Abstract
Zinc is an essential micronutrient playing fundamental roles in cellular metabolism. It acts mostly through binding a wide range of proteins, thus affecting a broad spectrum of biological processes, which include cell division, growth and differentiation. Full annotation of zinc-binding proteins showed them to represent about 10 % of the human proteome, with over 300 enzymes containing zinc ions within their catalytic domains. Also, hundreds of key regulatory proteins, including transcription factors, require zinc for their activity. In this study, the whole set of zinc-binding proteins together with their direct interactors was listed and defined as the zinc proteome (ZNP). We interrogated pathway analysis tools to identify the cellular processes that are predicted to be affected by zinc availability. Network and functional enrichment analyses highlighted biological processes potentially affected by deregulated zinc homeostasis. This computational approach was also tested on a real case study: The possible involvement of ZNP network proteins in Crohn’s disease pathogenesis was assessed on genes transcriptionally regulated in the intestine of patients affected by this condition. The analysis produced a network of pathways likely to be influenced by zinc and associated with Crohn’s disease. These results highlight a central role for zinc in the tissue remodeling process which occurs upon gut inflammation, pointing at novel disease pathways whose effect could be worsened by zinc dyshomeostasis and impaired zinc fluxes in specific damaged areas. Overall, our computational approach could provide novel insights into pathological conditions and could therefore be used to drive mechanistic research in under-investigated fields of research. An interactive version of the determined ZNP network is available at URL http://93.63.165.11/ZNnetwork/.
Electronic supplementary material
The online version of this article (doi:10.1007/s12263-014-0436-0) contains supplementary material, which is available to authorized users.
Keywords: Zinc, Pathway analysis, Crohn’s disease
Introduction
The group XII metal zinc is an essential micronutrient playing fundamental housekeeping roles in physiology, cellular metabolism and gene expression. After iron, zinc is the most abundant transition metal ion in living organisms, being strictly required for a wide variety of biological functions. Zinc needs to be taken up from the diet through specific membrane transporters (Huang and Tepaamorndech 2013; Liuzzi et al. 2004; Liuzzi and Cousins 2004), whose expression and/or membrane localization is tightly regulated in a tissue/cell type-specific manner. It is especially enriched in protein-rich foods such as meat, seafood and legumes. The World Health Organization (WHO) recommends a daily zinc intake of 10 mg/day for children, 12 mg/day for women and 15 mg/day for men (WHO and Organization 1996). Specific population groups such as children and adolescents, pregnant women and the elderly display higher zinc requirements and a corresponding increased risk of deficiency. Previous work reporting complete annotation of zinc-containing proteins has contributed to further understanding of the biological role of zinc, showing that zinc-containing proteins represent about 10 % of the human proteome, with over 300 enzymes containing zinc ions within their catalytic domains (Andreini et al. 2009; Bertini et al. 2010).
Several lines of evidence point to zinc and to its metabolism as important players in the onset and/or in the progression of multifactorial diseases. Due to its widespread functions throughout the body, zinc unbalance in specific tissues is likely to contribute to several diseases (Devirgiliis et al. 2007). Zinc dyshomeostasis was associated with Alzheimer’s disease and altered deposition of extracellular amyloid-β plaques (Craddock et al. 2012). Decreased zinc content was also described in post-mortem pancreata of diabetic individuals over 75 years ago (Scott and Fisher 1938), and the role of this metal ion in insulin secretion and glucose homeostasis was confirmed since then by several reports (Chimienti 2013; Sladek et al. 2007). Airways inflammation, one of the main hallmarks of asthma, has also been associated with deregulated zinc homeostasis (Zalewski et al. 2005). Gut mucosal integrity also requires zinc, and zinc dyshomeostasis has been associated with several intestinal pathologies. Inflammatory bowel diseases (IBD) represent complex disorders, including both Crohn’s (CD) and ulcerative colitis (UC), which are both characterized by chronic inflammation of the gastrointestinal tract. CD and UC display a strong genetic component, but they are also influenced by environmental factors, including the diet (Ferguson 2010; Tysk et al. 1988). Current clinical interventions to treat IBD are based on anti-inflammatory steroid therapy; positive results in controlling CD symptoms have also been obtained with therapeutic strategies including specific monoclonal antibodies that neutralize the effect of the inflammatory cytokine TNFα (Chowers and Allez 2010). The management of CD is rapidly changing with the development of a number of promising drugs that will broaden and improve the spectrum of treatment options (Cheifetz 2013). Nutritional management of IBD through the application of controlled diets to restraint gut inflammation would also be highly desirable, but it is still difficult to achieve mainly because limited information is presently available on the mechanisms of action of the specific food components that were suggested to exert positive effects in CD-affected patients (Ferguson et al. 2007). It is therefore extremely important to gain further knowledge on the modulatory role of specific nutrients in gut homeostasis, both in healthy and in pathological conditions.
