Abstract
A botanical extract from Artemisia dracunculus L., termed PMI 5011, has been shown previously to improve insulin sensitivity by increasing cellular insulin signaling in in vitro and in vivo studies. These studies suggest that PMI 5011 effects changes in phosphorylation levels of proteins involved in insulin signaling. To explore effects of this promising botanical extract on the human skeletal muscle phosphoproteome, changes in site-specific protein phosphorylation levels in primary skeletal muscle cultures from obese, insulin resistant individuals were evaluated with and without insulin stimulation. Insulin resistance is a condition in which a normal or elevated insulin level results in an abnormal biologic response, e.g., glucose uptake. Using isobaric tagging for relative and absolute quantification (iTRAQ™) followed by phosphopeptide enrichment and liquid chromatography – tandem mass spectrometry, 125 unique phosphopeptides and 159 unique phosphorylation sites from 80 unique proteins were identified and quantified. Insulin stimulation of primary cultured muscle cells from insulin resistant individuals resulted in minimal increase in phosphorylation, demonstrating impaired insulin action in this condition. Treatment with PMI 5011 resulted in significant up regulation of 35 phosphopeptides that were mapped to proteins participating in the regulation of transcription, translation, actin cytoskeleton signaling, caveolae translocation and GLUT4 transport. These data further showed that PMI 5011 increased phosphorylation levels of specific amino acids in proteins in the insulin resistant state that are normally phosphorylated by insulin (thus, increasing cellular insulin signaling) and PMI 5011 also increased the abundance of phosphorylation sites of proteins regulating anti-apoptotic effects. Thus, the phosphoproteomics analysis demonstrated conclusively that PMI 5011 effects changes in phosphorylation levels of proteins and identified novel pathways by which PMI 5011 exerts its insulin sensitizing effects in skeletal muscle.
Keywords: skeletal muscle, insulin resistance, botanicals, quantitative phosphoproteomics, iTRAQ
Introduction
Insulin resistance is a key factor in the development of metabolic syndrome and type 2 diabetes mellitus (T2DM) and is characterized by normal or elevated insulin levels with reduced biological response [1]. Insulin resistance typically develops 5 – 10 years before the onset of diabetes and is therefore considered a pre-diabetic condition. Successful clinical interventions are an important tool to improve insulin sensitivity so as to reduce the risk of developing T2DM and improve glycemic control in those with Type 2 diabetes. In this regard, lifestyle modifications, i.e. weight loss and increased exercise, can improve insulin sensitivity on a clinical level; however, such changes are often difficult to maintain permanently. Pharmaceutical drugs are commonly used to improve insulin sensitivity but they are often accompanied with adverse side effects. In contrast, nutritional supplementation with botanical extracts is postulated as an alternative to pharmaceutical intervention. Many botanical extracts have been used historically for specific ailments and diseases [2,3], but there is a paucity of data on mechanisms of action for most botanicals. Due to their complex compositions, it is important to understand the full range of their biological effects and the efficacy of particular preparations.
Cultivars of the species Artemisia dracunculus L. find extensive culinary and medicinal use around the world [4]. Chemical compositions of Artemisia dracunculus L. cultivars vary greatly depending on the geographical origin of the plant source [4,5]. For example, French tarragon and Russian tarragon vary in their composition of essential oils which results in their different usage. Based on its aroma and anise-flavored taste, French tarragon is often used as culinary herb [4] whereas Russian tarragon is bitter and more often used in medicinal preparations that have anti-inflammatory, anti-cancer, anti-bacterial, anti-fungal, anti-hyperglycemic, and hypolipidaemic properties [4,6].
PMI 5011, an ethanolic extract from Russian tarragon (Artemisia dracunculus L.) is currently studied extensively to determine its composition and the resulting insulin sensitizing properties in vitro and in vivo [7-14]. Previous proteomics studies show that PMI 5011 treatment increases abundance of proteins involved in glycolysis pathway and increases glucose uptake and metabolism via enhanced translocation of glucose transporter 4 (GLUT4) into the plasma membrane. Both gel-based and gel-free proteomics analyses also showed that PMI 5011 exhibits anti-inflammatory action by reducing levels of proteins participating in the NFkB pathway [15,16]. As recognized, protein phosphorylation is an important post-translational modification that controls activation and deactivation of proteins and their subcellular localization to regulate metabolic processes. Targeted protein analysis and global gene expression studies suggest changes in protein phosphorylation levels and activity of skeletal muscle phosphatases are modulated by PMI 5011 [13,14]. To further understand and determine regulation of protein phosphorylation by PMI 5011, quantitative phosphoproteomic analysis of primary human skeletal muscle culture derived from obese, insulin resistant individuals was performed. Using isobaric tagging for relative and absolute quantification (iTRAQ™) combined with titanium dioxide based affinity chromatography enrichment, phosphorylated peptides and phosphorylation sites were identified and their abundance quantified using liquid chromatography – tandem mass spectrometry (LC-MS/MS). This approach allowed study of quantitative changes in the phosphoproteome of primary human skeletal muscle culture treated with PMI 5011 with or without insulin stimulation.
Material and Methods
Botanical Extract
Extracts from Artemisia dracunculus L. were produced from plants grown hydroponically in greenhouses maintained under uniform and strictly controlled conditions. Detailed information about the sourcing, growing conditions, quality control, stability, biochemical characterization and specific preparation of the Artemisia dracunculus L. extract (PMI 5011) tested in this study has been extensively reported [8,10]. Major compounds identified in the extract include chalcones and flavonoids [8,10].
Primary Human Skeletal Muscle Culture (HSkMC)
Primary HSkMC were prepared as described in detail previously [13,15]. Briefly, freshly removed muscle tissue from biopsies of vastus lateralis muscle from five obese diabetic patients was placed in Ham's F-10 media (HyClone Laboratories, Logan, UT) at 4 °C. After dissection, washing and centrifugation, it was placed in human skeletal growth medium (SkGM Bullet Kit, Cambrex). Cells were incubated at 37 °C with 95% air and 5% CO2, and myoblasts were subcultured and grown to 80 – 90 % confluence. Cells were then differentiated into fused myotubes by switching to culture media with 2% horse serum. After starvation, cells were treated with 10 μg/mL of PMI 5011 for 16 h. To evaluate effects of PMI 5011 on insulin signaling, cultures were stimulated with 100 nM insulin for five minutes prior to protein extraction. Thus, each experimental set included four HSkMC samples: baseline control, PMI 5011 treated, insulin stimulated control and insulin stimulated and PMI 5011 treated. All primary cultured cells used in this study were within five passages.
