Abstract
Diabetes mellitus is caused by absolute (type 1) or relative (type 2) deficiency of insulin-secreting islet β cells. An ideal treatment of diabetes would, therefore, be to replace the lost or deficient β cells, by transplantation of donated islets or differentiated endocrine cells or by regeneration of endogenous islet cells. Due to their ability of unlimited proliferation and differentiation into all functional lineages in our body, including β cells, embryonic stem cells and induced pluripotent stem cells are ideally placed as cell sources for a diabetic transplantation therapy. Unfortunately, the inability to generate functional differentiated islet cells from pluripotent stem cells and the poor availability of donor islets have severely restricted the broad clinical use of the replacement therapy. Therefore, endogenous sources that can be directed to becoming insulin-secreting cells are actively sought after. In particular, any cell types in the developing or adult pancreas that may act as pancreatic stem cells (PSC) would provide an alternative renewable source for endogenous regeneration. In this review, we will summarize the latest progress and knowledge of such PSC, and discuss ways that facilitate the future development of this often controversial, but crucial research.
Introduction
Diabetes mellitus is a major public health issue and has an increasing pandemic prevalence. This metabolic disorder currently affects over 382 million people, and this number is likely to increase to 592 million by 2035 (www.idf.org/diabetesatlas). Approximately 10% of these cases are of type 1 diabetes mellitus (T1D), caused by absolute deficiency of insulin-producing β cells resulting from autoimmune destruction. Should autoimmunity to β cells be controlled, a regenerative therapy would be a desirable avenue toward a cure of T1D, either by transplantation of hormone-secreting islets or by regeneration in situ of endogenous β cells. To achieve these ultimate goals, much attention has recently been paid to stem cells.
Stem cell is the term used to describe those undifferentiated cells that are capable of both self-renewal and giving rise to specialized functional cells. Stem cells are of pivotal importance for organ and tissue integrity and for injury and disease repair. Based on their developmental potential, stem cells are classified into four categories: (1) totipotent, (2) pluripotent, (3) multipotent, and (4) oligopotent/unipotent. Totipotent stem cells give rise to all three germ layers and extraembryonic tissues. Pluripotent stem cells are capable of generating the embryo proper, comprising all organs with ectoderm, mesoderm, and endoderm origins. Multipotent stem cells differentiate only into tissue-specific progenitors of a given organ. Unipotent or oligopotent stem/progenitor cells give rise only to one or a few functional cell types. Depending on the developmental stages of their origin, stem cells are known as embryonic stem cells (ESCs, generated from isolated inner cell mass of preimplanted embryos) [1,2]; epiblast stem cells (generated from postimplanted epiblast-stage embryos) [3,4]; germline-derived stem cells (generated from embryonic gonadal ridges or postnatal testes) [5–7]; induced pluripotent stem cells (iPSCs, originally induced from fetal or adult cells by the overexpression of defined transcription factors) [8–11] or tissue-specific stem cells (derived from postnatal tissues).
The ESCs and iPSCs theoretically have the ability to proliferate indefinitely and differentiate into all functional lineages of the body, including β cells. Earlier studies claimed successful differentiation of functional β cells from ESCs and iPSCs [12–15], and these have been summarized in recent reviews [16,17]. However, it is now clear from many studies employing diverse methods such as lineage-tracing, functional characterization, transplantation assays, and transcriptomic profiling, that this differentiation did not proceed beyond the pancreatic progenitor (PP), islet progenitor, and/or nonfunctional fetal β-cell stage [18–26]. Lack of breakthroughs in this area has diverted attention to tissue-specific stem cells.
Tissue-specific stem cells are a rare population residing in specific tissues, and show powerful potential for regeneration when required. They can be further divided based on the tissue origin into a number of categories such as neuronal stem cells, skin stem cells, hematopoietic stem cells, germline stem cells, mesenchymal stem cells (MSC) as well as gut stem cells. Unlike other tissue-specific stem cells, pancreatic stem cells (PSC) were proposed only relatively recently [27].