Low levels of plasma zinc have frequently been associated with IBD, while zinc supplementation was reported to improve intestinal barrier function in CD patients, as well as in animal models, by contributing to the resolution of alterations in permeability of the gut mucosa and to reducing the risk of relapse (Hering and Schulzke 2009; Matsui 1998; Sturniolo et al. 2001, 2002). Marginally, zinc-deficient diets were shown to worsen experimentally induced colitis in rats (Iwaya et al. 2011), and in vitro evidences have shown that mild zinc deficiency renders intestinal cells susceptible to inflammatory cytokine-induced apoptosis, therefore contributing to epithelial barrier disruption (Ranaldi et al. 2013).
In the study presented here, we aimed at characterizing the complex of biological processes enriched in zinc-binding proteins (ZNBP) and in their interactors, which we here define as the zinc proteome (ZNP). The network and functional enrichment analyses that we have performed highlighted physiological functions potentially affected by deregulated zinc homeostasis. To verify the applicability of our computational approach to pathological processes in real life, we performed a case study in which we re-analyzed the results of a previously published work on differentially regulated genes in the intestinal mucosa of CD patients, which validated the results of our ZNP network analysis.
Materials and methods
Determination of the zinc proteome (ZNP)
Human zinc-binding proteins were identified by mapping all known zinc-binding domains and structures on refseqs from the reference genome PRJNA168, utilizing the protocol implemented in software RDGB (Andreini et al. 2011). The protocol itself is detailed in Andreini et al. (2009). Briefly, the input to RDGB is a manually curated list of HMMs representing all Pfam domains reported in the literature as zinc binding. As an additional input, we provide a list of known zinc-binding patterns, automatically extracted from the 3D structures of zinc-binding proteins (Andreini et al. 2004), each of which is associated with the specific domain(s) present in the structure from which the pattern was derived. RDGB uses the patterns to filter the results obtained by mining the reference genome with the input domain list. This reduces the rate of false positives (Andreini et al. 2009). Refseq sequences harboring at least one zinc-binding pattern were selected. ZNBP refseq codes map 2,066 protein coding genes from Ensembl genome version 69.
Network building and functional enrichment analysis
The ZNP network was built with the MIMI bionet builder plug in of Cytoscape (Shannon et al. 2003) browsing experimentally validated protein–protein and gene–protein interactions from public databases. Enrichment of biological processes annotated in gene ontology was estimated with the DAVID web server (da Huang et al. 2009). Overrepresented BPs were selected by comparing the ZNP versus the starting reference genome. BPs annotated in gene ontology (level 5) were selected only if significantly enriched with a p value threshold <0.01 corrected with Benjamini method.
Pairwise semantic similarities between overrepresented BPs were calculated by the REVIGO server (Supek et al. 2011), utilizing the Resnik measure as metric distance.
PCA and cluster analysis
PCA was performed with FactoMineR (Le et al. 2008) package part of R software on ZNBPs further characterized by degree, topological coefficient and number of enriched biological processes in which each ZNBP was involved. The degree and number of BPs were preliminarily scaled on the unit variance to make them comparable with topological coefficient.
Hierarchical clustering was performed with the clustering utility included in FactoMineR, selecting the Ward method and the Euclidean distance as distance metric. Best number of clusters was determined by silhouette score.
Results
Constitution of the zinc proteome network
Our analysis highlighted 3,643 proteins in 2,066 human genes harboring at least one zinc-binding domain, corresponding to 9.4 % of the total number of protein coding genes annotated in the reference genome (PRJNA168). We reasoned that protein functions affected by zinc availability and intracellular zinc fluxes would not be represented only by proteins that bind the metal ion directly, but also by their interactors. Therefore, we expanded the zinc proteome by including proteins previously shown to interact with at least one ZNBP. The final zinc proteome (ZNP) consisted of 6,680 proteins (supplementary table S1a) connected in a network of 68,358 interactions including both protein–protein and protein–gene interactions as annotated in public databases. An interactive version of this ZNP network is available at URL http://93.63.165.11/ZNnetwork/.
Properties of the ZNP network
To evaluate biological processes most likely affected by zinc balance, we characterized our ZNP network by performing functional enrichment analysis of biological processes (BP) annotated in the gene ontology database (GO).
Functional analysis resulted in enrichment of 308 BP, all annotated in GO (supplementary data table S1b and http://93.63.165.11/ZNnetwork/). We calculated pairwise semantic similarities between the overrepresented BPs and built a network in which BPs are nodes connected by edges indicating the semantic distance within the lowest 3 % of the entire range of observed distances.
