Abstract
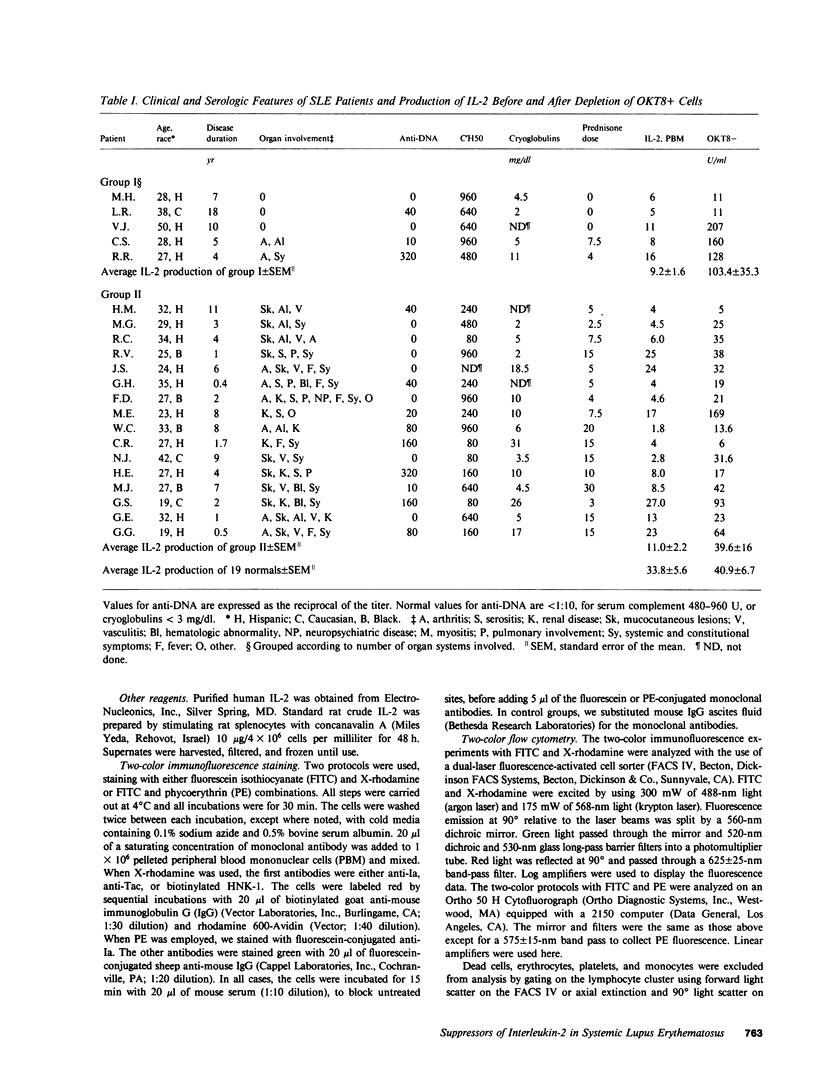
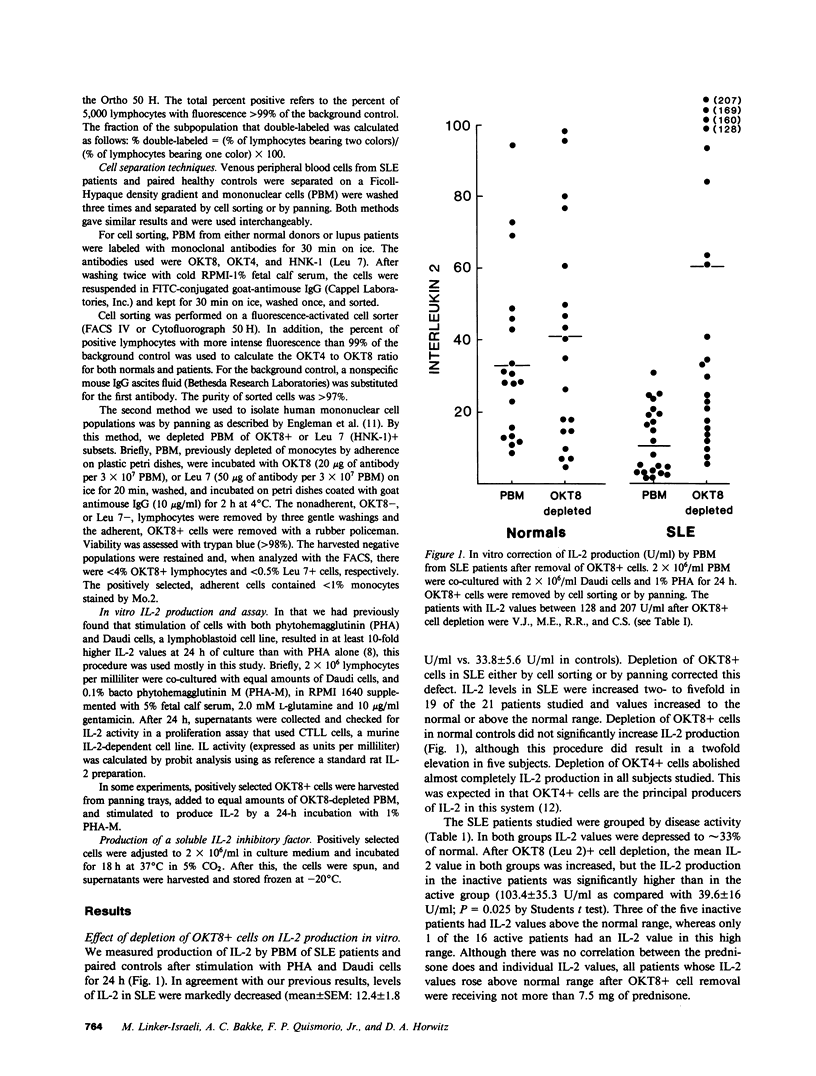
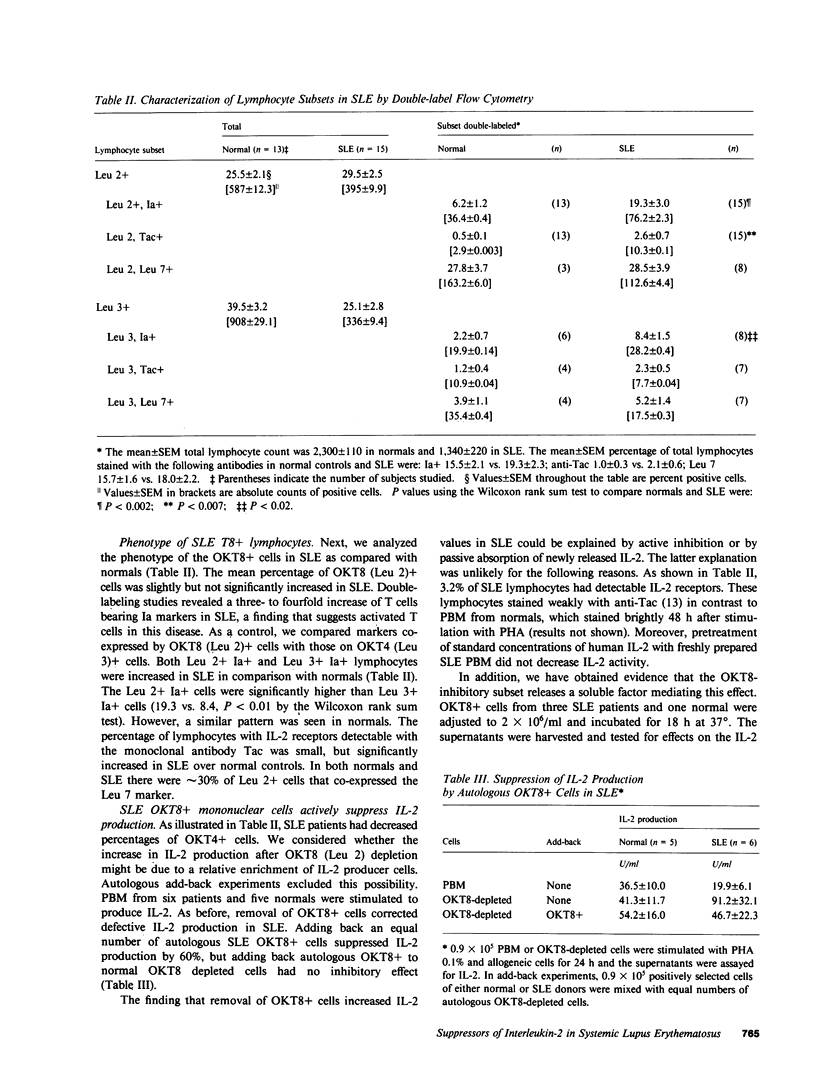
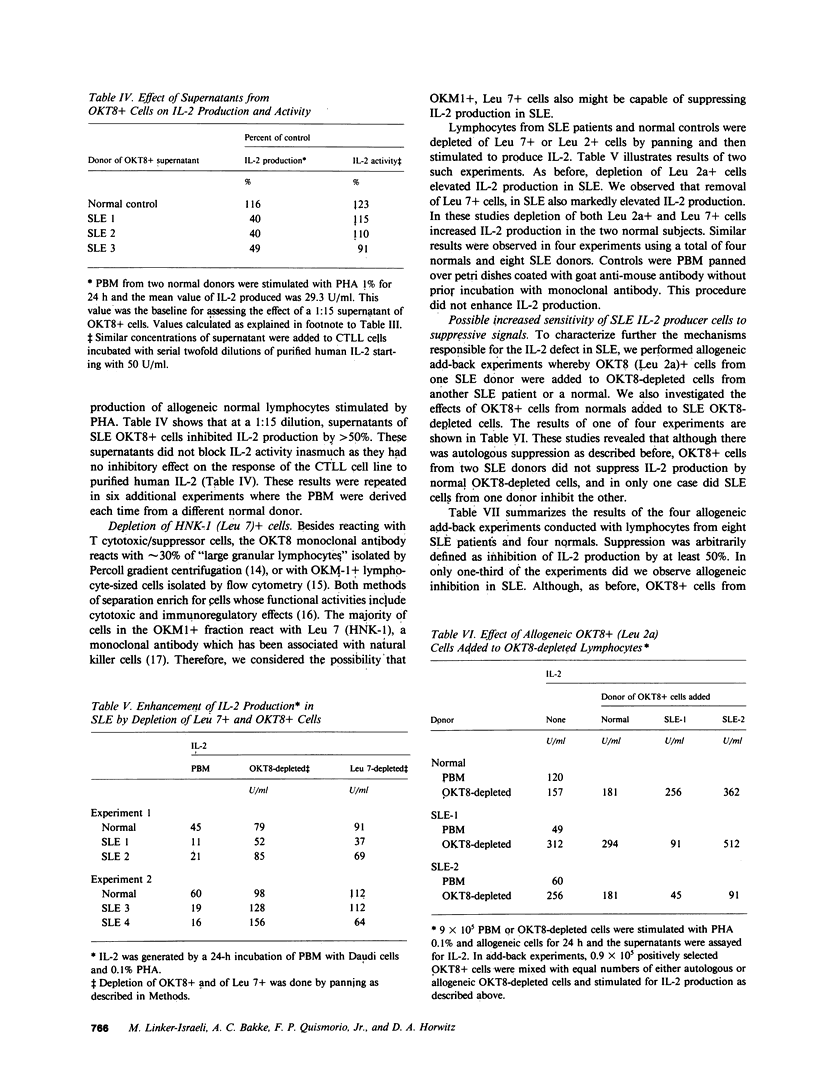
Interleukin-2 (IL-2) production in vitro is depressed in systemic lupus erythematosus (SLE) patients. It is not known whether this abnormality is caused by a defect in the producer lymphocytes or by excessive suppression. We report that removal of OKT8 (Leu 2a)+ cells increased the IL-2 production by in vitro-stimulated lymphocytes to normal or above normal levels in 19 of 21 SLE patients. This increase was more apparent in those patients with clinically inactive disease and/or receiving less than 7.5 mg of prednisone. Removal of OKT8+ cells from normals did not significantly increase IL-2 activity. SLE, but not normal, OKT8+ cells decreased IL-2 production when added back to autologous OKT8-depleted cells. In some experiments, OKT8+ cells from normal donors also suppressed IL-2 production in SLE. This result suggests that the defect in IL-2 production is complex and may involve multiple cell interactions. Three lines of evidence suggest that the SLE OKT8+ cells actively inhibit the production of IL-2 rather than passively absorb this lymphokine: (a) only 3.2% of SLE lymphocytes expressed IL-2 receptors as detected with anti-Tac; (b) freshly prepared SLE lymphocytes did not absorb IL-2; and (c) cell-free supernatants from SLE OKT8+ cells inhibited IL-2 production, but not IL-2 activity. Double-labeling studies by flow cytometry revealed that 19.3% of SLE OKT8+ cells were also Ia-positive, and approximately 33% co-expressed the natural killer cell marker, HNK-1 (Leu 7). Removal of Leu 7+ cells also significantly elevated IL-2 production in SLE. These studies suggest that one or more circulating mononuclear cell subsets in SLE patients can suppress IL-2 production and that one subset may possibly belong to a non-T, non-B "third mononuclear population."