Sample Preparation
After treatments, culture media was removed and cultures were washed three times with ice cold phosphate buffered saline (PBS). Then, proteins from all culture samples were extracted by adding 1 mL of lysis buffer (5M Urea, 2M Thiourea, 2% CHAPS, 2% SB3-10, 0.2% Bio-Lyte (pH 3-10), 2% n-dodecyl-b-d-maltoside, 40 mM Tris, 5 mM PMSF, 2 mM TBP and 150U Benzonase) containing 3x final concentration of HALT phosphatase inhibitor (100x solution, Pierce Thermo Fisher, Rockford, IL) and 50 mM dithiothreitol (DTT) as described previously [15-17]. Samples were centrifuged for 30 min at 20,800 × g, and the supernatant was acetone precipitated and resolubilized in 0.5 M triethylammonium bicarbonate buffer (TEAB; pH 8.5) and 0.8 M urea. Protein concentrations were determined using Bradford Protein Assay (Bio-Rad, Hercules, CA).
iTRAQ Labeling
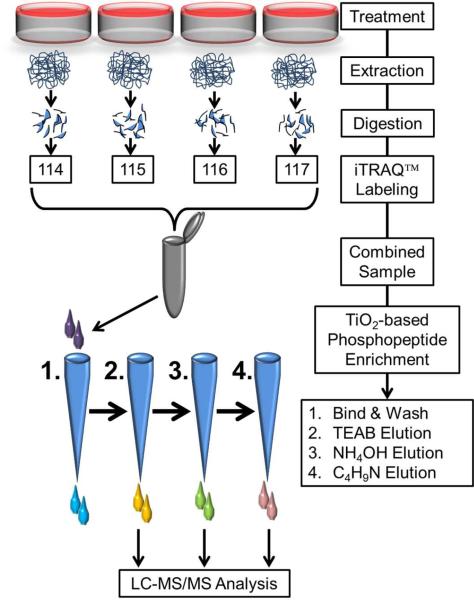
Fifty microgram of protein from each sample was digested with trypsin and labeled with iTRAQ™ labeling reagents as per manufacturer's instructions (AB SCIEX, Foster City, CA) [16]. Briefly, protein extracts in TEAB buffer (2 νg/μL) from each of the four HSkMC samples were denatured using 2% SDS and reduced with 50 mM Tris (2-carboxyethyl) phosphine (TCEP). Cysteine residues were blocked using 200 mM methyl methane thiosulfonate (MMTS). Samples were digested for 3 h using trypsin (Sequencing grade trypsin, Promega, Madison, WI) at an enzyme to substrate ratio of 1:50. A series of digestion times (30 min – 20 h) and enzyme to substrate ratios (1:25 – 1:100) were tested. The conditions used in the studies presented here proved to be most effective in achieving maximum phosphopeptide detection. All reagents for labeling were provided in the iTRAQ™ kit unless stated otherwise. Peptides derived from baseline control, PMI 5011 treated, insulin stimulated control and insulin stimulated and PMI 5011 treated samples were labeled with iTRAQ™ tags 114, 115, 116 and 117, respectively. After labeling, excess iTRAQ labels were hydrolyzed by addition of 100 μL of water to each sample. All four labeled samples were then combined to yield one 4plex iTRAQ™ sample which was concentrated under vacuum prior to phosphopeptide enrichment (Fig. 1).
Figure 1.
Schematic workflow of sample preparation for quantitative phosphoproteomics. Cultures were treated with or without PMI 5011 in the presence or absence of insulin and proteins were extracted and digested with trypsin. After iTRAQ™ labeling, the combined peptide samples were enriched for phosphopeptides. In order to avoid overloading of the TiO2 columns, the iTRAQ™ sample was split in four equal parts and loaded on four microcolumns. All four columns were first washed and then eluted sequentially with triethyl ammonium bicarbonate, ammonium hydroxide and pyrolidine solutions in acetonitrile. Same solvent elutions from the four columns were combined, dried under vacuum and resuspended in 97% H2O, 3% acetonitrile and 0.1% formic acid prior to mass spectrometry analysis.
Phosphopeptide Enrichment
Phosphopeptides from each 4plex iTRAQ™ sample set were enriched using TiO2 microcolumns (TitanSphere Phos-TiO Kit, GL Sciences). Prior to enrichment, four TiO2 microcolumns per 4plex iTRAQ™ sample were conditioned using wash buffer (80% acetonitrile and 5% trifluoroacetic acid (TFA)) followed by loading buffer. The 4plex iTRAQ™ sample was resuspended in loading buffer labeled as solution 1 (1M lactic acid, 80% acetonitrile with 5%, TFA) and loaded evenly onto the four conditioned columns by centrifugation (1000 × g for 10 min). Columns were washed twice using loading buffer and wash buffer, respectively. Phosphopeptides were then sequentially eluted from columns using 50% acetonitrile with 50 mM TEAB (solution 2), 50% acetonitrile with 500 mM ammonium hydroxide (solution 3) and 50% acetonitrile with 5% pyrrolidine (solution 4) (Sigma, St. Louis). All elutions were collected separately (Fig. 1). Elutions using solution 2 from all four columns were pooled. Similarly, elutions from solution 3 and from solution 4 from all four columns were pooled to generate three fractions per sample. These fractions were dried under vacuum and resuspended in 50 μL of H2O containing 3% acetonitrile and 0.1% formic acid prior to LC-MS/MS analysis.
LC-MS/MS
Individual fractions from TiO2 enrichment were analyzed using reverse phase liquid chromatography (2D nano LC, Eksigent, Dublin, CA) coupled to nano electrospray ionization source of a hybrid triple quadrupole linear ion trap mass spectrometer (4000 QTRAP, AB SCIEX). Peptides in each fraction were loaded on a SGE Reprosil C18-AQ 5 μm trap column and washed with 97% mobile phase A and 3% mobile phase B (mobile phase A: 0.1% formic acid; mobile phase B: 0.1% formic acid in acetonitrile) for 10 minutes at 2 μL/min. Phosphopeptides were then eluted from the trap column and separated on an Acclaim PepMap C18 analytical column (3 μm, 100 Å, 75 μm ID × 150 mm, Dionex) using a linear gradient of 3-40% mobile phase B over 150 minutes at 300 nL/min. Eluting peptides were introduced into the MS via nano-electrospray ionization source using a fused silica taper tip (20 μm tip diameter; New Objective, Woburn, MA). The mass spectrometer was operated in positive ion mode, interface heater temperature 150 °C, ion source voltage 2.7kV, declustering potential 80 V and collision energy spread of 5. Tandem mass spectra (MS/MS) were acquired using Analyst® 1.5.2 software (AB SCIEX) in an information dependent acquisition (IDA) mode using both dynamic fill time (DFT) and fixed fill time (FFT) of 20 ms with Q0 trapping. These acquisition modes are complementary and result in quantitative information for larger number of phosphopeptides than either of the methods alone. For both modes, enhanced MS scans were acquired from mass/charge (m/z) 400 to 1200 at 4000 Da/s followed by enhanced resolution scans at 250 Da/s to determine exact m/z and charge states. Enhanced product ion scans (MS/MS) from m/z 80-1500 at 4000 Da/s were triggered for the three most abundant ions between charge states 2 and 5. Rolling collision energies were applied based on charge states to promote fragmentation. All three phosphopeptide fractions were analyzed in triplicates using both DFT and FFT, resulting in 18 LC-MS/MS runs per 4plex iTRAQ™ set.