As expected, ZNP network proteins are involved in a wide range of diverse functional processes, some of which were already known to be affected by zinc fluxes. For example, pathways enriched in the ZNP network were implicated in controlling apoptosis and cell proliferation/survival. The enrichment analysis also showed an involvement of ZNP proteins in development and differentiation pathways in several tissues. Several BPs regulating DNA metabolism and repair were also found to be enriched in ZNP network proteins (Table S1b). Specific examples of this latter group of proteins are PARP1, a poly (ADP-ribose) polymerase and primary sensor of DNA breakage though specialized zinc-finger protein domains (Ali et al. 2012), and the zinc-finger transcription factor SP1, which also plays a central role in DNA double-strand break recognition.
Topological analysis of the ZNP network
Topological analysis of the ZNP network reveals extensive connections between nodes, with a global network density of 0.03 and an average clustering coefficient between nodes of 0.22, suggesting overall grouping of nodes in short branches (http://93.63.165.11/ZNnetwork/). Within the total network of 6,680 nodes, only 723 are completely isolated, with an overall diameter of 10 nodes and a characteristic path length of 3.18 nodes. Such features suggest high degree of communication between the nodes, implying that disturbance in a local network district can be quickly propagated, affecting in turn the connected nodes.
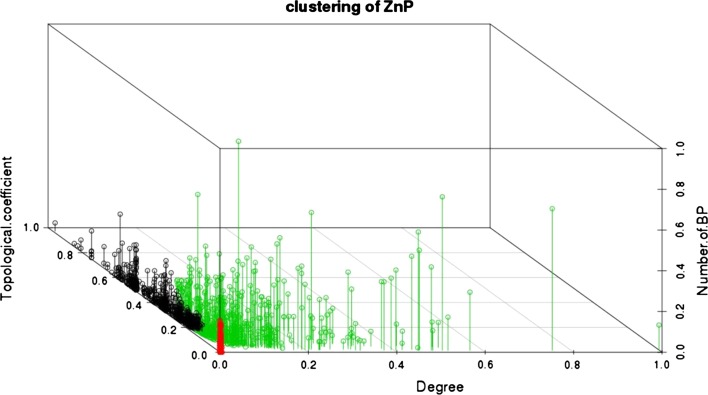
The 2,066 ZNBP encoding genes were further characterized by estimating their degree and topological coefficient, where the degree represents the number of interactions linked to a single node and the topological coefficient is a relative measure of the extent to which a node shares neighbors with other nodes. A node with many shared neighbors is in a central position within the analyzed network. To more accurately estimate the importance of ZNBPs in mediating a biological response, we estimated also the number of overrepresented BPs to which each node belongs. To identify the most relevant properties of ZNBPs in relation to these three above-mentioned parameters, we grouped the nodes through hierarchical cluster analysis based on the variance along the principal components identified by principal component analysis (PCA). The first two principal components capture 81 % of the variance in the three parameters monitored.
Clustering highlighted three major clusters (Fig. 1; table S2) separating the central ZNBPs that share several interactors (cluster 1), from ZNBPs displaying fewer interactions, located in the peripheral regions of the network (cluster 2), and ZNBPs present in most of the overrepresented BPs with a high number of “specific” interactors (cluster 3).
Fig. 1.
Cluster analysis of ZNBPs. The three parameters employed to characterize topological properties of ZNBPs are represented along the three major axes. Colored dots indicate different clusters of ZNBPs. Members of cluster 1 that play a central role in network topology with lower number of BPs are shown in black, cluster 2 characterized by few interactors at the edge of the network is shown in red and cluster 3 characterized by many interactors and present in a high number of BPs is shown in green
Although sharing several interactions, proteins in cluster 1 belong to few BPs, suggesting that they play a central role in specific pathways. Due to their high topological coefficient, we expected that functional alteration of these proteins would result in a systemic effect.
An example of the descriptive potential of our analysis is provided by the distribution of the poly [ADP-ribose] polymerase 1 (PARP) family among the three clusters. This protein family includes a group of related enzymes that detect and signal single-strand DNA breaks, and catalyze post-translational modification of proteins by adding multiple ADP-ribose moieties. Ribose addition is a crucial step in the repair of single-strand DNA nicks. This process is called base excision repair (BER), and both PARP1 and PARP2 are involved in the mechanism as a functional hetero-dimer. Of the two monomers, PARP1 alone is known to be associated with increased apoptosis in response to genotoxic stress and to modulate transcription of several genes. Our analysis places PARP1 in cluster 3, while PARP2, centrally involved only in the BER process, is listed in cluster 1 (Ali et al. 2012).