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Alcocer-Varela J., Alarcón-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982 Jun;69(6):1388–1392. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azogui O., Gluckman E., Fradelizi D. Inhibiton of IL 2 production after human allogeneic bone marrow transplantation. J Immunol. 1983 Sep;131(3):1205–1208. [PubMed] [Google Scholar]
- Bakke A. C., Kirkland P. A., Kitridou R. C., Quismorio F. P., Jr, Rea T., Ehresmann G. R., Horwitz D. A. T lymphocyte subsets in systemic lupus erythematosus. Correlations with corticosteroid therapy and disease activity. Arthritis Rheum. 1983 Jun;26(6):745–750. doi: 10.1002/art.1780260607. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Fradelizi D. The mechanism of inhibition of human IL 2 production. J Immunol. 1982 Dec;129(6):2463–2468. [PubMed] [Google Scholar]
- Cohen P. L., Litvin D. A., Winfield J. B. Association between endogenously activated T cells and immunoglobulin-secreting B cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 1982 Feb;25(2):168–173. doi: 10.1002/art.1780250209. [DOI] [PubMed] [Google Scholar]
- Delfraissy J. F., Segond P., Galanaud P., Wallon C., Massias P., Dormont J. Depressed primary in vitro antibody response in untreated systemic lupus erythematosus. T helper cell defect and lack of defective suppressor cell function. J Clin Invest. 1980 Jul;66(1):141–148. doi: 10.1172/JCI109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Horwitz D. A., Stastny P., Ziff M. Circulating deoxyribonucleic acid--synthesizing mononuclear leukocytes. I. Increased numbers of proliferating mononuclear leukocytes in inflammatory disease. J Lab Clin Med. 1970 Sep;76(3):391–402. [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- Malkovsky M., Asherson G. L., Stockinger B., Watkins M. C. Nonspecific inhibitor released by T acceptor cells reduces the production of interleukin-2. Nature. 1982 Dec 16;300(5893):652–655. doi: 10.1038/300652a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Convit J., Rubinstein A., Bloom B. R. Activated suppressor T cells in leprosy. J Immunol. 1982 Nov;129(5):1946–1951. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Penta A. C., Fitzgerald K. A., Stadler B. M., Schlossman S. F., Reinherz E. L. Cellular origin of interleukin 2 (IL 2) in man: evidence for stimulus-restricted IL 2 production by T4+ and T8+ T lymphocytes. J Immunol. 1982 Sep;129(3):1076–1079. [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Schlossman S. F., Schur P. H., Mills J. A., Steinberg A. D. Alterations in immunoregulatory T cell subsets in active systemic lupus erythematosus. J Clin Invest. 1980 Nov;66(5):1171–1174. doi: 10.1172/JCI109948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff H., Carter C., Oppenheim J. J. Inhibition of concanavalin A-induced human lymphocyte mitogenic factor (Interleukin-2) production by suppressor T lymphocytes. J Immunol. 1980 Oct;125(4):1823–1828. [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B., Holden H. T. Natural cell-mediated cytotoxicity in mice against non-lymphoid tumor cells and some normal cells. Int J Cancer. 1977 Sep 15;20(3):381–387. doi: 10.1002/ijc.2910200309. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Puck J. M., Rich R. R. Regulatory interactions governing the proliferation of T cell subsets stimulated with pokeweed mitogen. J Immunol. 1984 Mar;132(3):1106–1112. [PubMed] [Google Scholar]
- Smolen J. S., Chused T. M., Leiserson W. M., Reeves J. P., Alling D., Steinberg A. D. Heterogeneity of immunoregulatory T-cell subsets in systemic lupus erythematosus. Correlation with clinical features. Am J Med. 1982 May;72(5):783–790. doi: 10.1016/0002-9343(82)90544-7. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tilden A. B., Abo T., Balch C. M. Suppressor cell function of human granular lymphocytes identified by the HNK-1 (Leu 7) monoclonal antibody. J Immunol. 1983 Mar;130(3):1171–1175. [PubMed] [Google Scholar]
- Tilden A. B., Balch C. M. A comparison of PGE2 effects on human suppressor cell function and on interleukin 2 function. J Immunol. 1982 Dec;129(6):2469–2473. [PubMed] [Google Scholar]
- Tsokos G. C., Balow J. E. Suppressor T cells in systemic lupus erythematosus: lack of defective in vitro suppressor cell generation in patients with active disease. J Clin Lab Immunol. 1982 Jun;8(2):83–90. [PubMed] [Google Scholar]