Mass Spectrometry Data Analysis
Mass spectrometry data were analyzed and searched against a UniProt/SwissProt database limited to human proteins (UniProt Release 2011_09 with 532,146 entries) using ProteinPilot™ 4.0 software (AB SCIEX). Search parameters were set as follows: quantification, iTRAQ™ labeled peptides; bias and background correction on to correct for isotopic impurities in the iTRAQ™ reporter ions; enzyme, trypsin; cysteine alkylation, MMTS; special factors, phosphorylation emphasis; and protein detection threshold > 0.05. Peptide identifications with a local false discovery rate (FDR) of < 5% were considered for further analysis. If necessary, mass spectra were investigated manually to verify correct assignment of phosphorylation sites. Correct assignments were verified by the presence of a 69/167 Da difference between fragments ions for phosphoserine and an 83/181Da difference for phosphothreonine. Phosphotyrosine peptides were validated by the presence of the immonium ion at 216 Da. Peptide sequences, phosphorylation sites, protein accession number, precursor mass to charge ratios, charge states and iTRAQ™ quantification results for each 4plex iTRAQ™ set were exported, merged and processed in Microsoft Excel 2007 (Microsoft) to yield one comprehensive dataset. SAS® software was utilized to calculate relative iTRAQ™ ratios for phosphopeptides detected two or more times. Ratios from iTRAQ™ tags were calculated for the following pair-wise comparisons to determine change in levels of the detected phosphopeptides: 1) baseline control vs. insulin stimulated control; 2) baseline control vs. PMI 5011 treated; 3) PMI 5011 treated vs. PMI 5011 treated and insulin stimulated; 4) insulin stimulated baseline control vs. PMI 5011 treated and insulin stimulated; and 5) baseline control vs. PMI 5011 treated and insulin stimulated. The student t-test was applied to calculate p-values to determine whether the phosphopeptides were significantly differentially expressed. Supplementary Table 1 includes protein accession number, protein name, phosphopeptide sequence, phosphorylation site, number of times the peptide was detected, relative ratios and p-values.
Cellular Signaling Network and Pathway Analysis
A protein list containing all phosphopeptides, protein accession numbers, relative fold change and p-values for all five comparisons were uploaded to Ingenuity Pathway Analysis (IPA) software to map proteins into biological networks and to retrieve their functions and subcellular locations. Only proteins matched to differentially regulated phosphopeptides (± 1.2 fold, p-values < 0.05) were considered for network and pathway analyses. Submitted accession numbers were assigned by the IPA software to their respective genes based on the curated Ingenuity Knowledge Base (IKB). The genes corresponding to the identified phosphoproteins were overlaid onto biological pathways developed from information contained in the IPKB. Networks of the identified genes were algorithmically generated based on their connectivity. Networks were scored based on the number of network eligible molecules within the specific network, size of the network, total number of network eligible molecules in the dataset and total number of molecules in the IPKB that could potentially be included in networks. The statistical significance values (p-value) for network and pathway analyses were calculated using right-tailed Fisher's Exact Test by the IPA software. These values are calculated based on the number of proteins that participate in a given pathway relative to the total number of occurrences of these proteins in all pathway annotations stored in the IPKB. These p-values provide a measure of the likelihood that proteins identified in our studies are associated with the pathways and networks due to random chance. Small p-values show that the association is significant, and it is less likely that the association is random. Generally, p-values less than 0.05 signify a statistically significant, non-random association.
Results
To identify effects of PMI 5011 on phosphoproteomics of HSkMC, iTRAQ™ labeled peptides from four treatment groups (baseline control, insulin stimulated control, PMI 5011 treated, PMI 5011 treated and insulin stimulated) were enriched using immobilized metal affinity chromatography (IMAC) and analyzed using LC-MS/MS (Fig. 1). iTRAQ™ ratios allowed comparison of levels of phosphopeptides in four treatment groups based on the abundance of their specific mass labels. In this study, 125 unique phosphopeptide sequences from 80 phosphoproteins (Supplementary Table 1) were identified. Of these, 79 peptides were singly phosphorylated, 38 were doubly phosphorylated, 7 were triply phosphorylated and 1contained four phosphorylation sites. In total we identified 159 unique phosphorylation sites of which 11 were not previously described in the literature. Peptide sequences, phosphorylation sites and representative tandem mass spectra of these novel phosphopeptides are presented in Supplementary Fig. 1.
Results from the five pair-wise comparisons (baseline control vs. insulin stimulated control; baseline control vs. PMI 5011 treated; PMI 5011 treated vs. PMI 5011 treated and insulin stimulated; insulin stimulated baseline control vs. PMI 5011 treated and insulin stimulated; and baseline control vs. PMI 5011 treated and insulin stimulated) were evaluated. 60 phosphopeptides were identified to be differentially regulated (±1.2 fold; p-value < 0.05) in at least one of the comparisons and were subjected to further analysis. These cut-off parameters were selected based on previous results using iTRAQ™ quantification [16]. A summary of the number of significantly up and down regulated phosphopeptides in each treatment comparison is provided in Table 1. Our results show that treatment with PMI 5011 induced the strongest response which resulted in up regulation of 35 phosphopeptides. In contrast, insulin stimulation of baseline control cultures alone induced up regulation of only 8 phosphopeptides (Table 1). These data are consistent with the insulin resistant state of the baseline culture and with our global proteomics studies demonstrating a minimal change in protein expression levels due to insulin treatment of the baseline cultured muscle cells.
Table 1.