Due to their great potential importance for diabetes regeneration therapy, PSCs have attracted intense research over the last decade. So too has the reprogramming or transdifferentiation of surrogate β cells [28], although this subject is not the central theme of this review. We have recently reviewed PSCs [29], but the presence and origin of such cells has remained unverified and hotly debated. In this review, we will first discuss potential PSCs along the islet lineage developmental pathway, discuss several types of tentatively defined PSCs in the pancreas, and provide an update on the latest progress. We will also explore future directions of research using these cells.
Embryology
The pancreas is an endoderm-derived organ. The endoderm is one of the three primitive germ layers originated from the inner cell mass during gastrulation. After gastrulation, the thickened endodermal epithelium along the dorsal and ventral surfaces of the posterior foregut gives rise to the pancreas. In mice, these thickenings can be identified histologically at embryonic day (E) 9.0–9.5 [30].
Subsequently, these epithelia evaginate into the surrounding mesoderm-derived mesenchymal tissue and form dorsal and ventral pancreatic buds. These buds continue to expand, branch and fuse as a result of the gut rotation that brings the buds together. The fused developing pancreas continues to proliferate, differentiate and, ultimately, give rise to the mature organ. The adult pancreas consists of digestive fluid-transporting ductal tissue, digestive enzyme-secreting acinar tissue, and hormone-secreting cells located in the islets of Langerhans.
Interestingly, human pancreas development displays some unique features. For example, the dorsal bud can be detected as early as 26 days postcoitum (dpc), an equivalent stage to E9.5 mouse embryos, but insulin-positive cells are not visible until 52 dpc, ∼2 weeks later than the equivalent stage at which they could be detected in mice. The appearance of human insulin-positive cells precedes that of glucagon-positive cells at 8–10 weeks of development [31]. All islet cells are detectable at the end of the first trimester in humans [31], but at later stages (E17.5) in mice [32]. These data indicate that the sequence of key developmental events in human growth is not identical to that observed during mouse development [33], and this is supported by differences in gene expression patterns during developmental and disease processes in these two species [34]. More details of human pancreas development can be found in reviews elsewhere [35–38].
Developmental Intermediates as Potential PSC
Definitive endoderm
One of three germ layers during embryogenesis, the definitive endoderm gives rise to numerous organs in a process that is summarized in Fig. 1. Recapitulating their developmental pathways in vivo, ESCs give rise to definitive endodermal (DE) cells in vitro in the presence of a high concentration of activin A, a member of transforming growth factor β superfamily. ESC-derived human expandable DE cells are known as endodermal progenitors [25]. Remarkably, they have been shown to self-renew in the presence of a group of growth factors, including bone morphogenetic protein 4, fibroblast growth factor 2, vascular endothelial growth factor, and epidermal growth factor [25]. These progenitors can be passaged at least 24 times with a population expansion of hundreds of thousands fold. Furthermore, reprogrammed fibroblast-derived DE-like cells were independently demonstrated to be capable of expanding ∼65,000-fold in the presence of activin A and LiCl [39]. Although further studies are required, the endodermal progenitors may indeed have an astonishing capacity for expansion and act as prepancreas progenitors.
FIG. 1.
