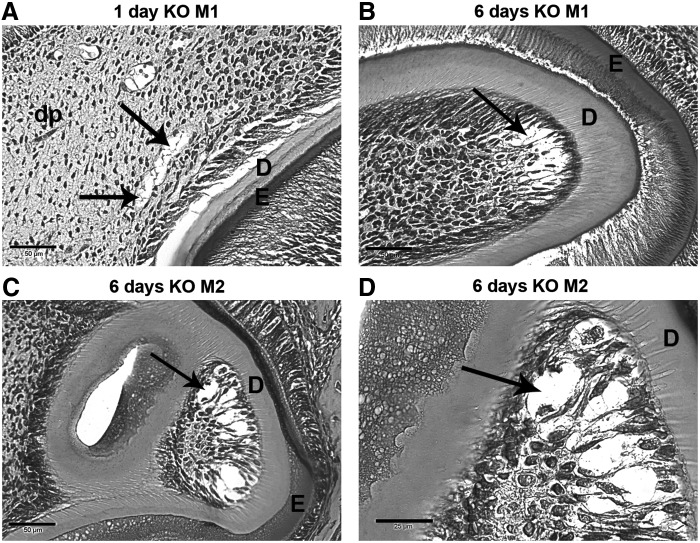
Columnar odontoblasts were located subjacent to the dentin layer in wt mouse M1 and M2 teeth (Fig. 2E, F). When we viewed highly magnified M1 and M2 teeth from DSPP-KO mice, we noticed the presence of what appeared to be chondrocyte-like cells in lacunae in the dental pulp and subjacent to the dentin layer (Fig. 5). For example, in 1-day M1 teeth (Fig. 5A, arrows) chondrocyte-like cells in lacunae are present in the dental pulp, while 6-day M1 teeth show these cells in lacunae subjacent to the dentin (Fig. 5B, arrow). Chondrocyte-like cells in lacunae are more easily seen in KO M2 teeth (Fig. 5C, D, arrows).
FIG. 5.
Appearance of chondrocyte-like cells in 1- and 6-day DSPP-KO M1 and M2 dental pulp. All samples were stained with H&E. (A) One-day DSPP-KO M1. Note chondrocyte-like cells in lacunae in the dental pulp (arrows). Scale bar=50 μm. (B) Six-day DSPP-KO M1. Note chondrocyte-like cells (arrow) in lacunae subjacent to the dentin. Scale bar=50 μm. (C) Six-day DSPP-KO M2. Note chondrocyte-like cells (arrow) in lacunae near the M2 cusp (400× magnification). Scale bar=50 μm. (D) The same section as in (C) at 1,000× magnification. Note chondrocyte-like cells in lacunae indicated by an arrow. Scale bar=25 μm.
Chondrocyte-like cells in postnatal 21-day DSPP-KO molars expressed collagen type II
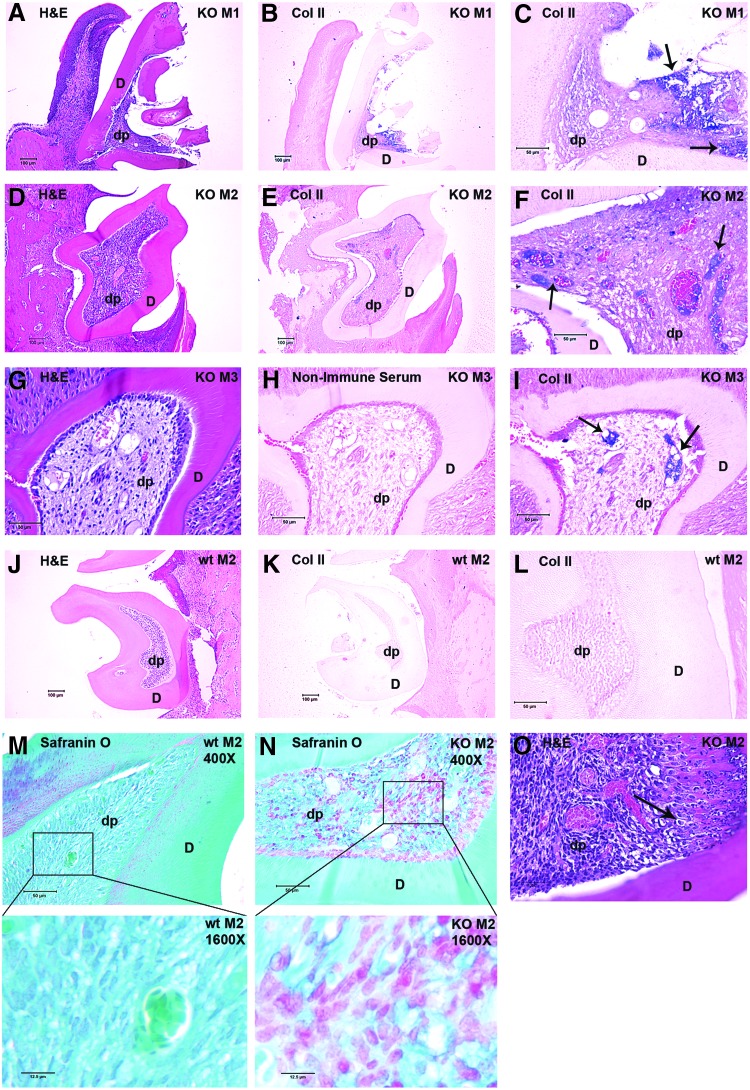
