Abstract
Contracting muscle cells release K ions into their surrounding interstitial fluid, and some of these ions, in turn, enter venous plasma. Thereby, intense or exhaustive exercise may result in hyperkalemia and potentially dangerous cardiotoxicity. Training not only reduces hyperkalemia produced by exercise but in addition, highly conditioned, long-distance runners may show resting hypokalemia that is not caused by K deficiency. To examine the factors underlying these changes, dogs were studied before and after 6 wk of training induced by running on the treadmill. Resting serum [K] fell from 4.2 +/- 0.2 to 3.9 +/- 0.3 meq/liter (P less than 0.001), muscle intracellular [K] rose from 139 +/- 7 to 148 +/- 14 meq/liter (P less than 0.001), and directly measured muscle cell membrane potential (Em) in vivo rose from -92 +/- 5 to -103 +/- 5 mV (P less than 0.001). Before training, resting Em of isolated intercostal muscle in vitro was -87 +/- 5 mV, and after incubation in 10(-4) M ouabain, Em fell to -78 +/- 5 mV. After training, resting Em of intercostal muscle rose to -95 +/- 4, but fell to -62 +/- 4 mV during incubation in 10(-4) M ouabain. The measured value for the Em was not completely explained by the increased ratio of intracellular to extracellular [K] or by the potassium diffusion potential. Skeletal muscle sarcolemmal Na,K-ATPase activity (microM inorganic phosphate mg-1 protein h-1) increased from 0.189 +/- 0.028 to 0.500 +/- 0.076 (P less than 0.05) after training, whereas activities of Mg2+ -dependent ATPase and 5'nucleotidase did not change. In untrained dogs, exercise to the point of exhaustion elevated serum [K] from 4.4 +/- 0.5 to 6.0 +/- 1.0 meq/liter (P less than 0.05). In trained dogs, exhaustive exercise was associated with elevation of serum [K] from 3.8 +/- 0.3 to 4.2 +/- 0.4 (NS). The different response of serum [K] to exercise after training was not explainable by blood pH. Basal insulin levels rose from 7.0 +/- 0.7 microU/ml in the untrained dogs to 9.9 +/- 1.0 microU/ml (P less than 0.05) after training. Although insulin might have played a role in the acquired electrical hyperpolarization, the reduced exercise-produced hyperkalemia after training was not reversed by blockade of insulin release with somatostatin. Although the fundamental mechanisms underlying the cellular hyperpolarization were not resolved, our observations suggest that increased Na-K exchange across the sarcolemmal membrane, the increase of Na,K-ATPase activity and possibly increased electrogenicity of the sodium pump may all play a role in the changes induced by training.
Full text
PDF

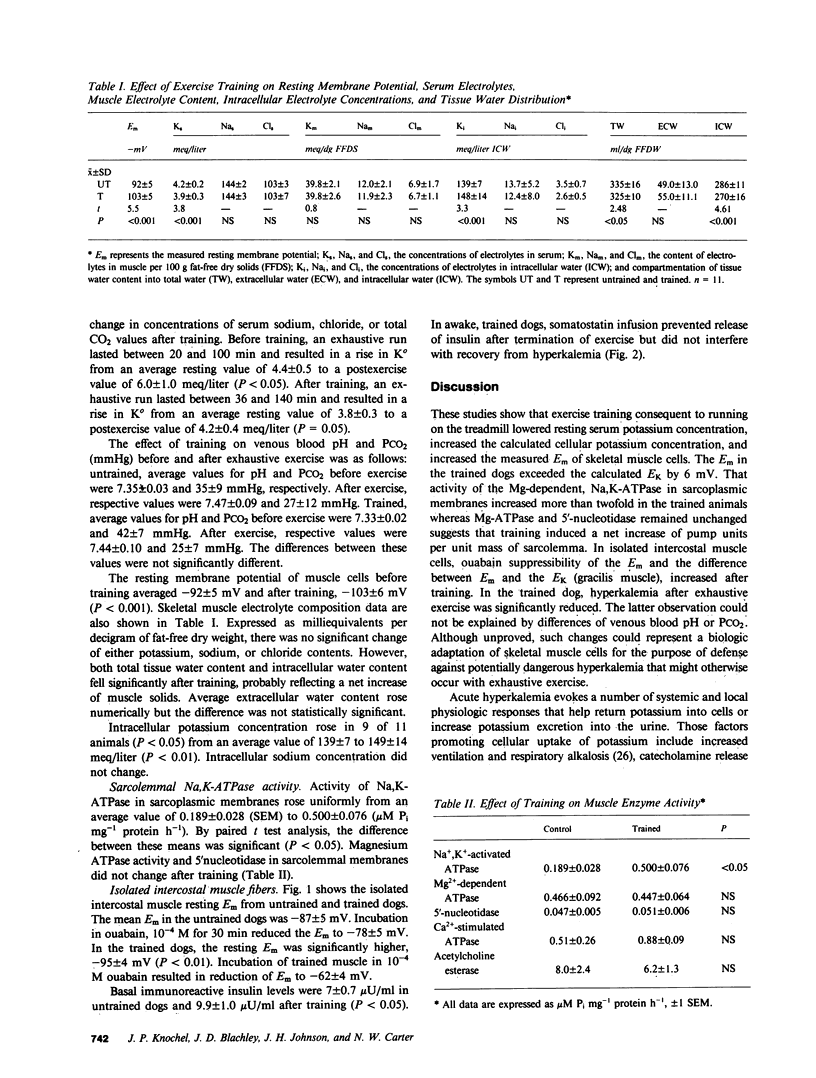
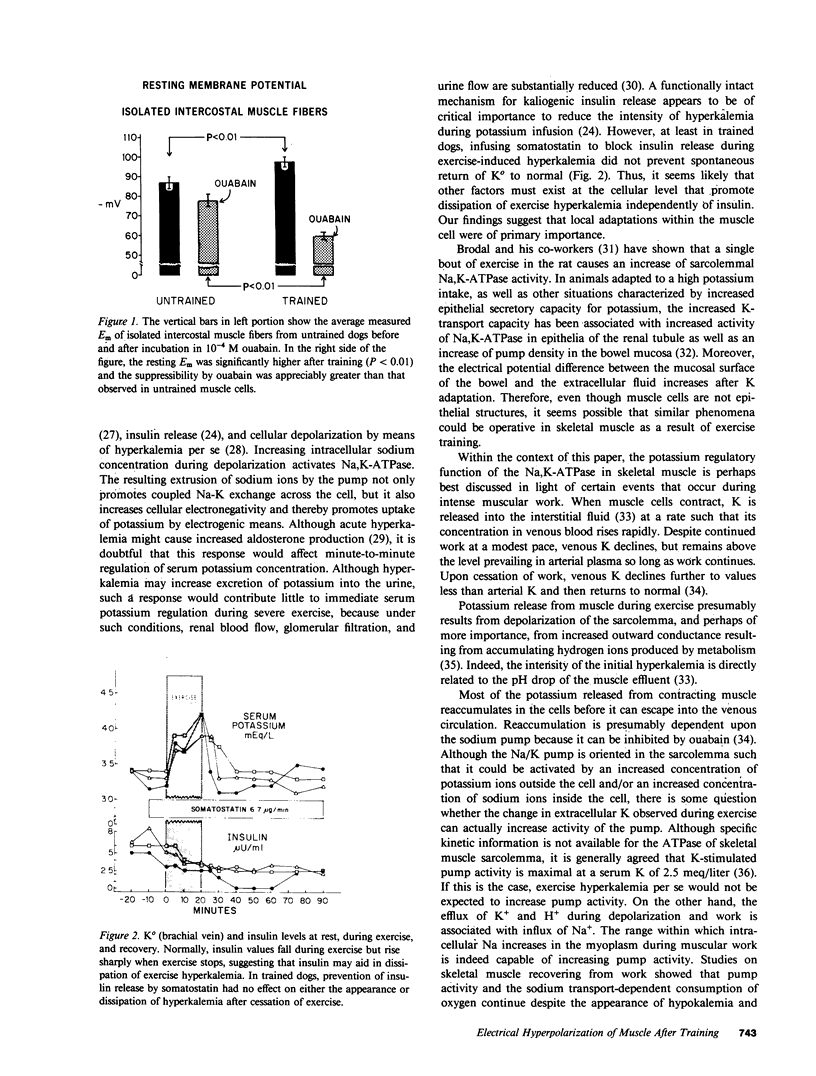


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrogué H. J., Madias N. E. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med. 1981 Sep;71(3):456–467. doi: 10.1016/0002-9343(81)90182-0. [DOI] [PubMed] [Google Scholar]