Number of Differentially Regulated Phosphopeptides
| Numbers of Differentially Regulated Phosphopeptides (± ± 1.2 fold, p<0.05) |
|||
|---|---|---|---|
| Treatment Comparison | Total | Up-Regulated | Down-Regulated |
| Baseline Control vs. Insulin Stimulated Control | 11 | 8 | 3 |
| Baseline Control vs. PMI 5011 Treated | 39 | 35 | 4 |
| PMI 5011 Treated vs. PMI 5011 Treated and Insulin Stimulated | 31 | 7 | 24 |
| Insulin Stimulated Control vs. PMI 5011 Treated and Insulin Stimulated | 19 | 6 | 13 |
| Baseline Control vs. PMI 5011 Treated and Insulin Stimulated | 15 | 12 | 3 |
Cellular location and function of all identified phosphoproteins were retrieved by IPA software. Molecular and cellular functions and cellular locations of matched phosphoproteins determined using IPA clearly show that our extraction of proteins and enrichment of phosphopeptides was not biased toward any peptide species, functional classes or sub-cellular locations (Supplementary Figure 2A and 2B).
Using this list of phosphoproteins, IPA identified networks and canonical pathways that are most significant to this dataset. This analysis determined that of the 60 differentially regulated phosphopeptides, 41 phosphopeptides are in the proteins participating in canonical pathways such as AKT/insulin signaling, transcription and translation, actin cytoskeleton signaling, caveolar and vesicle transport, and regulation of apoptosis. A list of these 41 significantly differentially regulated phosphopeptides and the level of change with respect to treatment is provided in Table 2. In this subset of 41 significantly differentially regulated phosphopeptides, treatment with PMI 5011had the strongest effect on phosphorylation levels. 24 phosphorylation sites were significantly up regulated after treatment of the baseline cultures with the botanical extract. Phosphorylation of EIF2S2 at aspartic acid (D4) identified to be significantly differentially regulated due to insulin stimulation and due to botanical treatment has not been reported in the literature. A representative tandem mass spectrum (MS/MS) confirming the identified phosphorylation of aspartic acid site with high confidence is included in Supplementary Fig. 1. Peptides containing novel phosphorylation sites in both Cavin 3 and A-kinase anchor protein 12 are also upregulated due to PMI 5011 treatment. Thus, in addition to providing insight on the mechanisms by which PMI 5011 improves insulin resistance, the phosphoproteomics data presented here have also uncovered novel phosphorylation sites potentially involved in insulin signaling.
Table 2.
Differentially regulated phosphopeptides involved in AKT/insulin signaling (1), transcription/translation (2), actin cytoskeleton signaling (3), caveolar and vesicle transport (4) and regulation of apoptosis (5).
| Function | ID | Protein Name | Phosphorylation Site in Protein | Baseline Control vs. Insulin Stimulated Control | Baseline Control vs. PMI 5011 Treated | PMI 5011 Treated vs. PMI 5011 Treated and Insulin Stimulated | Insulin Stimulated Control vs. PMI 5011 Treated and Insulin Stimulated | Baseline Control vs. PMI 5011 Treated and Insulin Stimulated |
|---|---|---|---|---|---|---|---|---|
| 1 | P04792 | Heat shock protein beta-1 (HSPB1) | S(82) | 1.00 | 1.24 | −1.02 | 1.22 | 1.22 |
| 1 | P07900 | Heat shock protein HSP 90-alpha (HSP90AA1) | S(263) | 1.05 | 1.27 | −1.10 | 1.11 | 1.16 |
| 1 | P08670 | Vimentin | S(39) | 1.01 | −1.03 | 1.30 | 1.24 | 1.26 |
| 1 | P50502 | Hsc70-interacting protein | S(75), S(76), S(79) | 1.23 | 1.42 | −1.41 | −1.22 | 1.01 |
| 1 | Q02952 | A-kinase anchor protein 12 (AKAP12) | S(645), S(648), S(651) | 1.25 | 1.33 | −1.22 | −1.15 | 1.09 |
| 1 | Q15121 | Astrocytic phosphoprotein PEA-15 | S(116) | −1.03 | 1.23 | −1.09 | 1.16 | 1.12 |
| 1 | P02765 | Alpha-2-HS-glycoprotein | S(138) | 1.45 | 1.00 | 1.30 | −1.11 | 1.30 |
| 2 | O95218 | Zinc finger Ran-binding domain protein 2 | S(153) | 1.22 | 1.52 | −1.28 | −1.03 | 1.18 |
| 2 | P05387 | 60S acidic ribosomal protein P2 | S(101), S(104) | 1.03 | 1.37 | −1.13 | 1.18 | 1.21 |
| 2 | P05388 | 60S acidic ribosomal protein P0 | S(304), D(317) | 1.11 | 1.03 | 1.23 | 1.15 | 1.27 |
| 2 | P07910 | Heterogeneous nuclear ribonucleoproteins C1/C2 | S(260) | −1.28 | −1.58 | 1.29 | 1.04 | −1.23 |
| 2 | P17096 | High mobility group protein HMG-I/HMG-Y | S(102), S(103) | −1.33 | −1.10 | −1.05 | 1.15 | −1.16 |
| 2 | P19338 | Nucleolin | S(145), S(153) | −1.11 | 1.37 | 1.02 | 1.55 | 1.40 |
| 2 | P29692 | Elongation factor 1-delta (EEF1D) | S(162) | 1.02 | 1.22 | −1.19 | 1.01 | 1.03 |
| 2 | P29692 | Elongation factor 1-delta (EEF1D) | T(147), S(162) | −1.02 | 1.21 | −1.33 | −1.07 | −1.09 |
| 2 | Q13263 | Transcription intermediary factor 1-beta | S(19) | −1.18 | −1.95 | −1.27 | −2.10 | −2.48 |
| 2 | Q13283 | Ras GTPase-activating protein-binding protein 1 | S(232) | 1.11 | 1.19 | −1.33 | −1.24 | −1.12 |
| 2 | Q15185 | Prostaglandin E synthase 3 | S(113) | 1.33 | 1.42 | −1.39 | −1.30 | 1.02 |
| 2 | Q15637 | Splicing factor 1 | S(80), S(82) | −1.14 | −1.65 | 1.60 | 1.11 | −1.03 |
| 2 | P20042 | Eukaryotic translation initiation factor 2 subunit 2 (EIF2S2) | D(4) | 1.44 | 1.82 | −1.58 | −1.24 | 1.16 |
