Abstract
Facial epidermal pigmentation and skin tumors can be caused by UV exposure and other physical and chemical irritations. In this report we describe the primary culture of melanocytes from human face skin. The ability to culture these melanocytes will enable their morphological and biological properties to be investigated. Skin specimens were obtained from patients who had undergone lower blepharoplasty procedures. Digestion with neutral protease and trypsin was used to obtain single cell suspensions of epidermal cells. The cells were cultured in M254 medium supplemented with human melanocyte growth solution. Cell morphology was observed using inverted microscopy. Melanocytes were positively identified using both l-DOPA staining and S-100 protein immunohistochemical staining. Immunofluorescence was used to confirm the expression of tyrosinase-related protein-1, a melanocyte-specific protein. The cellular ultrastructure of the melanocytes was observed by transmission electron microscopy. The cultured human melanocytes from face skin were multi-dendritic, and many mature melanosomes were observed. Therefore, using a specific culture medium, melanocytes from face skin can be successfully cultured and made available for further investigations.
Keywords: Cell culture, Melanocytes, Pigmentation, Morphology, Tyrosinase-related protein-1
Introduction
Melanocytes are dendritic cells of neural crest cell origin. Anatomically, melanocytes exist as relatively minor populations in certain tissues: including skin, in the basal layer of the epidermis; the eye, in the retinal pigment epithelium and uveal tract; the hair, in the matrix or outer root sheath; the ear, in the stria vascularis; and in mucous membranes and the central nervous system. Melanocytes account for approximately 2–4 % of epidermal cells, and 8–10 % of cells in the basal layer of the adult epidermis. Melanocytes are occasionally present in the dermis (Sulaimon and Kitchell 2003). In the vertebrate epidermis, melanocytes are in close contact with surrounding keratinocytes via their dendritic processes. This close association allows melanocytes to accomplish their primary function of producing and delivering melanin to keratinocytes, thereby providing skin pigmentation and hair coloration (Furuya et al. 2009). Hyperpigmentation results from an increase in melanin, and its treatment remains a challenge. Many types of treatment are available for acquired skin hyperpigmentation, such as alpha hydroxy acid or laser therapies, but none are completely satisfactory. To be effective, potential treatments for pigment disorders need to influence melanin formation; depigmenting compounds would have to act selectively on hyperactivated melanocytes, without producing any short- or long-term side-effects, and induce permanent removal of the undesired pigment. Identification of such a depigmenting compound would require an understanding of the biology of melanocytes, and the mechanism of melanin synthesis (Oiso et al. 2008). The ability to culture melanocytes would be beneficial in the understanding of pigment cell biology and the pathology of pigment diseases.
The culture of melanocytes has proven to be difficult, due to the low percentage of melanocytes in the total epidermal cell population and their low mitotic activity, compared with the other main skin cell types. This has resulted in melanocyte cultures being rapidly overgrown by keratinocytes and fibroblasts (Pittelkow and Shipley 1989). Eisinger and Marko solved this problem by using the tumor-promoting phorbol ester 12-O-tetradecanoyl phorbol-13-acetate (TPA), which enhances the proliferation and attachment of melanocytes and is toxic to keratinocytes. Furthermore, use of cyclic AMP (cAMP)-inducers, such as cholera toxin (CT) and isobutyl-methylxanthine (IBMX), which have a mitogenic effect on melanocytes, can be used to promote melanotcyte cell division in cell culture (Eisinger and Marko 1982). Over the past decade, these advances, along with recent technological improvements in culture systems, mean that major progress has been made in the culture of skin melanocytes. Basic fibroblast growth factor (bFGF), nerve growth factor (NGF), and endothelin-1 (ET-1) are keratinocyte-derived factors that enhance melanocyte proliferation, tyrosinase activity and dendricity (Akio et al. 2004). At present, either TPA or ET-1 is essential for the successful culture of melanocytes.
Melanocyte densities in foreskin and scalp hair follicles are relatively high, and, consequently, these are the cell types that are predominantly used for melanocyte cell culture. However, pigmentation diseases that affect appearance occur on the face, so the specimens used for melanocyte cell culture in this study were obtained from face skin samples obtained from patients who had undergone lower blepharoplasty.