- Asano Y., Liberman U. A., Edelman I. S. Thyroid thermogenesis. Relationships between Na+-dependent respiration and Na+ + K+-adenosine triphosphatase activity in rat skeletal muscle. J Clin Invest. 1976 Feb;57(2):368–379. doi: 10.1172/JCI108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal B. P., Eeg-Larsen N. L., Iversen O. J., Jebens E., Roed A. Enhanced (Na+, K+)-activated ATPase activity after indirect electric stimulation of rat skeletal muscle in vivo. Life Sci. 1975 Aug 1;17(3):329–331. doi: 10.1016/0024-3205(75)90480-4. [DOI] [PubMed] [Google Scholar]
- Brodal B. P., Jebens E., Oy V., Iversen O. J. Effect of insulin on (Na+, K+)-activated adenosine triphosphatase activity in rat muscle sarcolemma. Nature. 1974 May 3;249(452):41–43. doi: 10.1038/249041a0. [DOI] [PubMed] [Google Scholar]
- Cannon P. J., Ames R. P., Laragh J. H. Relation between potassium balance and aldosterone secretion in normal subjects and in patients with hypertensive or renal tubular disease. J Clin Invest. 1966 Jun;45(6):865–879. doi: 10.1172/JCI105402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. C. Dependence of cellular potential on ionic concentrations. Data supporting a modification of the constant field equation. Biophys J. 1983 Aug;43(2):149–156. doi: 10.1016/S0006-3495(83)84335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Hansen O. Active Na-K transport and the rate of ouabain binding. The effect of insulin and other stimuli on skeletal muscle and adipocytes. J Physiol. 1977 Sep;270(2):415–430. doi: 10.1113/jphysiol.1977.sp011959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Kohn P. G. The effect of insulin on the transport of sodium and potassium in rat soleus muscle. J Physiol. 1977 Feb;265(1):19–42. doi: 10.1113/jphysiol.1977.sp011703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J. M., Srikant C. B., Ipp E., Schusdziarra V., Vale W., Unger R. H. Properties of endogenous somatostatin-like immunoreactivity and synthetic somatostatin in dog plasma. J Clin Invest. 1978 Dec;62(6):1187–1193. doi: 10.1172/JCI109238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton J. R., Woodard T., Carter N. W., Knochel J. P. Resting skeletal muscle membrane potential as an index of uremic toxicity. A proposed new method to assess adequacy of hemodialysis. J Clin Invest. 1979 Mar;63(3):501–506. doi: 10.1172/JCI109328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J. N., Jr, Carter N. W., Rector F. C., Jr, Seldin D. W. Resting transmembrane potential difference of skeletal muscle in normal subjects and severely ill patients. J Clin Invest. 1971 Jan;50(1):49–59. doi: 10.1172/JCI106483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S. The vaso-dilator action of potassium. J Physiol. 