Given the appearance of chondrocyte-like cells in DSPP-KO M1 and M2 teeth, we next stained teeth obtained from 21-day wt and DSPP-KO mice with anti-collagen type II antibodies, to determine whether molar dental pulp cells express collagen type II, a chondrocyte marker [24]. In 21-day KO mice, H&E-stained M1 tooth sections show broken dentin with residual odontoblasts and abnormal dental pulp (Fig. 6A). Using anti-collagen type II antibodies, we find strong collagen type II staining (as shown by blue staining) in the dental pulp (Fig. 6B, C). H&E staining of 21-day DSPP-KO M2 teeth demonstrates an intact dentin layer (Fig. 6D). However, in subsequent sections from this tooth (not shown) the dentin layer was broken. The tightly packed cells within the dental pulp area may be the result of an influx of inflammatory cells that breached the broken dentin layer. Collagen type II (as shown by blue staining) is present in M2 dental pulp (Fig. 6E at 100× magnification and Fig. 6F at 400× magnification). The third-molar tooth (M3) is the last to develop and therefore the dentin layer in H&E-stained 21-day KO M3 sections appears to be intact (Fig. 6G). Using anti-collagen type II antibodies, blue-stained chondrocyte-like cells are easily identified in KO M3 tooth sections (Fig. 6I), indicating the presence of collagen type II. No staining is observed in KO tooth sections treated with nonimmune serum (Fig. 6H). No staining by anti-collagen II antibody is observed in wt 21-day M2 teeth (Fig. 6K, at 100× magnification; Fig. 6L, at 400× magnification). These wt control sections confirmed the specificity of our anti-collagen type II antibodies.
FIG. 6.