Materials and methods
Materials
M254 medium (Item: M-254-500) and human melanocyte growth supplement (HMGS2) (Item: S-016-5; GIBCO Cascade Biologics, Portland, OR, USA) were used for cell culture. 0.25 % neutral protease (Solarbio Company, Shanghai, China) and 0.25 % trypsin + 0.02 % EDTA (Gino Company, Hangzhou, China) was used for digestion to obtain single cell suspensions of epidermal cells. Newborn calf serum (Holly Leaf Company, Hangzhou, China) was used for terminate the digestion of protease. 0.1 % l-DOPA (Item: D9628, Sigma, St. Louis, MO, USA) was used for melanocytes staining. MTT (Sigma) colorimetric test was used to plot growth curves of the melanocytes. S-100 monoclonal antibody (Item: BMO120) and SABC-AP immunohistochemistry kits (Item: SA1052; Boster Company, Wuhan, China) were used for cell identification. Anti-TRP1 antibody (Item: ab83774) and secondary antibodies (Item: ab150077; Abcam, Cambridge, UK) were used for detection of TRP-1 expression.
Primary culture of melanocytes from face skin
The skin specimens were obtained from patients following lower blepharoplasty procedures. This study was approved by the Institutional Research Ethics Committee of the General Hospital of Guangzhou Military Command, and informed consent was obtained from all patients. The skin specimens were immersed in an iodine solution for 5 min, then washed extensively with cold normal saline. The subcutaneous tissue and dermis were removed, and the remaining skin was cut into small sections (0.5 mm thick) and placed in 0.25 % neutral protease overnight at 4 °C to obtain the epidermis, which was then immersed in a solution of 0.25 % trypsin and 0.02 % EDTA at 37 °C for 5 min. This digestion was terminated by the addition of serum. Single cell suspensions were obtained by pipette blowing, filtered through a 200 mesh filter for screening and centrifuged twice at 1,500 rpm for 6 min. M254 medium, supplemented with 1 % (v/v) human melanocyte growth supplement (HMGS2), 100 U/ml penicillin and 50 μg/ml streptomycin, was added to the cells. The cells were then seeded into 25 cm2 culture flasks, at 5 × 105 cells per flask, and cultured at 37 °C in a humidified atmosphere with 5 % CO2. The culture medium was changed after 48 h. And cell morphology was observed using inverted microscopy.
Melanocyte subcultures
When the melanocytes had reached 70–80 % confluence, they were passaged at a ratio of 1:5. The adherent cells were released by digestion with a solution of 0.25 % trypsin and 0.02 % EDTA for 2 min at 37 °C, and single cell suspensions were obtained by gentle blowing with a pipette. Serum was added to terminate the digestion, and cells were centrifuged twice at 1,500 rpm for 6 min. The supernatant was discarded, and M254 medium supplemented with HMGS was added. The cells were seeded into 25 cm2 culture flasks at 1 × 105 cells per flask, and cultured at 37 °C with 5 % CO2. The medium was changed after 24 h, and thereafter every 3–4 days.
Melanocyte cell growth curves
Third generation logarithmic phase melanocytes were collected by trypsin digestion, adjusted to 2 × 104 cells/ml and seeded into 96 well plates (200 μl per well; approximately 4 × 103 cells in each well), cultured at 37 °C and 5 % CO2. After 24 h, five wells were selected each day to carry out the methyl thiazolyl tetrazolium (MTT) colorimetric test (observing absorbance at 490 nm). This test was performed every 24 h for a total of 10 days. The results of this test were used to plot growth curves for the melanocytes, using time as the horizontal axis and the average daily absorbance (OD value) as the vertical axis. Cells from foreskin samples after circumcision were used as controls to compare the proliferation character according to the same culture method of melanocytes from face skin.
Identification of melanocytes
The subcultured melanocytes were seeded onto glass coverslips placed in 6-well plates, and incubated at 37 °C and 5 % CO2. When cell growth reached 50–70 % confluence, the coverslips were taken off using fine forceps, and the cells were rinsed twice in phosphate-buffered saline (PBS). Cells were fixed by the addition of cold acetone for 20 min, and used for the following identification tests: (1) hematoxylin-eosin (HE) staining to observe cell morphology; (2) anti-S-100 protein immunohistochemistry to identify melanocytes, carried out in accordance with the manufacturer’s instructions of the kits; (3) DOPA staining to identify melanocytes. DOPA staining was performed by adding 1 g/l of 0.1 % l-DOPA to the cells, which were then incubated at 37 °C for 4 h, air dried and sealed with glycerol for observation. l-DOPA stains melanocytes gray or black because of tyrosinase in melanocytes, which synthesize melanin granules from l-DOPA.