1941 Jan 14;99(2):224–238. doi: 10.1113/jphysiol.1941.sp003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlij D., Grinstein S. The number of sodium ion pumping sites in skeletal muscle and its modification by insulin. J Physiol. 1976 Jul;259(1):13–31. doi: 10.1113/jphysiol.1976.sp011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller T. J., Carter N. W., Barcenas C., Knochel J. P. Reversible changes of the muscle cell in experimental phosphorus deficiency. J Clin Invest. 1976 Apr;57(4):1019–1024. doi: 10.1172/JCI108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P., Myketey N., Binder H. J., Aronson P. S. Mechanism of increased potassium secretion in potassium loading and sodium deprivation. Am J Physiol. 1980 Oct;239(4):F378–F382. doi: 10.1152/ajprenal.1980.239.4.F378. [DOI] [PubMed] [Google Scholar]
- Hazeyama Y., Sparks H. V. A model of potassium ion efflux during exercise of skeletal muscle. Am J Physiol. 1979 Jan;236(1):R83–R90. doi: 10.1152/ajpregu.1979.236.1.R83. [DOI] [PubMed] [Google Scholar]
- Kendig J. J., Bunker J. P. Extracellular space, electrolyte distribution, and resting potential in K depletion. Am J Physiol. 1970 Jun;218(6):1737–1741. doi: 10.1152/ajplegacy.1970.218.6.1737. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitasato H., Sato S., Murayama K., Nishio K. The interaction between the effects of insulin and ouabain on the activity of Na transport system in frog skeletal muscle. Jpn J Physiol. 1980;30(1):115–130. doi: 10.2170/jjphysiol.30.115. [DOI] [PubMed] [Google Scholar]
- Knochel J. P., Dotin L. N., Hamburger R. J. Heat stress, exercise, and muscle injury: effects on urate metabolism and renal function. Ann Intern Med. 1974 Sep;81(3):321–328. doi: 10.7326/0003-4819-81-3-321. [DOI] [PubMed] [Google Scholar]
- Knochel J. P., Dotin L. N., Hamburger R. J. Pathophysiology of intense physical conditioning in a hot climate. I. Mechanisms of potassium depletion. J Clin Invest. 1972 Feb;51(2):242–255. doi: 10.1172/JCI106809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochel J. P. Role of glucoregulatory hormones in potassium homeostasis. Kidney Int. 1977 Jun;11(6):443–452. doi: 10.1038/ki.1977.62. [DOI] [PubMed] [Google Scholar]
- Knochel J. P., Schlein E. M. On the mechanism of rhabdomyolysis in potassium depletion. J Clin Invest. 1972 Jul;51(7):1750–1758. doi: 10.1172/JCI106976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto V. A., Soman V., Conrad P., Hendler R., Nadel E., Felig P. Insulin binding to monocytes in trained athletes: changes in the resting state and after exercise. J Clin Invest. 1979 Oct;64(4):1011–1015. doi: 10.1172/JCI109537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H., Salmon J. H. Cable parameters, sodium, potassium, chloride, and water content, and potassium efflux in isolated external intercostal muscle of normal volunteers and patients with myotonia congenita. J Clin Invest. 1971 Oct;50(10):2091–2103. doi: 10.1172/JCI106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie J. K., Leary W. P., Joubert S. M. Some electrocardiographic and biochemical changes recorded in marathon runners. S Afr Med J. 1967 Aug 5;41(29):722–725. [PubMed] [Google Scholar]