The presence of the chondrocyte marker collagen type II in 21-day DSPP-KO molars 1, 2, and 3, and acidic proteoglycan in 21-day DSPP-KO M2. Panels (A–C) represent samples from 21-day DSPP-KO molar 1 (M1). (A) H&E staining of M1 at 100× magnification. The dentin is broken and the dental pulp is exposed. Few dental pulp cells remain, but those present are mixed with extracellular-matrix-like material. (B) IHC staining of M1 section with anti-collagen II antibody (100× magnification). Many cells within the remaining dental pulp are positively stained (blue). (C) Same section at 400× magnification. Collagen type II staining was blue as indicated by arrows. Panels (D–F) represent samples from 21-day DSPP-KO M2. (D) H&E-stained M2 section at 100× magnification. Dentin is intact in this section, but was broken in later sections (data not shown). Inflammatory cells are present in dental pulp. (E) IHC staining of M2 section with anti-collagen type II antibody (at 100× magnification). (F) The same section at 400× magnification. Note the presence of positively stained cells. Collagen type II staining was blue as indicated by arrows. Panels (G–I) represent sections from 21-day DSPP-KO M3. (G) H&E-stained M3 section at 400× magnification. (H) IHC staining of M3 using nonimmune serum as primary antibody (control) at 400× magnification. No staining was detected. (I) IHC staining of KO M3 using anti-collagen II antibody at 400× magnification. Blue staining, as indicated by arrows, was detected in dental pulp, which indicates the presence of collagen type II. Panels (J–L) represent control sections from 21-day wt M2. (J) H&E-stained wt M2 section at 100× magnification. (K) IHC-stained wt M2 section using anti-collagen type II antibodies at 100× magnification. Note that no staining was detected. (L) IHC-stained wt M2 section using anti-collagen type II antibodies at 400× magnification. Note that no staining was detected. Panels (M–O) represent sections from 21-day wt M2 and 21-day DSPP-KO M2 samples. (M–N) Sections were stained with Safranin O/fast green. (M) No Safranin O staining was detected in the dental pulp of 21-day wt M2 section (at 400× magnification). (N) Safranin O staining was observed in the dental pulp of 21-day DSPP-KO M2 section (at 400× magnification). Distinct difference in Safranin O staining was observed between wt pulp and DSPP-KO pulp. (O) H&E-stained section from 21-day DSPP-KO M2 (at 400× magnification). Note the presence of fibrocartilage and chondrocyte-like cell in lacuna (see arrow). IHC, immunohistochemical.
Fibrocartilage was detected in 21-day DSPP-KO molars
Safranin O is widely used to stain acidic proteoglycan in cartilage [25]. Because we observed chondrocyte-like cells in DSPP-KO molars, we also used Safranin O/fast green staining to test whether acidic proteoglycan (another characteristic marker of cartilage) was present in 21-day DSPP-KO molars. Safranin O staining demonstrates the presence of acidic proteoglycan in the dental pulp of 21-day DSPP-KO M2 teeth (Fig. 6N at 400× magnification). In contrast, no Safranin O staining is observed in the dental pulp of 21-day wt M2 teeth (Fig. 6M at 400× magnification). H&E staining in 21-day DSPP-KO M2 teeth (Fig. 6O at 400× magnification) shows the presence of fibrocartilage as well as chondrocyte lacunae (see arrow in Fig. 6O).
Chondrocyte-specific marker Sox9 and collagen type II mRNAs are present in DSPP-KO incisors
Sox9 is required for the commitment of chondrogenic cell lineage and col type II is a chondrogenic marker gene [24,26]. Using RT-PCR analyses of wt and DSPP-KO-derived RNAs, we found that Sox9 mRNA is expressed in DSPP-KO incisors but not expressed in wt incisors. We also found that col II mRNA is expressed in DSPP-KO incisors but not expressed in wt incisors (Fig. 7).
FIG. 7.
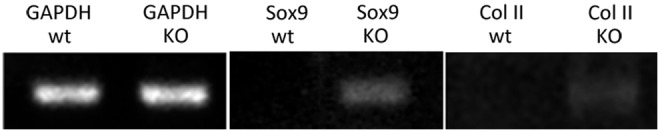
Sox9 and collagen type II mRNA expression in DSPP-KO mouse incisors. Dental pulps from 4-week-old wild type (WT) and DSPP-KO mouse incisors were cultured for 2 days. Total RNAs were extracted with Trizol and cDNA pools were generated with AMV reverse transcriptase. Primer sets for chondrocyte-specific markers were used to examine the presence of Sox9 and Col II. Sox9 primer set generated an expected 360-bp PCR fragment in DSPP-KO-cultured dental pulp. Col II primer set generated an expected 383-bp PCR fragment in DSPP-KO-cultured dental pulp. GAPDH was used as an internal control. No Sox9 and Col II mRNAs were detected in WT-cultured dental pulp. Col II, collagen type II. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PCR, polymerase chain reaction.