Immunofluorescent staining with melanocyte lineage-specific monoclonal antibodies
Melanocyte antigen expression was studied using immunofluorescent (IF) staining with melanocyte lineage-specific monoclonal antibodies. Briefly, the cells were grown in 6-well chamber slides (Nunc, Roskilde, Denmark), washed with PBS, and fixed with absolute methanol at −20 °C for 15 min. Then the cells were washed three times with Triton X-100 (Boehringer Mannheim, Germany) and PBS. Fixed cells were incubated overnight at 4 °C with primary antibodies directed towards tyrosinase-related protein (TRP-1; polyclonal rabbit anti-human TRP-1, working dilution 1:50; Abcam), a melanocyte-specific protein that is involved in black eumelanin synthesis and tyrosinase stabilization. Negative controls were incubated as above, but in the absence of the primary antibody. Cells were washed three times with PBS and incubated with fluorochrome-conjugated species-specific secondary antibodies (working dilution 1:100; Abcam). Finally, the slides were washed three times with PBS and rinsed with water. For each slide, a minimum of ten fields or, if possible, all the fields on the slide, were examined and photographed with a fluorescence microscope (OLYMPUS Company, Tokyo, Japan).
Transmission electron microscopic analysis of melanocyte ultrastructure
Cultured melanocytes were collected, fixed with 3 % glutaraldehyde and 1 % osmic acid, and ultrathin sections were prepared in accordance with standard procedures to observe melanocyte ultrastructure via transmission electron microscopy.
Results
Morphology of the cultured cells
Primary culture of cells from the skin samples produced cells that were adherent after 24 h, with some multi-dendritic cells scattered among the epidermal cells under inverted microscopy. Most multi-dendritic cells were round and small, had two or three dendrites, and an orbicular-ovate nucleus. For a small number of cells, the cell body was larger, with a circular nucleus and more than four short dendrites. Epidermal cells did not grow well and appeared to be slightly adherent in the melanocyte culture medium, which was discarded after medium exchange. The primary cultured cells could be passaged after being cultured for 20–30 days. A short time after being passaged (2–4 h) cells were able to adhere and grow (Figs. 1, 2).
Fig. 1.
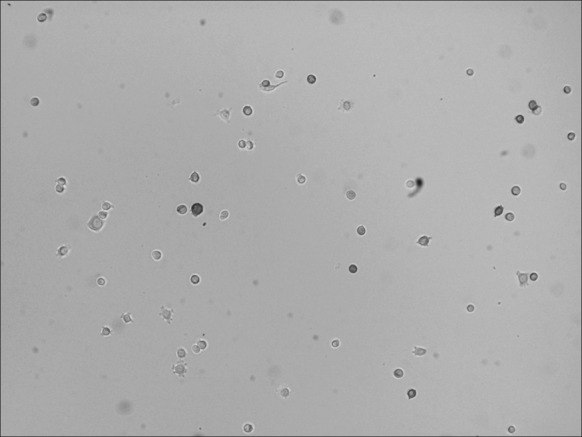
Primary culture of melanocytes. The medium was changed 48 h after inoculation. A proportion of the cells were adherent, and dendrites could be observed in some cells (inverted microscopy ×100)
Fig. 2.