Simplified developmental pathway toward the pancreas islet lineages. Whereas inner cell mass (ICM) commits to three germ layers (the ectoderm, mesoderm, and endoderm) during gastrulation, embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC) preferentially differentiate into definitive endodermal (DE) cells [marked by the expression of Sox17 (the Sry-related HMG box transcription factor 17) and Foxa2 (foxhead homeobox 2a)] in the presence of activin A. Along the anterior–posterior axis, the DE is divided into foregut (giving rise to the lung, thyroid, and esophagus), posterior foregut [PF, marked by the expression of the transcription factor Hnf4a (hepatocyte nuclear factor 4a)], and hindgut (committing the intestine and colon). In vitro, retinoid acid would direct the DE cells to PF cells. Rather than to the stomach, liver, and gallbladder, the PF cells preferentially give rise to pancreatic progenitors [PP, marked by the expression of the transcription factor pancreas and duodenum homeobox 1 (Pdx1)] in the presence of retinoid acid and fibroblast growth factor 10. In addition to the ductal and acinar tissues, the PP commits to progenitors of the endocrine islet lineages [IP, marked by the expression of Ngn3 (neurogenin 3, also known as neurog3), as well as NeuroD (neural differentiation 1), IA1 (insulinoma associated 1), Isl1 (Islet 1), Pax6 (paired box factor 6) and Rfx6]. The IP then differentiates into five types of islet cells [α, β, δ (somatostatin), Ppy (pancreatic polypeptide), and ɛ (ghrelin)].
Pancreatic progenitors
A group of special cells in the thickened DE epithelium at E9.0–9.5 along the dorsal and ventral surfaces of the posterior mouse foregut expresses the gene named Pdx1 (pancreas and duodenum homeobox 1). Pdx1 is a member of the parahox homeobox transcription factor family and is essential for both the expansion of pancreas primordial populations [40] and the function of adult β cells [41,42].
Genetic lineage tracing experiments demonstrated that Pdx1-expressing (Pdx1+) cells give rise to duct, acinar, and endocrine tissues in the pancreas [43]. These progenitors are located at the tip of the branching pancreatic tree marked by Pdx1+Ptf1a+(pancreas transcription factor 1a) Cpa1+(carboxypeptidase 1) [44]. The Pdx1+ cells are able to take up bromodeoxyuridine [45], a thymidine analogue incorporated into DNA during S-phase of the cell cycle, indicating that these cells are proliferative. We previously developed protocols for dissociated fetal pancreatic cells committed from Pdx1+ progenitors to generate cystic epithelial colonies containing β cells in the presence of laminin 1,1,1 and growth factors [46–48]. Recently, lineage tracing demonstrated that the expandable cystic epithelial colonies are only generated from Pdx1+ progenitor-derived Sox9+ cells [49,50]. Future studies are required to determine whether all cystic epithelial colonies stochastically commit to various lineages or only certain fractions of cystic epithelial colony cells have defined differentiation potential.
Following in vivo developmental pathways, ESCs can be directed to give rise to Pdx1+ cells that are able to proliferate 16-fold in the presence of pancreas-derived mesenchymal cells [23]. Independent reproduction of these results will be essential. Some caution should be exercised because no data were presented to demonstrate that these ESC-derived Pdx1+ cells are indeed the equivalent of pancreatic Pdx1+ progenitors; this is important to consider, since Pdx1 is also expressed in other nonpancreas endoderm-derived tissues [51].
In humans, numerous PDX1+ cells can be readily detected in the pancreas between 8 and 21 weeks of age [52,53]. The number of PDX1+ cells that also express insulin or somatostatin progressively increases during this period of development [52]. An unanswered fundamental question is whether the PDX1+ cells are generated by self-renewal or commitment from their endodermal progenitors or both.
Identification of a specific marker that allows the purification of ESC-derived pancreatic Pdx1+ cells would be necessary. The capacity of Pdx1+ progenitors to proliferate and self-renew in vitro needs to be evaluated and established. Recently, reserpine and tetrabenzine, both inhibitors of vesicular monoamine transporter-2, were shown to be able to differentiate ESC-derived Pdx1+ cells into Ngn3-expressing cells [54]. Again, it would be interesting to address whether all or a fractional Pdx1+ population commits along the endocrine pathway.