These data further confirm the presence of chondrocyte-like cells in the dental pulp of DSPP-KO mice. Therefore these results indicate that some dental pulp cells in 21-day DSPP-KO mice appear to differentiate into chondrocyte-like cells. Dental pulp stem cells are known to have the plasticity to convert into odontoblasts, osteoblasts, and chondrocytes under both in vitro and in vivo conditions [13–17]. The absence of DSPP in DSPP-KO mouse molars likely resulted in the appearance of chondrocyte-like cells, which is supported by the emergence of chondrocyte-shaped cells located inside the lacunae, the expression of collagen type II, and the presence of fibrocartilage as well as Sox9 and col II mRNA expression in the KO dental pulp. Taken together, these data suggest that DSPP protein expression is required for maintaining the odontoblast lineage and preventing stem cell differentiation into chondrocyte-like cells.
Discussion
The dental pulp consists of mesenchymal stem cells, which under normal developmental conditions differentiate to become odontoblasts [1–3] (and http://bite-it.helsinki.fi/). Our studies of DSPP protein suggest that this protein can control the lineage of the pulp mesenchymal cells. Using wt and DSPP-KO C57BL/6 mice our studies reported here suggest that DSPP is required for normal odontoblast lineage differentiation and that deletion of this protein results in the appearance of chondrocyte-like cells in the dental pulp.
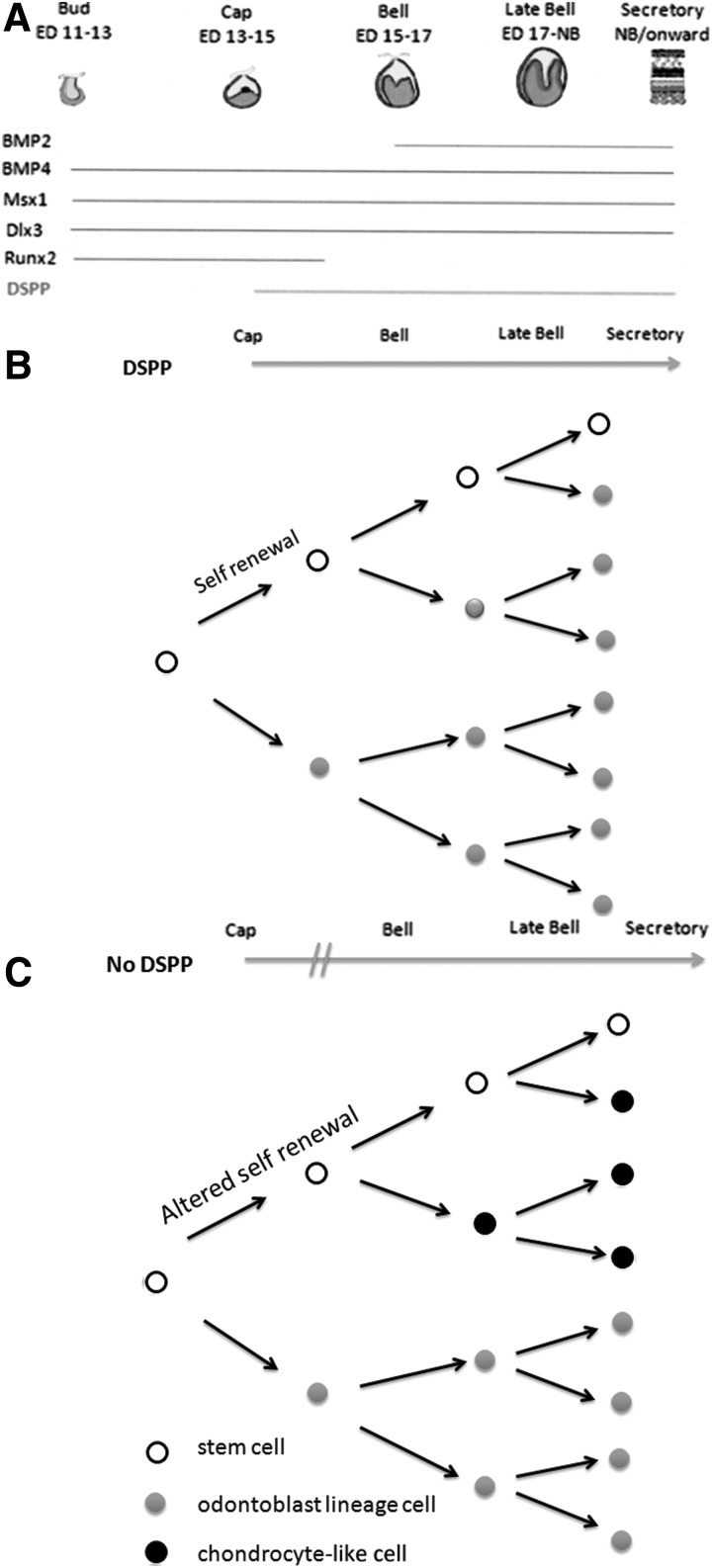
The key morphogens/growth factors that participate in E-M interaction include bone morphogenetic protein (BMP)4, as well as Wnt and sonic hedgehog (Shh) protein [27–32]. Specific transcription factors residing in mesenchymal cells that are necessary for maintaining dental E-M interactions leading to mature odontoblast formation (ie, odontoblastic lineage) include Runx2 and Msx1, as outlined in Figure 8A. Further upstream control, mediated by gene regulatory networks, most likely includes specific micro-RNAs (miRNAs) that target and thereby regulate the presence of these transcription factors, as has been shown for Runx2 expression in osteoblast mesenchymal lineage regulation [33,34]. Thus, the maintenance of the odontoblast lineage (and by extension normal tooth development) requires an exquisitely balanced dental molecular signaling apparatus.
FIG. 8.