Subcultured melanocytes showing that two or more dendrites could be seen to extend from the cells (inverted microscopy ×100)
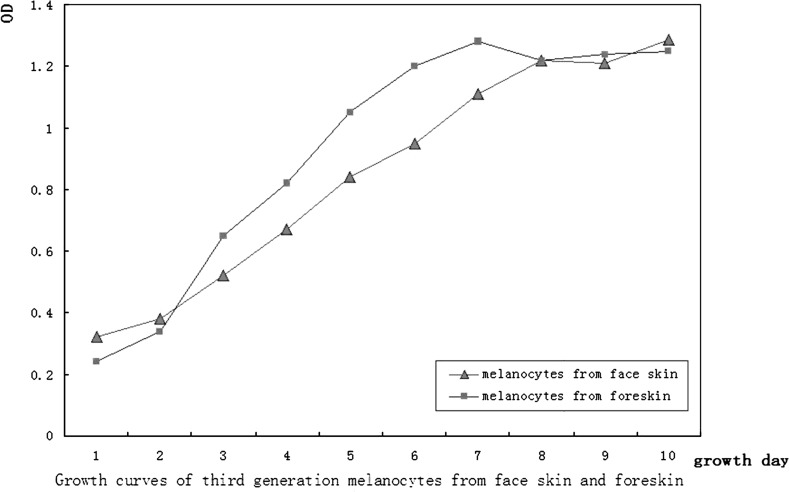
Melanocyte growth curves
To begin with, at days 1–4 after passage, cell proliferation was slow; by days 5–6 the cells proliferated rapidly and entered the logarithmic growth phase. After 10 days, the proliferation plateaued. After being cultured for 8–10 generations, the cells began to proliferate slowly and differentiate. However, the melanocytes from foreskin entered the logarithmic growth phase at days 3–4 after passage (Fig. 3).
Fig. 3.
Growth curves of third generation melanocytes in logarithmic phase originating from face skin and foreskin
Biological identification of melanocytes and cellular ultrastructure
The melanocytes were stained purple with HE (Fig. 4). The cytoplasm and dendrites of the cells were grayish or black under inverted light microscopy when stained with l-DOPA (Fig. 5). Immunohistochemical staining with anti-S-100 stained the cytoplasm and dendrites of the melanocytes brown (Fig. 6). Many mature melanosomes were observed in the melanocytes (Fig. 7).
Fig. 4.

Melanocytes after the third passage observed by HE staining (light microscopy ×200)
Fig. 5.
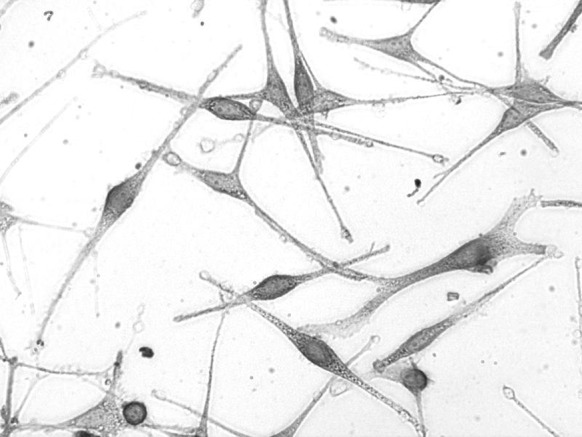
Melanocytes after the third passage observed by l-DOPA staining. Melanocytes are stained black; pigment granules can be observed (inverted microscopy ×400)
Fig. 6.

S-100 protein is positively expressed in melanocyte cytoplasm and dendrites appear brown by immunohistochemical staining in third passage of melanocytes (light microscope ×100)
Fig. 7.
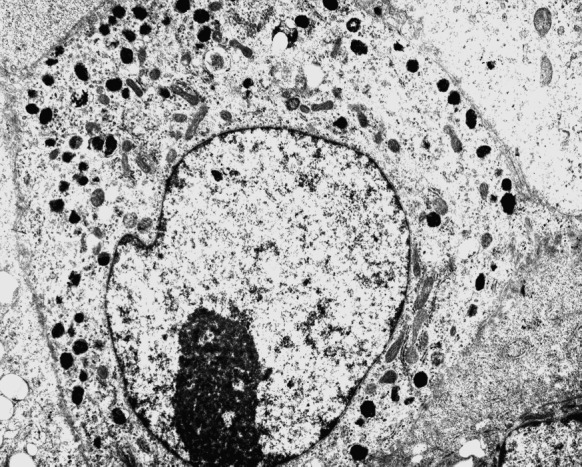
Mature melanosomes are observed in melanocytes (transmission electron microscopy ×12,000)
Immunofluorescent staining of melanocytes
To confirm the identity of the cultured melanocytes, we immunostained the cells with melanocyte-specific antibodies. After the second passage, immunostaining showed that all of the melanocytes expressed high levels of TRP-1, confirming the cells were melanocytes (Fig. 8).