Islet progenitors
At around E9.5 in mice, a small group of cells in the thickened DE epithelium begins to express the basic helix-loop-helix transcription factor Ngn3 (neurogenin 3, also known as neurog3) [43,55,56]. Earlier studies demonstrated that these Ngn3+ cells are islet progenitors because they can give rise to all islet lineage cells (Fig. 2). A number of observations support the importance of Ngn3 in islet development: islet cells do not develop in Ngn3 knockout mice [55]; gene lineage tracing shows that Ngn3+ cells give rise to all pancreatic endocrine cells [43]; in adult pancreas, purified Ngn3+ cells activated by partial duct ligation can, after injection into a fetal pancreas in vitro, differentiate into all islet cell types [56]. Again, it remains to be clarified whether the ESC-derived Ngn3-expressing cells [54,57] were bona fide islet progenitors.
FIG. 2.
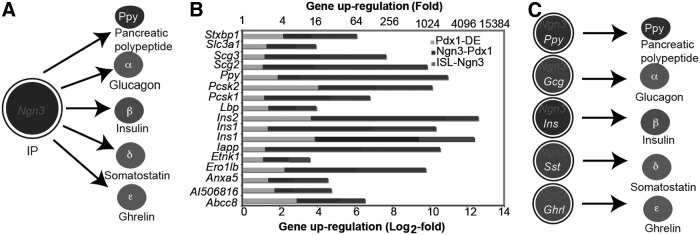
Ngn3+ cells are multipotent islet progenitors or unipotent islet precursors. (A) Ngn3+ islet progenitors are multipotent and give rise to all five types of mature islet cells. (B) Dynamic expression of islet genes during development. Modified from published data [72]. (C) Ngn3+ cells are unipotent and each give rise to one type of mature islet cells.
In mouse, Ngn3 mRNA expression peaks around E15.5 [58], which is equivalent to week 9 in humans. However, human NGN3 expression is low before 9 weeks, when its expression increases sharply and remains high until 17 weeks [53]. Although some studies seemed to show that Ngn3+ cells could proliferate [59,60], the recent genetic clonal assays by mosaic analysis with double marker (MADM) demonstrated that Ngn3+ cells are quiescent and each cell gives rise to only a single islet cell type [61]. Consistent with this, molecular analyses showed that Ngn3 inhibits proliferation by inducing cyclin-dependent kinase inhibitor 1a (Cdkn1a) [62]. The apparent contradiction between the earlier and latest studies may arise from different interpretations of the available data, and requires further studies to be reconciled.
Ghrelin (ɛ)-expressing cells
Ghrelin is a 28-amino acid polypeptide hormone and named due to its ability to stimulate growth hormone (GH) secretion (GH-relin). It has been shown to inhibit insulin secretion in mice, rats, and humans [63]. Ghrelin (ɛ)-expressing cells were originally identified in the stomach [64]. They are detectable in mid-gestation in the developing pancreas and their number peaks at late gestation or in neonates [65]. Adult islets of humans, but not of any other known species, contain a substantial number of ɛ cells [65], suggesting that these cells may play an unidentified role in the function of human islets.
The number of mouse ɛ cells can be drastically decreased by deletion of the Arx gene [66]. In contrast, numbers of ɛ cells were significantly increased in mice with deletions of Pax4, Pax6, or Nkx2.2, although this is at the expense of other islet cell types [67,68]. Interestingly, lineage-tracing studies demonstrated that ɛ cells are not terminally differentiated endocrine cells, because in the adult mouse pancreas they can give rise to α, Ppy and, to a lesser extent, β cells [69]. Whereas being redolent of progenitor cells, it remains to be established whether ɛ cells are facultative stem cells of functional islets.