- Moore R. D. Effect of insulin upon the sodium pump in frog skeletal muscle. J Physiol. 1973 Jul;232(1):23–45. doi: 10.1113/jphysiol.1973.sp010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D. Stimulation of Na:H exchange by insulin. Biophys J. 1981 Feb;33(2):203–210. doi: 10.1016/S0006-3495(81)84881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. D., Ursick J. A., Maca R. D. Exercise and serum potassium flux: Myocardial metabolic implications. Recent Adv Stud Cardiac Struct Metab. 1972;1:673–683. [PubMed] [Google Scholar]
- Rose L. I., Carroll D. R., Lowe S. L., Peterson E. W., Cooper K. H. Serum electrolyte changes after marathon running. J Appl Physiol. 1970 Oct;29(4):449–451. doi: 10.1152/jappl.1970.29.4.449. [DOI] [PubMed] [Google Scholar]
- Santeusanio F., Faloona G. R., Knochel J. P., Unger R. H. Evidence for a role of endogenous insulin and glucagon in the regulation of potassium homeostasis. J Lab Clin Med. 1973 Jun;81(6):809–817. [PubMed] [Google Scholar]
- Seiler S., Fleischer S. Isolation of plasma membrane vesicles from rabbit skeletal muscle and their use in ion transport studies. J Biol Chem. 1982 Nov 25;257(22):13862–13871. [PubMed] [Google Scholar]
- Skou J. C. The (Na++K+) activated enzyme system and its relationship to transport of sodium and potassium. Q Rev Biophys. 1974 Jul;7(3):401–434. doi: 10.1017/s0033583500001475. [DOI] [PubMed] [Google Scholar]
- Tibes U., Hemmer B., Böning D., Schweigart U. Relationships of femoral venous [K+], PO2, osmolality, and [orthophosphate) with heart rate, ventilation, and leg blood flow during bicycle exercise in athletes and non-athletes. Eur J Appl Physiol Occup Physiol. 1976 Aug 12;35(3):201–214. doi: 10.1007/BF02336194. [DOI] [PubMed] [Google Scholar]
- Tibes U., Hemmer B., Schweigart U., Böning D., Fotescu D. Exercise acidosis as cause of electrolyte changes in femoral venous blood of trained and untrained man. Pflugers Arch. 1974 Jan 11;347(2):145–158. doi: 10.1007/BF00592396. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Hník P., Rehfeldt H., Vejsada R., Ujec E. The measurement of K+e concentration changes in human muscles during volitional contractions. Pflugers Arch. 1983 Nov;399(3):235–237. doi: 10.1007/BF00656721. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Effect of insulin on membrane potential and potassium content of rat muscle. Am J Physiol. 1959 Sep;197:515–523. doi: 10.1152/ajplegacy.1959.197.3.515. [DOI] [PubMed] [Google Scholar]