| 2 | O76021 | Ribosomal L1 domain-containing protein 1 | S(361) | 2.99 | 1.31 | −1.32 | −3.02 | −1.01 |
| 3 | P46821 | Microtubule-associated protein 1B (MAP1B) | S(831), S(832) | 1.01 | 1.24 | −1.04 | 1.18 | 1.19 |
| 3 | P50479 | PDZ and LIM domain protein 4 | S(112) | 1.11 | 1.27 | −1.31 | −1.15 | −1.04 |
| 3 | Q66K74 | Microtubule-associated protein 1S (MAP1S) | T(638), S(640) | 1.08 | 1.34 | −1.53 | −1.24 | −1.15 |
| 3 | Q9UHB6 | LIM domain and actin-binding protein 1 (LIMA1) | S(686) | 1.19 | 1.28 | −1.04 | 1.04 | 1.23 |
| 4 | O43493 | Trans-Golgi network integral membrane protein 2 | S(71) | 1.13 | 1.38 | −1.35 | −1.11 | 1.02 |
| 4 | O60763 | General vesicular transport factor p115 | S(942) | −1.06 | 1.45 | −1.37 | 1.12 | 1.06 |
| 4 | P08648 | Integrin alpha-5 | S(127) | −1.24 | −1.21 | 1.27 | 1.29 | 1.04 |
| 4 | P13861 | cAMP-dep. protein kinase II-a regulatory subunit | S(78), S(80) | 1.17 | −1.06 | 1.02 | −1.22 | −1.04 |
| 4 | P27824 | Calnexin | S(583) | 1.04 | 1.25 | −1.23 | −1.02 | 1.02 |
| 4 | P35606 | Coatomer subunit beta (COBP2) | S(859) | 1.08 | −1.56 | 1.20 | −1.40 | −1.30 |
| 4 | P51636 | Caveolin-2 | S(20), S(23) | 1.02 | 1.39 | −1.54 | −1.13 | −1.11 |
| 4 | Q14247 | Src substrate cortactin | T(401), S(405) | 2.13 | −1.21 | 1.16 | −2.21 | −1.04 |
| 4 | Q14315 | Filamin-C | S(2233) | 2.19 | 1.26 | 2.01 | 1.15 | 2.53 |
| 4 | Q6NZI2 | Polymerase I and transcript release factor | S(300) | −1.31 | 1.08 | −1.42 | −1.00 | −1.32 |
| 4 | Q969G5 | Protein kinase C delta-binding protein (Cavin 3) | S(165), S(166) | 1.36 | 1.71 | −1.51 | −1.21 | 1.13 |
| 4 | Q969G5 | Protein kinase C delta-binding protein (Cavin 3) | S(151), S(166) | 1.42 | 2.57 | −1.76 | −1.01 | 1.56 |
| 4 | Q969G5 | Protein kinase C delta-binding protein (Cavin 3) | S(151), S(165) | 1.15 | 1.57 | −1.76 | −1.29 | −1.12 |
| 5 | P25788 | Proteasome subunit alpha type-3 | S(250) | −1.11 | 1.26 | −1.33 | 1.05 | −1.05 |
| 5 | P46937 | Yorkie homolog (YAP) | S(105), S(109) | 1.23 | 1.78 | −1.82 | −1.26 | −1.02 |
| 5 | Q9H3N1 | Thioredoxin-related transmembrane protein 1 | S(247) | −1.14 | 1.22 | −1.37 | 1.02 | −1.12 |
Shaded values signify statistically significant ratios (p-value < .05).
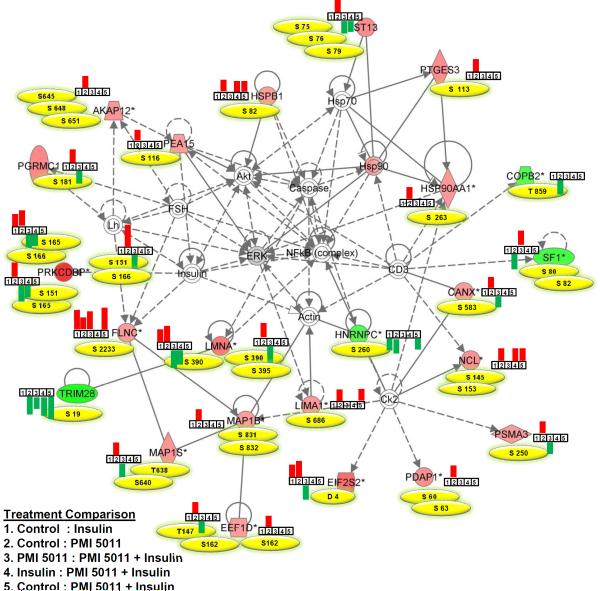
To help identify mechanistically relevant relationships between the phosphopeptides identified in this study, IPA identified several inter-related networks related to cellular assembly, organization, function, maintenance and death. Figure 2 presents the highest scoring network containing 23 phosphoproteins significantly differentially regulated due to PMI 5011 treatment of the baseline control culture. Figure 2 also lists phosphorylation sites identified in each protein in the network and bar graphs showing whether a phosphopeptide was up or down-regulated based on a specific treatment. Phosphopeptides from only 5 proteins involved in this network were differentially regulated after insulin stimulation of baseline control samples. In contrast, treatment with PMI 5011 resulted in significant up regulation of phosphorylation levels of specific amino acids in 19 proteins in insulin resistant state that are normally phosphorylated by insulin. In addition, this network included NFkB, insulin, Akt, actin and heat shock proteins consistent with our global proteomics data [16] demonstrating that PMI 5011 modulates phosphorylation levels of proteins related to inflammatory networks and insulin signaling previously identified in proteomic analyses.
Figure 2.
Top connectivity network with p-value 10−65 showing changes in expression of phosphopeptides involved in NFκB network in basal and insulin stimulated states with and without PMI 5011 treatment. Proteins labeled with filled red or green colors were identified to contain differentially regulated phosphopeptides in PMI 5011 treated baseline control cultures. All other proteins are imported from the IPA knowledge base. Phosphorylation sites identified in each protein in the network are listed in yellow, and bar graphs show whether a phosphopeptide was up or down-regulated based on a specific treatment comparison. Up-regulated and down-regulated peptides are presented in red and green, respectively. A line indicates interactions, and the dotted lines indicate an inferred or indirect interaction. The abbreviations are: AKAP12: A kinase (PRKA) anchor protein 12, CANX: calnexin, COBP2: coatomer subunit beta, EEF1D: eukaryotic translation elongation factor 1 delta, EIF2S2: eukaryotic translation initiation factor 2 subunit 2, FLNC: filamin C, HNRNPC: heterogeneous nuclear ribonucleoprotein C, HSP90AA1: heat shock protein 90kDa alpha, HSPB1: heat shock 27kDa protein 1, LIMA1: LIM domain and actin binding 1, LMNA: lamin A/C, MAP1B: microtubule-associated protein 1B, MAP1S: microtubule-associated protein 1S, NCL: nucleolin, PDAP1: PDGFA associated protein 1, PEA15: phosphoprotein enriched in astrocytes 15, PGRMC1: progesterone receptor membrane component 1, PRKCDBP: protein kinase C, delta binding protein (Cavin-3), PSMA3: proteasome subunit alpha 3, PTGES3: predicted pseudogene 9769, SF1: splicing factor 1, ST13: Hsp70 interacting protein, TRIM28: tripartite motif containing 28.