A proposed model summarizing DSPP effects on odontoblast lineage differentiation. (A) Selective transcription factors that are expressed in mesenchymal cells during tooth development (see http://bite-it.helsinki.fi/). Transcription factors BMP4, Msx1, Dlx3, and Runx2 are first expressed at bud stage. Runx2 expression declines during bell stage. DSPP expression begins at the cap stage. BMP2 expression begins at the bell stage. (B) Proposed model showing that DSPP expression is likely required for dental pulp stem cell renewal and odontoblast lineage differentiation. Dental pulp stem cells initially produce odontoblast lineage cells, which secrete DSPP resulting in continued stem cell renewal, odontoblast lineage differentiation, and normal dentin formation. (C) In DSPP-KO mice, dental pulp stem cells initially produce odontoblast lineage cells, which synthesize collagen type I, Dmp1, and other dentin proteins but not DSPP protein. We propose that the loss of DSPP alters pulp stem cell renewal and results in the appearance of chondrocyte-like cells. In KO mice, both odontoblasts and chondrocyte-like cells are present in the dental pulp. BMP, bone morphogenetic protein.
Our initial findings from DSPP-KO mice show fewer dental pulp cells (Supplementary Fig. S2) and thicker dentin in 1- and 6-day molars (Fig. 1B, D) as compared with wt animals (Fig. 1A, C), and in addition there are more well-differentiated odontoblasts in KO mice (Fig. 1B, D), which correlates with increased dentin thickness. The data suggest that a more rapid rate of odontoblast differentiation occurs in late bell stage (ie, postnatal 1 day) and early secretory stage in DSPP-KO mice as compared with wt mice. Given that there are more differentiated odontoblasts present in KO mice compared with wt animals, there may be a disturbance in stem cell self-renewal such that more stem cells differentiate and the pool of self-renewing stem cells is depleted.
Postnatal 1-day DSPP-KO mice also displayed abnormal odontoblast and ameloblast layers; in that, they were positioned in the dental pulp and formed a circular structure in M2 samples (Fig. 2B). In 6-day KO M1 and M2 teeth, thick dentin was present and circular dentin was also visible (Fig. 2A, C). Abnormal cusp formation was found in some DSPP-KO molars with missing enamel and very thin dentin (Fig. 2G, H) making these teeth more vulnerable to pulp exposure. One- and 6-day DSPP-KO mice showed separated enamel and dentin layer (Fig. 1B, D). These findings suggest that during early development, a lack of DSPP in the KO mice resulted in abnormal E-M interactions.