Fig. 8.
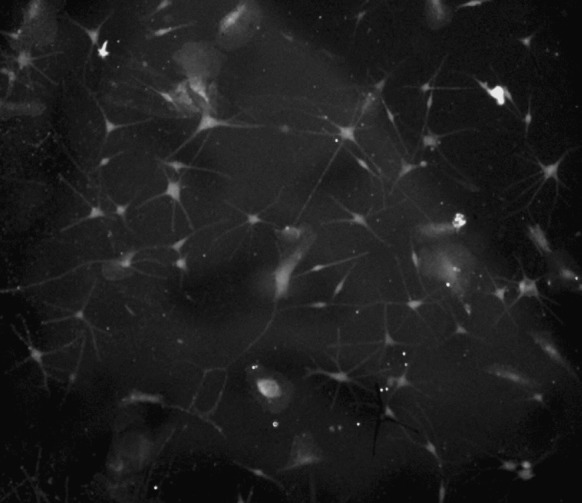
Positive expression of TRP-1 in cultured melanocytes observed by immunofluorescence (fluorescence microscopy ×400)
Discussion
Epidermal melanocytes are skin cells that are specialized in melanin production. In vivo, melanocytes exist in a close anatomical and functional relationship with keratinocytes in the epidermal melanin unit, and it is possible that melanocytes are capable of transferring melanosomes to keratinocytes. The ability to culture melanocytes would be helpful for investigation of the pathology of pigment diseases. In this experiment, we succeeded in the in vitro primary culture of melanocytes taken from samples of human face skin. Melanocytes are found at higher densities in the foreskin and scalp, so samples from these tissues have previously been used in attempts to culture melanocytes. Some researchers have also successfully cultured hair follicle melanocytes and choroidal melanocytes (Valtink and Engelmann 2007; Zhu et al. 2004). The pigmentation diseases that affect appearance, such as melisma and freckles, occur in the face and can often be improved with laser treatment (Wang and Chen 2012). In this study, melanocytes were successfully cultured using face skin samples obtained from lower blepharoplasty procedures.
The critical issue for culturing melanocytes is the choice of culture conditions and the preparation of the culture medium, as the growth of melanocytes is negligible under standard cell culture conditions. The first successful in vitro culture of adult human melanocytes was carried out by Nielsen and Don (1984), and was achieved by adding TPA and CT to the culture medium (Arita et al. 2000). CT is a neurotoxin that activates adenylyl cyclase and stimulates melanocyte proliferation. However, as CT is a toxic substance, researchers have been keen to develop an alternative reagent for use in melanocyte culture (Abdel-Naser 2003). M254 medium is a commercially available, complete medium produced by GIBCO cascade Biologics, and contains essential and non-essential amino acids, vitamins, other organic substances, trace elements and salts, but does not contain antibiotics, antifungal drugs, hormones, growth factors or other proteins. M254 medium also contains HEPES and bicarbonate buffer. To enable long-term cultures of melanocytes to be established, supplementation of M254 medium with HMGS is required. HMGS contains bovine pituitary extract (BPE), fetal bovine serum, bovine insulin, bovine transferrin, basic fibroblast growth factor (bFGF), hydrocortisone, heparin, and phorbol esters, including phorbol 12-myristate 13-acetate (PMA), 4-β-12-O-tetradecanoylphorbol 13-acetate (TPA), all of which are required for melanocyte growth (Swope et al. 1995). We used another commercially available medium complement (HMGS2), which does not contain PMA or TPA, but does contain endothelin-1, which was usually used in the culture of neonatal melanocytes or melanocyte stem cells (Hirobe 2001). The density of melanocytes in facial skin and hair follicles is lower than that in the foreskin, and a large proportion of these cells are mature melanocytes. After enzymatic digestion, low-speed centrifugation at approximately 800 rpm per min was used to harvest the cells. The differentiated cells or ageing cells in the supernatant were removed to increase the ratio of immature melanocytes. A 200 mesh filter was used to screen the cells before they were inoculated into the culture medium. HMGS2, which does not contain the gene mutation-inducing agents TPA or PMA, was used as a medium supplement, reducing the possibility of mutagenesis, and increasing the likelihood that the cultured cells would be suitable for clinical applications in the future.