Discussion
Insulin action in skeletal muscle and eventual glucose uptake is accomplished via effective insulin signaling initiated by binding of insulin to the transmembrane receptor followed by a cascade of intracellular molecular signaling pathways. One of the signaling proteins, e.g., phosphatidylinositol 3-kinase (PI3-K), binds to insulin receptor substrate (IRS) and further activates 3-phosphoinositide-dependent protein kinase 1 (PDK1), which activates Akt, a serine kinase. Akt activates glycogen synthesis and also stimulates glucose uptake by enhancing the translocation of glucose transporters (GLUT 4) to the cell surface. This is a rate limiting step for glucose uptake and further processing by glucose oxidation and glycogen synthesis. Attenuation in uptake and metabolism of glucose is a key feature of the skeletal muscle insulin resistance [1,18,19].
Our previous in vivo and in vitro studies have demonstrated that PMI 5011 lowers blood glucose and insulin levels and improves insulin receptor signaling (i.e. increased AKT phosphorylation, reduced protein tyrosine phosphatase 1B levels and increased PI3-K activity) [13,14]. To evaluate the molecular mechanisms resulting in enhanced insulin sensitizing effects of PMI 5011, both gel-based and gel-free global proteomics methods were utilized [15,16]. These studies demonstrated that the baseline control cultures had attenuation in insulin stimulation consistent with the phosphoproteomics data summarized in Table 1. Insulin-dependent phosphorylation generally occurs within the first five minutes of insulin stimulation [13,20,21]. Very little change in phosphorylation levels of proteins due to insulin stimulation of baseline control cultures was observed in the studies presented here. A strong effect on phosphorylation levels of proteins was obtained after insulin stimulation of primary cultured myotubes treated with PMI 5011. Primary HSkMC generated from biopsied skeletal muscle tissue from human subjects retain the intrinsic characteristics of skeletal muscle cells noted in the in vivo state, thus allowing a valuable in situ measure of whole body effects [22,23]. The cell cultures used in this study were obtained from biopsied skeletal muscle tissue from obese diabetic individuals and thus, phosphoproteomics data in this manuscript are consistent with the insulin resistant state of the individuals from which the cultures were obtained. Global proteomic and phosphoproteomic studies demonstrate that PMI 5011 altered baseline protein and site-specific phosphorylation levels in culture; thus, increasing the responsiveness of cells to insulin stimulation.
Out of the 125 unique phosphopeptides identified in the phosphoproteomic studies presented here, 41 are from proteins involved in AKT/insulin signaling, transcription and translation, actin cytoskeleton signaling and caveolar and vesicle transport (Table 2). 24 of these phosphopeptides are up-regulated due to treatment of baseline cultures with PMI 5011. Three of these sites are serine 151, 165 and 166 in Protein kinase C delta binding protein, Cavin-3. Cavin-3 is an important component of the caveolin signaling pathway and is involved in trafficking of caveolar vesicles to the plasma membrane [24]. Caveolae are membrane domains that occupy up to 40% of the plasma membrane surface [25] and are involved in translocation of proteins such as insulin receptor, insulin receptor substrate 1 and GLUT4 to the plasma membrane [26,27]. Furthermore, phosphorylation levels on two serines (20 and 23) in caveolin-2 were increased due to treatment with PMI 5011. Phosphorylation of serine 23 in caveolin-2 has been shown to be necessary for increased caveolae assembly in the plasma membrane by positively regulating caveolin-1 [24,28]. Data from our previous global proteomics analysis of PMI 5011 action in human skeletal muscle also show that caveolin-1 is significantly up regulated [16]. Together, these data indicate that PMI 5011 increases phosphorylation levels of proteins involved in caveolae translocation and their fusion with the plasma membrane, a mechanism which explains the previously measured increase in GLUT4 abundance in the plasma membrane after PMI 5011 treatment.
Additionally, LIM domain and actin-binding protein 1 (LIMA1, synonyms: EPLIN, SREBP3) phosphorylation at serine 686 was increased due to PMI 5011 treatment. LIMA1 has been shown previously to interact with actin through cross-linking and stabilization of the filaments, supporting the development of stress fibers [29] which have been indicated in the insulin-stimulated transport of GLUT4 [30]. Phosphorylation levels of a number of proteins involved in actin signaling including filamin C, microtubule-associated proteins 1B and 1S were also affected due to PMI 5011 treatment supporting our previous findings that PMI 5011 treatment increases the abundance of filamentous actin in human skeletal muscle culture [16]. Also, phosphorylation level of serine 942 in general vesicular transport factor P115 was up regulated in the presence of PMI 5011. Transport factor P115 is involved in insulin-dependent translocation of GLUT4 storage vesicles from the cytoplasm to the plasma membrane [31]. Thus, the observed increase in phosphorylation levels of a number of proteins (cavin-3, caveolin-2, actin binding protein and P115) clearly indicates role of PMI 5011 in increased caveolae-mediated GLUT4 translocation to the plasma membrane.