DSPP may participate in dental pulp stem cell self-renewal. As shown in Table 1, dentin thickness in KO animals appears to plateau at 13 days, since at 21 days teeth from wt molars have approximately twice the dentin thickness as found in the DSPP-KO animals. Moreover, in DSPP-KO molars, chondrocyte-like cells appeared in the dental pulp area. This finding suggests that the absence of DSPP has altered the dental pulp stem cell fate, such that the stem cells can no longer maintain the odontoblast lineage differentiation program. These data lead us to propose a model to show that in wt animals, the constant supply of odontoblasts needed to sustain a continuous formation of dentin is dependent upon the presence of DSPP as depicted in Figure 8B. In DSPP-KO mice the number of dental pulp cells is reduced (Supplementary Fig. S2A), suggesting that loss of DSPP results in altered pulp stem cell self-renewal as proposed in Figure 8C. In turn, this will reduce the number of stem cells differentiating into odontoblasts available for tooth dentin formation, which we observed after postnatal day 13 and later at days 21 in DSPP-KO teeth that exhibited enlarged pulp chambers (Figs. 3 and 4E–H).
Studies that utilize BMP2 [35] KO mice also support our hypothesis (ie, that DSPP protein is required for normal odontoblast lineage development). For example, DSPP expression in preodontoblasts and during odontoblast lineage differentiation also coincides with BMP2, which is first expressed in early bell stage mesenchymal cells (Fig. 8A). BMP2 was demonstrated to upregulate DSPP expression. A BMP2-conditional KO [Bmp2-cKO(od)] mouse model was obtained by crossing Bmp2-floxed mouse (Bmp2-fx/fx) with a 3.6Col1a1-Cre mouse [35]. Teeth obtained from Bmp2-cKO(od) mice possess a large pulp chamber, thinner dentin, and less DSPP. This group concluded that BMP2 is required for odontoblast differentiation and for DSPP expression [35]. DSPP expression is also induced by Dlx3, which itself is expressed at the bud stage of tooth development. Dlx3-KO mice showed reduced DSPP expression, enlarged pulp chambers, and thinner dentin [36]. These Dlx3-KO mice also showed abnormal E-M interactions, similar to our results from DSPP-KO mice (Fig. 1B, D). Taken together, DSPP protein is required for proper E-M interactions and odontoblast lineage differentiation.
The mechanism illuminating chondrocyte-like cell appearance in dental pulp remains to be determined. Transforming growth factors (TGFs)β1–3 were reported to enhance chondrogenesis, in vitro [17,37–39] and in vivo [40]. It is known that TGFβ1–3 are expressed in dental pulp mesenchymal cells (http://bite-it.helsinki.fi/). In future studies, it would be interesting to examine how DSPP interacts with TGFβ during chondrogenesis in wt mice (ie, not DSPP-KO mice). For this kind of study, the wt mice will be treated with TGFβ. Alternatively, the absence of DSPP could affect the production of growth factors, which in turn might promote chondrogenic cell differentiation. For example, overexpression of Runx2 was reported to downregulate DSPP expression [41]. This suggests that there are direct or indirect interactions between DSPP and Runx2. It would be interesting to examine whether DSPP interacts with Runx2, and whether this interaction promotes the appearance of chondrocyte-like cells.
To summarize, a progression of developmental defects in DSPP-KO mice, starting with lowered cellular density inside the dental pulp, and abnormal dentinogenesis and amelogenesis at cusps, compromises the structural integrity of the enamel and dentin layers and leads to exposure of the dental pulp with subsequent bacterial infiltration and inflammation. The resultant dental pulp necrosis further decreases the odontoblast population, and damages the enamel and dentin layers in the tooth crown and root. Additionally, chondrocyte-like cells appear in the dental pulp from KO-mice teeth. Thus these studies comparing wt and DSPP-KO mice suggest that DSPP precursor protein is required for normal odontoblast lineage differentiation and absence of DSPP allows the appearance of chondrocyte-like cells. Thus, in DSPP-KO mice molars, neural-crest-derived pulp mesenchymal stem cells likely differentiate into odontoblasts and chondrocyte-like cells. Overall, our findings suggest that DSPP is a regulatory protein for dental pulp stem cell identity and fate.