As a further example, cluster 3 includes the tumor suppressor protein TP53 (or P53), a zinc-finger transcription factor with a crucial role in tumor suppression. Mutations in this gene are associated with a variety of human cancers, including hereditary cancers. TP53 responds to diverse cellular stresses by regulating the expression of specific target genes, thereby inducing cell cycle arrest, apoptosis, senescence, DNA repair or metabolic changes (Dai and Gu 2010; Lane 1992).
An example of cluster 1 protein is the F-box protein FBOX11, which interacts with the E3 ubiquitin–protein ligase complex that mediates ubiquitination and subsequent proteasomal degradation of target proteins involved in diverse biological process (Duan et al. 2012), which therefore can be predicted to be sensitive to zinc availability. Of the 20 top ZNBPs in cluster 3, twelve are involved in gene expression either directly, as transcription factors (tumor suppressor genes P53, P63, BCL6, SSH, PML, SMAD2, SMAD3, SMAD4, SMAD7), or indirectly, as regulators of the DNA dwelling or DNA repair processes (HDAC4, HDAC5, BRCA1).
The top 20 ZNBPs in cluster 1, on the other hand, are represented by enzymes involved in hydrolytic and proteolytic activities (KLK10, MAN2A1, GNL1, GNL2, CCDC42, NEIL3, ZDHCC12), or by structural proteins of larger protein complexes (ZNF189, ZCRB1, ZRSR2, ZNF254, RNF26, BCL2L14, TRIM54, TRIM59, GAS2L1), G-protein coupled receptors (MC3R, MC2R, NPY5R) and only one transcription factor (CDCA7).
Case study application of the ZNP network/functional enrichment analysis
To test the potential of our analysis in unraveling novel aspects of physiology in healthy and pathological conditions, as well as to link it with the underlying biological effects, we applied the ZNP network/functional analysis to a real case scenario. Zinc was shown to represent a key factor in maintaining the integrity of the intestinal epithelium, both in vitro and in vivo (Iwaya et al. 2011; Sturniolo et al. 2001, 2002). Several lines of evidence suggest that intestinal epithelial cells play a critical role in gut homeostasis in chronic inflammatory bowel disorders such as Crohn’s disease (CD) and ulcerative colitis (UC). Therefore, we explored the possible involvement of ZNP network proteins in CD pathogenesis by re-analyzing previously reported differential expression profiles resulting from transcriptomic studies and by comparing gene expression in healthy tissues with that in inflamed and non-inflamed CD tissues. To this aim, we selected microarray gene expression profiles from the published study described by Noble et al. (Noble et al. 2010), which was performed on 172 biopsies from endoscopic samples of CD and control subjects, taken at ileo-colonoscopy from five specific anatomical locations (Table 1).
Table 1.
Number of genes encoding ZNP network proteins and number of total genes modulated in inflamed and non-inflamed tissue from CD patients
| Parallels | Modulated ZNP | Modulated genes | Hypergeometric distribution (ZNP MA vs. ZNP in genome) p value |
|---|---|---|---|
| 1. Inflamed CD versus non-inflamed CD | 152 | 382 | 0.003 |
| 2. Non-inflamed CD versus control | 179 | 511 | 0.56 |
| 3. CD versus control terminal ileum | 591 | 1,667 | 0.49 |
| 4. Inflamed CD versus control | 87 | 311 | 0.99 |
The p values listed in the last column were estimated by hypergeometric distribution and express the likelihood that ZNP network genes might be overrepresented within the modulated expression profiles compared to their occurrence within the human genome
Expression values of differentially expressed probe-sets were re-mapped on the reference genome reflecting the ZNP network. We estimated the likelihood of enrichment in ZNP genes modulated in diseased tissues, comparing by hypergeometric distribution the fraction of ZNP network genes represented in the expression profiles under examination, with the percentage of ZNP network genes present in the human genome. The results of such analysis revealed ZNP network genes as significantly overrepresented within the profile of differentially expressed genes in samples collected from the inflamed mucosa of CD patients, when compared with samples collected from the non-inflamed area. This observation highlights the involvement of the ZNP in inflammatory processes occurring in the intestine of CD patients.