Melanocytes are stained black after l-DOPA incubation. l-DOPA positive staining confirms the presence of DOPA oxidase (tyrosinase) activity in the melanosomes (Munoz–Munoz et al. 2010), and is used to positively identify melanocytes, as following protease digestion, melanocytes are the only DOPA oxidase-positive cells in the epidemis. The S-100 proteins are widely distributed in cells of neural crest origin and tumor cells. The observation of positive staining using both l-DOPA and S-100 proves that the cells we cultured were melanocytes (Petersson et al. 2009). Tyrosinase (TYR) and tyrosinase-related proteins-1 and -2 (TRP-1 and TRP-2) are related enzymes expressed in melanocytes and involved in melanin synthesis, which can also be used to identify melanocytes. The expression of TRP-1 is closely related to melanin synthesis and abnormal proliferation of melanocytes (Kedlaya et al. 2011).
At present, the in vitro culture of melanocytes has been widely used in the laboratory and clinical research of a variety of pigmentation diseases, such as post-traumatic pigmentation, depigmentation and acquired pigmentation disorders, such as melasma, senile plaques and vitiligo. In vitro melanocyte cultures are also used to study the etiology and pathology of malignant melanoma, and for studies examining the efficacy and toxicity of pigmentation removal drugs (Kumar et al. 2012). Cultured melanocytes have also been used in transplantations as a treatment for vitiligo (Chen et al. 2000). The research of melanocytes stem cells has made important progress (Davids et al. 2009).
Conclusion
In this study, we produced a primary culture of melanocytes using skin samples obtained from patients who had undergone a lower blepharoplasty; however, the amount of these skin specimens is limited. The cultured melanocytes were identified by l-DOPA and S-100 immunohistochemical staining. Immunofluorescence was used to demonstrate TRP-1 expression. Many mature melanosomes could be observed in the cultured melanocytes under transmission electron microscopy. The proliferation capacity of cultured melanocytes from face skin is less than those cultured from foreskin indicated from the cell growth curves. After eight to ten generations in culture, the cells begin to proliferate slowly and differentiate. Using a medium containing M254 and HMGS2, which does not contain TPA or CT, decreases the risk of gene mutations and potential toxicity if these cells were to have a clinical use. Further study of melanocytes cultured from face skin would be beneficial for investigations into the pathogenesis of melasma and other pigmentation disorders.
Acknowledgments
This work was supported by the CMA- L’OREAL CHINA SKIN GRANT 2010 (S2010070816).
References
- Abdel-Naser MB. Mitogen requirements of normal epidermal human melanocytes in a serum and tumor promoter free medium. Eur J Dermatol. 2003;13:29–33. [PubMed] [Google Scholar]
- Akio M, Hajime I, Hideo O, Norio K. Tyrosinase induction in normal human cultured melanocytes by endothelin-1. J Cardiovasc Pharmacol. 2004;44:S439–S442. doi: 10.1097/01.fjc.0000166321.76376.bb. [DOI] [PubMed] [Google Scholar]
- Arita Y, Santiago-Schwarz F, Coppock DL. Survival mechanisms induced by 12-O-tetradecanoylphorbol-13-acetate in normal human melanocytes include inhibition of apoptosis and increased Bcl-2 expression. Melanoma Res. 2000;10:412–420. doi: 10.1097/00008390-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Chen YF, Chang JS, Yang PY, Hung CM, Huang MH, Hu DN. Transplant of cultured autologous pure melanocytes after laser-abrasion for the treatment of segmental vitiligo. J Dermatol. 2000;27:434–439. doi: 10.1111/j.1346-8138.2000.tb02201.x. [DOI] [PubMed] [Google Scholar]