Supplementary Material
Acknowledgments
The authors thank Dr. David G. Ritchie for helpful discussions and critiques during the preparation of this article. This work was supported by NIH DE18901 grant to H.H.R.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thesleff I. (2003). Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci 116:1647–1648 [DOI] [PubMed] [Google Scholar]
- 2.Thesleff I. (1991). Tooth development. Dental Update November:382–387 [PubMed] [Google Scholar]
- 3.Tucker A. and Sharpe P. (2004). The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet 5:499–508 [DOI] [PubMed] [Google Scholar]
- 4.D'Souza RN, Aberg T, Gaikward J, Cavender A, Owen M, Karsenty G. and Thesleff I. (1999). Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development 126:2911–2920 [DOI] [PubMed] [Google Scholar]
- 5.Satokata I. and Maas R. (1994). Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 6:348–356 [DOI] [PubMed] [Google Scholar]
- 6.Bleicher F, Couble M, Farges J, Couble P. and Maglorie H. (1999). Sequential expression of matrix protein genes in developing rat teeth. Matrix Biol 18:133–143 [DOI] [PubMed] [Google Scholar]
- 7.D'Souza RN, Cavender A, Sunavala G, Alvarez J, Oshima T, Kulkarni AB. and MacDougall M. (1997). Gene expression of murine dentin matrix protein 1 (Dmp1) and dentin sialoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res 12:2040–2049 [DOI] [PubMed] [Google Scholar]
- 8.Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Scott G, Bonewald LB. and Mishina Y. (2004). The dentin matrix protein 1 (Dmp1) is pecifically expressed in mineralized, but not soft tissues during development. J Dent Res 82:776–780 [DOI] [PubMed] [Google Scholar]
- 9.Godovikova V, Li X, Saunders T. and Ritchie H. (2006). A rat 8 kb DSP-PP promoter directs cell-specific lacZ activity in multiple mouse tissues. Dev Biol 289:507–516 [DOI] [PubMed] [Google Scholar]
- 10.Webb P, Moxham B, Ralphs J. and Benjamin M. (1998). Immunolocalisation of collagens in the developing rat molar tooth. Eur J Oral Sci 106Suppl 1:147–155 [DOI] [PubMed] [Google Scholar]
- 11.Ritchie HH, Yee CT, Tang XN, Dong ZH. and Fuller RS. (2012). DSP-PP precursor protein cleavage by tolloid-related-1 protein and by bone morphogenetic protein-1. PLoS One 7:e41110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linde A. (1989). Dentin matrix proteins: composition and possible functions in calcification. Anat Rec 224:154–156 [DOI] [PubMed] [Google Scholar]
- 13.Ariffin S, Kermani S, Wahab R, Senafi S, Ariffin Z. and Razak M. (2012). In vitro chondrogenesis transformation study of mouse dental pulp stem cells. ScientificWorldJournal 2012:827149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guimaraes E, Cruz G, de Jesus A, de Carvalho A, Rogatto S, Pereira L, Ribeiro-dos-Santos R. and Soares M. (2011). Mesenchymal and embryonic characteristics of stem cells obtained from mouse dental pulp. Arch Oral Biol 56:1247–1255 [DOI] [PubMed] [Google Scholar]
- 15.Miura M, Gronthos S, Zhao M, Lu B, Fisher L, Robey P. and Shi S. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S. and Gronthos S. (2003). Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 8:696–704 [DOI] [PubMed] [Google Scholar]
- 17.Sloan A. and Waddington R. (2009). Dental pulp stem cells: what, where, how?. Int J Paediatric Dent 19:61–70 [DOI] [PubMed] [Google Scholar]
- 18.Åberg T, Wang X, Kim J, Yamashiro T, Bei M, Rice R, Ryoo H. and Thesleff I. (2004). Runx2 mediates FGF signalling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol 270:76–93 [DOI] [PubMed] [Google Scholar]
- 19.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, MacDougall M, Sauk J. and Kulkarni AB. (2003). Dentin sialophosphoportein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentiogenesis imperfecta type III. J Biol Chem 278:24874–24880 [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Lim G, Dong Z, Lee A, Yee C, Fuller R. and Ritchie H. (2013). The efficiency of dentin sialoprotein-phosphophoryn processing is affected by mutations both flanking and distant from the cleavage site. J Biol Chem 288:6024–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Marschall Z. and Fisher L. (2010). Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix 2 products by three isoforms of bone morphogenetic protein-1 (BMP1). Matrix Biol 29:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamakoshi Y, Hu J, Iwata T, Kobayashi K, Fukae M. and Simmer J. (2006). Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J Biol Chem 281:38235–38243 [DOI] [PubMed] [Google Scholar]