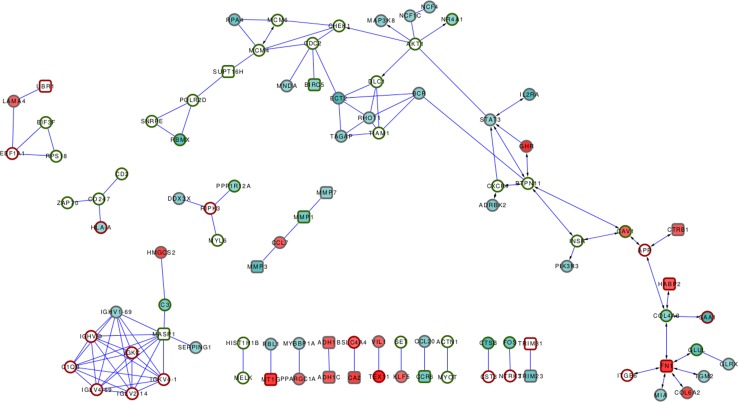
The genes encoding ZNP network proteins and their corresponding expression profiles in both inflamed and non-inflamed tissue biopsies from CD patients are listed in Table S3 (supplementary information). Mapping of the modulated genes on the overall ZNP network (Fig. 2) highlighted a group of 101 genes connected by 117 interactions and displaying two clearly identifiable major groups of interactions. The largest group constitutes the right arm of the interaction graph and includes six extracellular matrix proteins, all of which interact with fibronectin 1 (FN1). FN1 is a ZNBP thought to regulate epithelial response to injury during inflammation (Kolachala et al. 2007). It is a major component of the extracellular matrix interacting with adhesion receptors such as integrins, and it mediates cell anchorage and triggers specific signaling pathways regulating survival and differentiation. FN1 is expressed in the gut, where it promotes cell adhesion and wound healing, and it shows as a down-regulated gene in inflamed CD with respect to non-inflamed CD tissue, while GLUL, GLRX and TGM2 (involved in promoting glutamine biosynthesis) are up-regulated (Noble et al. 2010). FN1 pathway is, therefore, potentially affected by altered zinc homeostasis.
Fig. 2.
Network of ZNP interactions extracted from the microarray experiments of Noble et al. (2010). Genes are represented by nodes and interactions by edges. Green-colored nodes indicate down-regulated genes, red color variations refer to up-regulated genes. Border colors indicate genes modulated in CD versus control tissues. Fill colors correspond to genes modulated in inflamed versus non-inflamed CD tissues. Arrows point at pairs of ZNP network proteins whose direct interaction is annotated in public databases
On the left arm of the largest gene cluster shown in Fig. 2, we detected a group of seven genes encoding proteins that operate key steps in DNA replication: SUPT16H affects nucleosome disassembly, POLR2D encodes RNA polymerase 2, SNRPE functions in the U7 snRNP complex involved in histone 3′-end processing, RBMX promotes mRNA splicing, MCM4, MCM6 and CDC2 encode DNA unwinding enzymes. Almost all of them (except RBMX) were down-regulated in CD versus control biopsies.
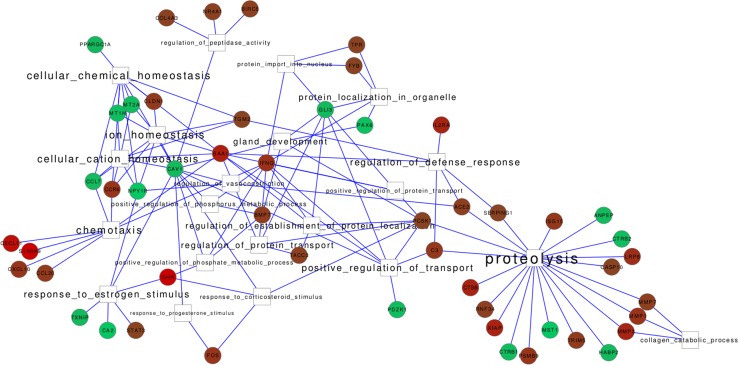
Further indications that the ZNP network is involved in tissue remodeling and repair in the inflammation process occurring in the gut of CD patients came from the analysis of ZNP BPs enriched in genes that are modulated in the inflamed intestine, as compared to BPs enriched in genes regulated in the non-inflamed mucosa (Fig. 3). In inflamed CD, 20 BPs are significantly enriched in the ZNP. The three most overrepresented BPs (proteolysis, regulation of defense response and collagen catabolic processes) underlie a shared set of 23 ZNP coding genes, 12 of which are specifically up-regulated in inflamed CD. This latter group includes nine genes encoding peptidases which inhibit complement activation (SERPING1), regulate apoptosis (CASP10 and RNF34) and vasodilatation (ACE2), determine breakdown of the extracellular matrix (MMP3, MMP7), or are involved in protein degradation as ubiquitin modifiers (TRIM5) or as proteasome subunit (PSMB9).
Fig. 3.