Phosphorylation sites determined to be up regulated by PMI 5011 treatment in this study also have been reported to be associated with the apoptosis signaling pathway. These proteins include PED/PEA15 (serine 116), heat shock protein 1b (serine 82), Yorkie homolog (YAP; serine 105 and 109) and thioredoxin-related transmembrane protein 1(serine 247). PED/PEA15 has been identified as phosphoprotein enriched in diabetes (PED) and protein enriched in astrocytes (PEA). Overexpression of PED/PEA15 in L6 muscle cells resulted in an increased abundance of GLUT1 transport in the plasma membrane and inhibited insulin-stimulated glucose transport by reducing GLUT4 translocation, thereby contributing to insulin resistance [32]. These findings are contrary to our observations showing that PMI 5011 increases levels of caveolae mediated transport proteins and GLUT4 in the plasma membrane. Other studies however show that the ability of PED/PEA15 to regulate both glucose transport and apoptosis is based on site-specific phosphorylation. Ser104 in PED/PEA15 is phosphorylated by protein kinase C and has been implicated in the negative regulation of glucose metabolism, i.e. insulin resistance. Ser116 in PEA/PED15 is phosphorylated by calcium/calmodulin-dependent protein kinase II or AKT [33] and is necessary for PED/PEA15 recruitment to the death-initiation signaling complex and for binding to Fas-associated death domain, resulting in the anti-apoptotic effects of PED/PEA15 [34,35]. Our data show up regulation of serine 116 phosphorylation levels in PEA/PED15 suggesting that PMI 5011 exerts anti-apoptotic effects. This is further supported by our findings that phosphorylation of serine 82 in heat shock protein 1b was down regulated. Phosphorylation of HSP1B at serine 82 has been shown to induce dissociation of HSP1B from AKT, leading to an inactivation of AKT and an increase in apoptosis in neutrophiles [36]. Reduced phosphorylation on this site therefore suggests that PMI 5011 promotes AKT activation through stabilization of the HSP1B-AKT complex. Together, these data suggest that PMI 5011 induces differential phosphorylation of proteins, triggering anti-apoptotic cellular signaling.
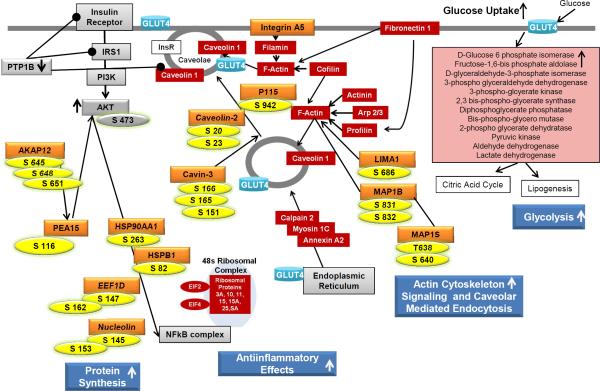
An overview of the current phosphoproteomics data in context of our previous in vitro and in vivo studies using both targeted and global protein and gene expression analyses is presented in Figure 3 [13-16]. Figure 3 demonstrates a comprehensive evaluation of mechanisms by which PMI 5011 increases glucose uptake and metabolism resulting in enhanced insulin sensitivity. Previous studies showed that PMI 5011 reduces PTP1B abundance and increases AKT phosphorylation, indicating improved insulin signaling [13,14]. Global proteomics data clearly demonstrated that PMI 5011 modulates cellular mechanisms that regulate glucose uptake and metabolism 3 [15,16]. These include inflammation, actin-cytoskeleton signaling, protein synthesis, caveolar-mediated endocytosis and glycolysis. These findings suggested that PMI 5011 increased GLUT4 translocation and incorporation into the plasma membrane to facilitate glucose uptake. Current phosphoproteomics studies show that PMI 5011 increases phosphorylation of proteins that are involved in protein synthesis, actin cytoskeleton signaling, and translocation of proteins such as GLUT4 to the plasma membrane, thereby increasing the cellular response to insulin which results in increased glucose uptake and utilization. In addition, the current studies show that anti-apoptotic and anti-inflammatory effects are likely to contribute to the beneficial effects of PMI 5011, further improving cellular insulin sensitivity in vitro .
Figure 3.
Overview of identified PMI 5011 effects on human skeletal muscle culture derived from biopsies from obese, insulin resistant individuals. Global phosphoproteomics data from the current study (protein name: orange, phosphorylation sites: yellow) are overlaid onto results from previous studies [13-16]. Proteins listed in red boxes are involved in actin cytoskeleton signaling and caveolar mediated endocytosis and glycolysis and were identified to be up-regulated due to PMI 5011 treatment in global proteomic analysis [16]. Proteins listed in gray boxes were also identified to be regulated due to PMI 5011 treatment in previous studies [13,14]. Phosphoproteomics data from this study show that PMI 5011 increases phosphorylation of proteins involved in protein synthesis, actin cytoskeleton signaling, and translocation of proteins such as GLUT4 to the plasma membrane. Combined, these data show that PMI 5011 improves insulin signaling and GLUT4 translocation to the plasma membrane, resulting in increased glucose uptake and utilization, thereby ameliorating insulin resistance.
Supplementary Material
Acknowledgements
This work was supported by the grant P50AT002776 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) which funds the Botanical Research Center of Pennington Biomedical Research Center and by the T-32 postdoctoral fellowship award (AT004094) to P.S. L.K. was supported by the Louisiana Biomedical Research Network (LBRN) which is supported by grants from the National Center for Research Resources (P20RR016456) and the National Institute of General Medical Sciences (P20GM103424) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cefalu WT. Insulin resistance: cellular and clinical concepts. Exp Biol Med (Maywood) 2001;226:13–26. doi: 10.1177/153537020122600103. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I. A natural history of botanical therapeutics. Metabolism. 2008;57:S3–9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cefalu WT, Ye J, Wang ZQ. Efficacy of dietary supplementation with botanicals on carbohydrate metabolism in humans. Endocr Metab Immune Disord Drug Targets. 2008;8:78–81. doi: 10.2174/187153008784534376. [DOI] [PubMed] [Google Scholar]
- 4.Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M. Artemisia dracunculus L. (tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem. 2011;59:11367–11384. doi: 10.1021/jf202277w. [DOI] [PubMed] [Google Scholar]
- 5.Eisenman SW, Poulev A, Struwe L, Raskin I, Ribnicky DM. Qualitative variation of anti-diabetic compounds in different tarragon (Artemisia dracunculus L.) cytotypes. Fitoterapia. 2011;82:1062–1074. doi: 10.1016/j.fitote.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meepagala KM, Sturtz G, Wedge DE. Antifungal constituents of the essential oil fraction of Artemisia dracunculus L. Var. dracunculus. J Agric Food Chem. 2002;50:6989–6992. doi: 10.1021/jf020466w. [DOI] [PubMed] [Google Scholar]
- 7.Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol Endocrinol Metab. 2007;293:E1503–1510. doi: 10.1152/ajpendo.00420.2007. [DOI] [PubMed] [Google Scholar]