- 23.Onishi T, Ooshima T, Sobue S, Tabata M, Kurisu K. and Wakisaka S. (2000). Calbindin D28k-like immunoreactivity during the formation of the enamel-free area in the rat molar teeth. Anat Rec 258:384–390 [DOI] [PubMed] [Google Scholar]
- 24.Akiyama H, Chaboissier M-C, Martin J, Schedl A. and de Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6 Genes Dev 16:2813–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg L. (1971). Chemical basis for the histological use of Safranin O in the study of articular cartilage. J Bone Joint Surg 53:69–82 [PubMed] [Google Scholar]
- 26.Akiyama H. (2008). Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol 18:213–219 [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Lan Y, Baek J, Gao Y. and Jiang R. (2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev biol 334:174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dassule H, Lewis P, Bei M, Maas R. and McMahon A. (2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Dev 127:4775–4785 [DOI] [PubMed] [Google Scholar]
- 29.Handrigan G. and Richman J. (2010). A network of Wnt, hedgehog and BMP signaling pathways regulates tooth replacement in snakes. Dev Biol 348:130–141 [DOI] [PubMed] [Google Scholar]
- 30.Koussoulakou D, Margaritis L. and Koussoulakos S. (2011). Antagonists of retinoic acid and BMP4 affect fetal mouse osteogenesis and odontoblast differentiation. Pathophysiology 18:103–109 [DOI] [PubMed] [Google Scholar]
- 31.Lin M, Li L, Liu C, Liu H, He F, Yan F, Zhang Y. and Chen Y. (2011). Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev Dyn 240:432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohazama A, Tucker A. and Sharpe P. (2005). Organized tooth-specific cellular differentiation stimulated by BMP4. J Dent Res 84:603–606 [DOI] [PubMed] [Google Scholar]
- 33.Hassan M, Gordon J, Beloti M, Croce C, van Wijnen A, Stein J, Stein G. and Lian J. (2010). A network connecting Runx2, SATB2, and the miR-23a∼27a∼24–2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci U S A 107:19879–19884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Xie R, Croce C, Stein J, Lian J, van Wijnen A. and Stein G. (2011). A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A 108:9863–9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W, Harris M, Cui Y, Mishina Y, Harris S. and Gluhak-Heinrich J. (2012). Bmp2 is required for odontoblast differentiation and pulp vasculogenesis. J Dent Res 91:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duverger O, Zah A, Isaac J, Sun H, Bartels A, Lian J, Berdal A, Hwang J. and Morasso M. (2012). Neural crest deletion of Dlx3 leads to major dentin defects through down-regulation of Dspp. J Biol Chem 287:12230–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry F, Boynton R, Liu B. and Murphy J. (2001). Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 268:189–200 [DOI] [PubMed] [Google Scholar]
- 38.Johnstone B, Hering T, Caplan A, Goldberga V. and Yo J. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238:265–272 [DOI] [PubMed] [Google Scholar]
- 39.Puetzer J, Petitte J. and Loboa E. (2010). Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 16:435–444 [DOI] [PubMed] [Google Scholar]
- 40.Bian L, Zhai D, Tous E, Rai R, Mauck R. and Burdick J. (2011). Enhanced MSC chondrogenesis following delivery of TGF-b3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32:6425–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Kong H, Yao N, Yu Q, Wang P, Lin Y, Wang J, Kuang R, Zhao X, et al. (2011). The role of runt-related transcription factor 2 (Runx2) in the late stage of odontoblast differentiation and dentin formation. Biochem Biophys Res Commun 410:698–704 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.