Overrepresented ZNP BPs within the expression profiles of inflamed versus non-inflamed CD tissues. Red and green nodes indicate up-regulated and down-regulated ZNP network genes, respectively. Nodes are connected through edges to the corresponding BPs
A group of five genes belonging to proteolytic pathways (ANPEP, MST1, HABP2, CTRB1, CTRB2) were shown to be down-regulated in the inflamed gut of CD patients in comparison with the non-inflamed portions of terminal ileum (TI). It is worth noting that these genes encode specific functions of the differentiated and functional intestinal enterocyte. The ANPEP protein is located within the microvillar membrane and plays a role in the final digestion of small peptides generated by gastric and pancreatic proteases. The CTRB1 and CTRB2 genes encode 2 of the 3 chymotrypsin isozymes, including a serine protease which also acts in the final stages of protein digestion. HABP2 is an extracellular serine protease that binds hyaluronic acid and is involved in cell adhesion. As for MST1, its specific receptor is the RON tyrosine kinase, which is associated with significantly increased crypt cell proliferation, possibly leading to susceptibility to tumor initiation in the non-transformed intestinal epithelium. All of the above-described, down-regulated zinc-dependent proteins are typical of the enterocytes, and their function may be impaired in the terminally differentiated epithelium of the heavily inflamed mucosa of CD patients. On the other hand, the five genes up-regulated in inflamed CD tissue (C3, MMP1, LRP8, CTSB, XIAP) encode proteins acting in proteolytic pathways. In particular, the inhibitor of caspases XIAP, whose activity leads to repression of apoptosis and promotion of cell survival, is known to be rapidly degraded under zinc-deficient conditions (Makhov et al. 2008; Ranaldi et al. 2013).
Discussion
The application of systems biology to nutrition aims at highlighting molecular links between diet and health in an integrated manner (van Ommen et al. 2008). Interactome models have been increasingly used, especially protein–protein interaction (PPI) networks which provide an abstraction of the complex relationships among different molecular components—spanning from nutrients and their metabolites to diet-modulated transcription factors (Nguyen et al. 2011). Nutritional systems biology has a tremendous potential to provide comprehensive predictions of nutrient dyshomeostasis in biological processes (Doring and Rimbach 2014). Zinc, being involved in such a large number of protein activities, is an ideal micronutrient to be studied in an integrated framework. Complete annotation of zinc-binding protein genes revealed that this micronutrient is bound to 2,066 proteins (Bertini et al. 2010). Using these findings as a starting point, we have reported here a bioinformatics analysis extending the zinc proteome network to protein–protein interactions, with the aim of providing more holistic basis toward the identification of potentially zinc-regulated pathways in health and disease. Pathway analysis highlighted biological processes that are likely affected by zinc fluxes. While most BPs were already known to be influenced by zinc, the power of our analysis drew the attention on biological functions not yet experimentally linked to zinc availability and therefore needing further investigation.
We observed that several pathways involved in regulating apoptosis and cell survival were enriched in ZNP network proteins, confirming a number of previously published experimental evidences linking zinc availability to apoptosis, among which our own studies (Ranaldi et al. 2013; Truong-Tran et al. 2003), as well as reports from other group (Allen-Redpath et al. 2013). Expansion of these observations in the directions suggested by our computational analysis can therefore strengthen the proposed central role of zinc in the balance between cell death and survival. The enrichment analysis reported in our work also highlighted involvement of ZNP network proteins in development and differentiation pathways in several tissues, which is likely due to the key role played by zinc-binding transcription factors that are largely represented in these pathways. Much less information is presently available on the role of zinc in the DNA damage response, although we identified several BPs involved in DNA metabolism and repair and zinc-binding proteins among those enriched in the ZNP network. For example, PARP1, a poly (ADP-ribose) polymerase and a primary sensor of DNA breakage though specialized zinc-finger protein domains (Ali et al. 2012). Therefore, we showed the ZNP network to be significantly enriched in proteins involved in DNA damage response pathways, although the biological effect on DNA repair of deregulated zinc homeostasis is yet to be investigated.
ZNBPs were clustered according to the number of interactions, the number of biological processes in which they are involved and their topological position within the network. Thirty percentage of the identified ZNBPs were found to be involved in a limited number of biological processes, even though some of them displayed a high number of interactions (cluster 1). We take this as an indication that they could play a central role in specific pathways. Decreased zinc availability is therefore likely to affect the overall process to which these ZNBP participate. On the other hand, another 30 % of the described ZNBPs are characterized by involvement in a high number of biological processes with a high number of interactions (cluster 3). Impaired zinc fluxes affecting these latter proteins may thus cause a systemic effect. The remaining proportion of ZNBPs shows involvement in a small number of processes and interactions (cluster 2). This group is likely to have minor effects on modulation of specific biological process although we cannot rule out that the features which led to their listing in cluster 2 might derive to incomplete annotation in public databases.