- Davids LM, du Toit E, Kidson SH, Todd G. A rare repigmentation pattern in a vitiligo patient: a clue to an epidermal stem-cell reservoir of melanocytes? Clin Exp Dermatol. 2009;34:246–248. doi: 10.1111/j.1365-2230.2008.02793.x. [DOI] [PubMed] [Google Scholar]
- Eisinger M, Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci USA. 1982;79:2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya R, Yoshida Y, Moro O, Tsunenaga M, Aoki H, Kishimoto J, Ifuku O, Hirobe T. Immunohistochemical survey of the distribution of epidermal melanoblasts and melanocytes during the development of UVB-induced pigmented spots. J Dermatol Sci. 2009;55:99–107. doi: 10.1016/j.jdermsci.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Hirobe T. Endothelins are involved in regulating the proliferation and differentiation of mouse epidermal melanocytes in serum-free primary culture. J Investig Dermatol Symp Proc. 2001;6:25–31. doi: 10.1046/j.0022-202x.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- Kedlaya R, Kandala G, Liu TF, Maddodi N, Devi S, Setaluri V. Interactions between GIPC-APPL and GIPC-TRP1 regulate melanosomal protein trafficking and melanogenesis in human melanocytes. Arch Biochem Biophys. 2011;508:227–233. doi: 10.1016/j.abb.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Parsad D, Kanwar A, Kaul D. Development of melanocye-keratinocyte co-culture model for controls and vitiligo to assess regulators of pigmentation and melanocytes. Indian J Dermatol Venereol Leprol. 2012;78:599–604. doi: 10.4103/0378-6323.100567. [DOI] [PubMed] [Google Scholar]
- Munoz–Munoz JL, Acosta-Motos JR, Garcia-Molina F, Varon R, Garcia-Ruiz PA, Tudela J, Garcia-Canovas F, Rodriguez-Lopez JN. Tyrosinase inactivation in its action on dopa. Biochim Biophys Acta. 2010;1804:1467–1475. doi: 10.1016/j.bbapap.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Nielsen HI, Don P. Culture of normal adult human melanocytes. Br J Dermatol. 1984;110:569–580. doi: 10.1111/j.1365-2133.1984.tb04680.x. [DOI] [PubMed] [Google Scholar]
- Oiso N, Tsuruta D, Imanishi H, Amatsu A, Kobayashi H, Kawara S, Kawada A. Spotted hyperpigmentation: disfigured melanosomes in melanocytes and keratinocytes. J Eur Acad Dermatol Venereol. 2008;22:876–878. doi: 10.1111/j.1468-3083.2007.02415.x. [DOI] [PubMed] [Google Scholar]
- Petersson S, Shubbar E, Enerback L, Enerback C. Expression patterns of S100 proteins in melanocytes and melanocytic lesions. Melanoma Res. 2009;19:215–225. doi: 10.1097/CMR.0b013e32832c6358. [DOI] [PubMed] [Google Scholar]
- Pittelkow MR, Shipley GD. Serum-free culture of normal human melanocytes: growth kinetics and growth factor requirements. J Cell Physiol. 1989;140:565–576. doi: 10.1002/jcp.1041400323. [DOI] [PubMed] [Google Scholar]
- Sulaimon SS, Kitchell BE. The biology of melanocytes. Vet Dermatol. 2003;14:57–65. doi: 10.1046/j.1365-3164.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- Swope VB, Medrano EE, Smalara D, Abdel-Malek ZA. Long-term proliferation of human melanocytes is supported by the physiologic mitogens alpha-melanotropin, endothelin-1, and basic fibroblast growth factor. Exp Cell Res. 1995;217:453–459. doi: 10.1006/excr.1995.1109. [DOI] [PubMed] [Google Scholar]
- Valtink M, Engelmann K. Serum-free cultivation of adult normal human choroidal melanocytes. Graefes Arch Clin Exp Ophthalmol. 2007;245:1487–1494. doi: 10.1007/s00417-007-0588-3. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chen CK. Effect of spot size and fluence on Q-switched alexandrite laser treatment for pigmentation in Asians: a randomized, double-blinded, split-face comparative trial. J Dermatol Treat. 2012;23:333–338. doi: 10.3109/09546634.2011.560929. [DOI] [PubMed] [Google Scholar]
- Zhu WY, Zhang RZ, Ma HJ, Wang DG. Isolation and culture of amelanotic melanocytes from human hair follicles. Pigment Cell Res. 2004;17:668–673. doi: 10.1111/j.1600-0749.2004.00190.x. [DOI] [PubMed] [Google Scholar]