- 8.Logendra S, Ribnicky DM, Yang H, Poulev A, Ma J, Kennelly EJ, Raskin I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry. 2006;67:1539–1546. doi: 10.1016/j.phytochem.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Obanda DN, Hernandez A, Ribnicky D, Yu Y, Zhang XH, Wang ZQ, Cefalu WT. Bioactives of Artemisia dracunculus L. Mitigate the Role of Ceramides in Attenuating Insulin Signaling in Rat Skeletal Muscle Cells. Diabetes. 2012;61:597–605. doi: 10.2337/db11-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribnicky DM, Kuhn P, Poulev A, Logendra S, Zuberi A, Cefalu WT, Raskin I. Improved absorption and bioactivity of active compounds from an anti-diabetic extract of Artemisia dracunculus L. Int J Pharm. 2009;370:87–92. doi: 10.1016/j.ijpharm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribnicky DM, Poulev A, O'Neal J, Wnorowski G, Malek DE, Jager R, Raskin I. Toxicological evaluation of the ethanolic extract of Artemisia dracunculus L. for use as a dietary supplement and in functional foods. Food Chem Toxicol. 2004;42:585–598. doi: 10.1016/j.fct.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13:550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZQ, Ribnicky D, Zhang XH, Raskin I, Yu Y, Cefalu WT. Bioactives of Artemisia dracunculus L enhance cellular insulin signaling in primary human skeletal muscle culture. Metabolism. 2008;57:S58–64. doi: 10.1016/j.metabol.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZQ, Ribnicky D, Zhang XH, Zuberi A, Raskin I, Yu Y, Cefalu WT. An extract of Artemisia dracunculus L. enhances insulin receptor signaling and modulates gene expression in skeletal muscle in KK-A(y) mice. J Nutr Biochem. 2011;22:71–78. doi: 10.1016/j.jnutbio.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kheterpal I, Coleman L, Ku G, Wang ZQ, Ribnicky D, Cefalu WT. Regulation of insulin action by an extract of Artemisia dracunculus L. in primary human skeletal muscle culture: a proteomics approach. Phytother Res. 2010;24:1278–1284. doi: 10.1002/ptr.3093. [DOI] [PubMed] [Google Scholar]
- 16.Scherp P, Putluri N, LeBlanc GJ, Wang ZQ, Zhang XH, Yu Y, Ribnicky D, Cefalu WT, Kheterpal I. Proteomic analysis reveals cellular pathways regulating carbohydrate metabolism that are modulated in primary human skeletal muscle culture due to treatment with bioactives from Artemisia dracunculus L. J Proteomics. 2012;75:3199–3210. doi: 10.1016/j.jprot.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherp P, Ku G, Coleman L, Kheterpal I. Gel-based and gel-free proteomic technologies. Methods Mol Biol. 2011;702:163–190. doi: 10.1007/978-1-61737-960-4_13. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 20.Schmelzle K, Kane S, Gridley S, Lienhard GE, White FM. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes. 2006;55:2171–2179. doi: 10.2337/db06-0148. [DOI] [PubMed] [Google Scholar]
- 21.Kruger M, Kratchmarova I, Blagoev B, Tseng YH, Kahn CR, Mann M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci U S A. 2008;105:2451–2456. doi: 10.1073/pnas.0711713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciaraldi TP, Abrams L, Nikoulina S, Mudaliar S, Henry RR. Glucose transport in cultured human skeletal muscle cells. Regulation by insulin and glucose in nondiabetic and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1995;96:2820–2827. doi: 10.1172/JCI118352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry RR, Abrams L, Nikoulina S, Ciaraldi TP. Insulin action and glucose metabolism in nondiabetic control and NIDDM subjects. Comparison using human skeletal muscle cell cultures. Diabetes. 1995;44:936–946. doi: 10.2337/diab.44.8.936. [DOI] [PubMed] [Google Scholar]
- 24.McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, Luby-Phelps K, Anderson RG. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28:1001–1015. doi: 10.1038/emboj.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parton RG, Howes MT. Revisiting caveolin trafficking: the end of the caveosome. J Cell Biol. 2010;191:439–441. doi: 10.1083/jcb.201009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson M, Thorn H, Danielsson A, Stenkula KG, Ost A, Gustavsson J, Nystrom FH, Stralfors P. Colocalization of insulin receptor and insulin receptor substrate-1 to caveolae in primary human adipocytes. Cholesterol depletion blocks insulin signalling for metabolic and mitogenic control. Eur J Biochem. 2004;271:2471–2479. doi: 10.1111/j.1432-1033.2004.04177.x. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson M, Thorn H, Parpal S, Stralfors P, Gustavsson J. Insulin induces translocation of glucose transporter GLUT4 to plasma membrane caveolae in adipocytes. FASEB J. 2002;16:249–251. doi: 10.1096/fj.01-0646fje. [DOI] [PubMed] [Google Scholar]
- 28.Sowa G, Pypaert M, Fulton D, Sessa WC. The phosphorylation of caveolin-2 on serines 23 and 36 modulates caveolin-1-dependent caveolae formation. Proc Natl Acad Sci U S A. 2003;100:6511–6516. doi: 10.1073/pnas.1031672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J Cell Biol. 2003;160:399–407. doi: 10.1083/jcb.200212057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong P, Khayat ZA, Huang C, Patel N, Ueyama A, Klip A. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J Clin Invest. 2001;108:371–381. doi: 10.1172/JCI12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosaka T, Brooks CC, Presman E, Kim SK, Zhang Z, Breen M, Gross DN, Sztul E, Pilch PF. p115 Interacts with the GLUT4 vesicle protein, IRAP, and plays a critical role in insulin-stimulated GLUT4 translocation. Mol Biol Cell. 2005;16:2882–2890. doi: 10.1091/mbc.E05-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condorelli G, Vigliotta G, Iavarone C, Caruso M, Tocchetti CG, Andreozzi F, Cafieri A, Tecce MF, Formisano P, Beguinot L, Beguinot F. PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J. 1998;17:3858–3866. doi: 10.1093/emboj/17.14.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trencia A, Perfetti A, Cassese A, Vigliotta G, Miele C, Oriente F, Santopietro S, Giacco F, Condorelli G, Formisano P, Beguinot F. Protein kinase B/Akt binds and phosphorylates PED/PEA-15, stabilizing its antiapoptotic action. Mol Cell Biol. 2003;23:4511–4521. doi: 10.1128/MCB.23.13.4511-4521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renganathan H, Vaidyanathan H, Knapinska A, Ramos JW. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem J. 2005;390:729–735. doi: 10.1042/BJ20050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bifulco G, Miele C, Pellicano M, Trencia A, Ferraioli M, Paturzo F, Tommaselli GA, Beguinot F, Nappi C. Molecular mechanisms involved in GnRH analogue-related apoptosis for uterine leiomyomas. Mol Hum Reprod. 2004;10:43–48. doi: 10.1093/molehr/gah002. [DOI] [PubMed] [Google Scholar]
- 36.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.