Another important advantage provided by our listing of ZNBPs and of their interactors is the potential to be analyzed within the context of pathological conditions, thus indicating a role for zinc fluxes in specific physio-pathological mechanisms. To test the usefulness of our computational approach in this direction, we performed a case study, in which we investigated the presence of ZNP network proteins encoded by differentially expressed genes in the intestine of CD patients (Noble et al. 2010). The output of this analysis was a model network of functional pathways associated with this pathology and likely affected by zinc, which can be used to drive mechanistic research in this area. In particular, our results highlighted a central role for zinc in tissue remodeling that occurs in patients affected by CD upon gut inflammation, pointing at novel pathways that could be worsened by zinc dyshomeostasis and impaired zinc fluxes in specific areas. For example, the FN1 network emerging from our analysis indicated a new possible role for zinc in the regulation of tissue architecture, which is potentially relevant in the mucosal inflammatory response occurring in CD. These results also suggested that marginal zinc deficiency, due to either insufficient dietary intake and/or a predisposing genetic make-up, might negatively affect the healing potential of inflamed intestinal mucosa in CD patients. An interesting case is represented by the caspase inhibitor protein XIAP, as our analysis highlighted that the corresponding gene is up-regulated in gut-inflamed mucosa of CD patients compared to non-inflamed tissue. This up-regulatory process might also be important for post-inflammation response in the damaged epithelium, as low zinc levels were demonstrated to lead to XIAP degradation in several cell types, including enterocytes (Makhov et al. 2008; Ranaldi et al. 2013). Zinc deficiency might thus impair crucial healing steps.
In conclusion, the zinc proteome emerging from our computational approach appears as a highly complex network with potential for further development, as many of the annotations regarding the direction of regulatory interactions are not yet present in public databases. This makes it still hard to predict the downstream effects that zinc may exert in pathological contexts. In this work, we have sought to provide a backbone model that can be used to address specific questions. The accompanying case study that we have performed provides an example of how the backbone can be used. We thus believe that the ZNP network resulting from a computational approach represents an important starting point to investigate the involvement and relevance of zinc in specific biological processes, as it can provide novel insights into pathological conditions. This approach could also be successfully extended to other nutrients.
Electronic supplementary material
Acknowledgments
We thank Dr. Gabriele Cavallaro for help with RDGB calculations. This work was supported by Grant “NUME” (DM 3688/7303/08) from the Italian Ministry of Agriculture Food and Forestry (MiPAAF) and by EU Marie Curie Actions—International Research Staff Exchange Scheme (IRSES); “Microgennet” funded by the EU Marie Curie action program IRSES Grant 26921 (www.microgennet.org).
Conflict of interest
None.
Abbreviations
- ZNBP
Zinc-binding proteins
- ZNP
Zinc proteome (ZNBP + interactors)
- BP
Biological processes
- IBD
Inflammatory bowel diseases
- CD
Crohn’s disease
- UC
Ulcerative colitis
- IECs
Intestinal epithelial cells
References
- Ali AA, et al. The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks. Nat Struct Mol Biol. 2012;19:685–692. doi: 10.1038/nsmb.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Redpath K, Ou O, Beattie JH, Kwun IS, Feldmann J, Nixon GF. Marginal dietary zinc deficiency in vivo induces vascular smooth muscle cell apoptosis in large arteries. Cardiovasc Res. 2013;99:525–534. doi: 10.1093/cvr/cvt114. [DOI] [PubMed] [Google Scholar]
- Andreini C, Bertini I, Rosato A. A hint to search for metalloproteins in gene banks. Bioinformatics. 2004;20:1373–1380. doi: 10.1093/bioinformatics/bth095. [DOI] [PubMed] [Google Scholar]
- Andreini C, Bertini I, Rosato A. Metalloproteomes: a bioinformatic approach. Acc Chem Res. 2009;42:1471–1479. doi: 10.1021/ar900015x. [DOI] [PubMed] [Google Scholar]
- Andreini C, Bertini I, Cavallaro G, Decaria L, Rosato A. A simple protocol for the comparative analysis of the structure and occurrence of biochemical pathways across superkingdoms. J Chem Inf Model. 2011;51:730–738. doi: 10.1021/ci100392q. [DOI] [PubMed] [Google Scholar]
- Bertini I, Decaria L, Rosato A. The annotation of full zinc proteomes. J Biol Inorg Chem. 2010;15:1071–1078. doi: 10.1007/s00775-010-0666-6. [DOI] [PubMed] [Google Scholar]
- Cheifetz AS. Management of active Crohn disease. JAMA. 2013;309:2150–2158. doi: 10.1001/jama.2013.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimienti F. Zinc, pancreatic islet cell function and diabetes: new insights into an old story. Nutr Res Rev. 2013;26:1–11. doi: 10.1017/S0954422412000212. [DOI] [PubMed] [Google Scholar]
- Chowers Y, Allez M. Efficacy of anti-TNF in Crohn’s disease: how does it work? Curr Drug Targets. 2010;11:138–142. doi: 10.2174/138945010790309876. [DOI] [PubMed] [Google Scholar]
- Craddock TJ, Tuszynski JA, Chopra D, Casey N, Goldstein LE, Hameroff SR, Tanzi RE. The zinc dyshomeostasis hypothesis of Alzheimer’s disease. PLoS One. 2012;7:e33552. doi: 10.1371/journal.pone.0033552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622:84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Doring F, Rimbach G. Nutri-informatics: a new kid on the block? Genes Nutr. 2014;9:394. doi: 10.1007/s12263-014-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S et al (2012) FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature 481:90–93. doi:10.1038/nature10688 [DOI] [PMC free article] [PubMed]
- Ferguson LR. Nutrigenomics and inflammatory bowel diseases. Expert Rev Clin Immunol. 2010;6:573–583. doi: 10.1586/eci.10.43. [DOI] [PubMed] [Google Scholar]
- Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. Genes, diet and inflammatory bowel disease. Mutat Res. 2007;622:70–83. doi: 10.1016/j.mrfmmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Hering NA, Schulzke JD. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis. 2009;27:450–454. doi: 10.1159/000233283. [DOI] [PubMed] [Google Scholar]
- Huang L, Tepaamorndech S. The SLC30 family of zinc transporters: a review of current understanding of their biological and pathophysiological roles. Mol Asp Med. 2013;34:548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Iwaya H, Kashiwaya M, Shinoki A, Lee JS, Hayashi K, Hara H, Ishizuka S. Marginal zinc deficiency exacerbates experimental colitis induced by dextran sulfate sodium in rats. J Nutr. 2011;141:1077–1082. doi: 10.3945/jn.111.138180. [DOI] [PubMed] [Google Scholar]
- Kolachala VL, et al. Epithelial-derived fibronectin expression, signaling, and function in intestinal inflammation. J Biol Chem. 2007;282:32965–32973. doi: 10.1074/jbc.M704388200. [DOI] [PubMed] [Google Scholar]
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Le S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis J Stat Softw 25(1):1–18
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhov P, et al. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ. 2008;15:1745–1751. doi: 10.1038/cdd.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T. Zinc deficiency in Crohn’s disease. J Gastroenterol. 1998;33:924–925. doi: 10.1007/s005350050205. [DOI] [PubMed] [Google Scholar]
- Nguyen TP, Scotti M, Morine MJ, Priami C. Model-based clustering reveals vitamin D dependent multi-centrality hubs in a network of vitamin-related proteins. BMC Syst Biol. 2011;5:195. doi: 10.1186/1752-0509-5-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble CL, et al. Characterization of intestinal gene expression profiles in Crohn’s disease by genome-wide microarray analysis. Inflamm Bowel Dis. 2010;16:1717–1728. doi: 10.1002/ibd.21263. [DOI] [PubMed] [Google Scholar]
- Ranaldi G, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFalpha. J Nutr Biochem. 2013;24:967–976. doi: 10.1016/j.jnutbio.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Scott DA, Fisher AM. The insulin and the zinc content of normal and diabetic pancreas. J Clin Invest. 1938;17:725–728. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885. doi:10.1038/nature05616 [DOI] [PubMed]
- Sturniolo GC, Di Leo V, Ferronato A, D’Odorico A, D’Inca R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm Bowel Dis. 2001;7:94–98. doi: 10.1097/00054725-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D’Inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311–315. doi: 10.1067/mlc.2002.123624. [DOI] [PubMed] [Google Scholar]
- Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Tran AQ, Grosser D, Ruffin RE, Murgia C, Zalewski PD. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochem Pharmacol. 2003;66:1459–1468. doi: 10.1016/S0006-2952(03)00498-2. [DOI] [PubMed] [Google Scholar]
- Tysk C, Lindberg E, Jarnerot G, Floderus-Myrhed B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29:990–996. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen B, Cavallieri D, Roche HM, Klein UI, Daniel H. The challenges for molecular nutrition research 4: the “nutritional systems biology level”. Genes Nutr. 2008;3:107–113. doi: 10.1007/s12263-008-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WH Organization (WHO) (1996) Trace element in human nutrition and health. WHO, Geneva
- Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets: a review. Pharmacol Ther. 2005;105:127–149. doi: 10.1016/j.pharmthera.2004.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.