Insulin+ stem cells
Earlier studies demonstrate that insulin-expressing cells in the developing pancreas gave rise to other islet cell types in addition to β cells [70], suggesting that the hormone-expressing cells are progenitors of other islet cells. Recently, by using genetic lineage-tracing techniques, the insulin+ stem cells (equivalent to PSCs herein) in the adult pancreas were derived from the embryonic pancreatic Pdx1+ cells, but not from the neural crest. These multipotent stem cells expressed an array of markers typical of islet progenitors and gave rise to all endocrine cells in vivo (Fig. 1). These stem cells are distinct from mature functional cells as they expressed a low level of insulin and a low level or absence of glucose transporter-2 [71]. However, given that insulin expression starts from Pdx1+ progenitors, through Ngn3+ progenitors to mature β cells [29,72] (Fig. 2B), further studies are required to verify whether the insulin+ stem cells are not only present in adult islets, but also in other developing stages.
Human counterparts of the mouse insulin+ stem cells have also been demonstrated [71]. Both mouse and human insulin+ stem cells could ameliorate hyperglycemia after transplantation to diabetic mice [71]. However, it is puzzling that the mouse insulin+ stem cells were also present in the fluorescence activated cell sorter (FACS)-sorted insulin-GFP− fractions. Critically, these clones have not yet been generated from single cells of fractionated populations.
The Presence of PSC in All Three Compartments
Substantial in vitro evidence from both rodents and humans has indicated that PSC may be present in all three major pancreas compartments, that is, the ductal epithelium [27,73,74], the acinar tissue, and the islets of Langerhans [75,76]. For example, a potential PSC candidate in the developing and adult mouse pancreas was purified by flow cytometry. These cells are defined by expression of the receptor for hepatocyte growth factor, c-Met, and the absence of blood cell surface markers such as CD45, TER119, c-Kit, and Flk-1. The isolated cells can differentiate into multiple pancreatic lineage cells in vitro and give rise to pancreatic acinar and endocrine cells in vivo after transplantation [77]. However, the in vivo localization and the molecular characteristics of these c-Met-expressing cells are largely unknown and single-cell clonogenesis has not been established.
Are PSCs present in the ductal epithelium?
In vitro and in vivo experiments have suggested the possibility that PSCs are localized to the ductal epithelium. Bonner-Weir et al. were the first to report that adult human pancreatic ductal epithelial cells give rise to islet-like clusters and differentiate into insulin-secreting β cells [78]. Ramiya et al. reported that in vitro generated islet-like structures from mouse PSC in the ductal epithelium were capable of reversing diabetes after transplantation [27]. In cultures of cellular aggregates after purification of human islets, fibroblast-like cells grew out as pancreatic mesenchymal stem cells (pMSC). The latter underwent at least 12 passages and expressed a range of bone marrow-derived MSC markers, including CD13, CD29, CD44, CD54, CD105, α6 integrin subunit (also known as CD49f), and Thy1 (also known as CD90). These pMSC were capable of generating at least two germ layer cells, including endoderm-derived cells, but there was no convincing evidence of pancreatic lineage cells [79]. Using culture conditions suitable for generating neurospheres ex vivo, mouse pancreatic ductal cells gave rise to neurosphere-like structures that differentiated into several types of islet cells, including β cells [76]. However, the molecular signature of these unique cells has not been defined. A limiting feature of all the above studies is their use of mixed cell populations, whereas most did not demonstrate clonogenesis.
Interestingly, after pancreatic duct ligation (PDL), numerous CK19+ ductal cells can be regenerated and give rise to islet cells [56]. Their lineage relationship has been independently addressed using the genetic Cre-loxP tracing system. In this system, Cre expression was directed by the promoter of carbonic anhydrase II, a marker of mature ductal cells, resulting in the excision of the stop cassette (Rosa-loxP-stop-loxP-lacZ) in transgenic Rosa26 (R26R) mice. This leads to β-galactosidase activity in cells expressing Cre (in this case, ductal cells). Four weeks later in normal or PDL pancreas, β-galactosidase was detectable in many ducts, patches of acinar cells, and 35%–40% of islet cells [80,81]. These data provided strong evidence that carbonic anhydrase II-expressing ductal epithelial cells are capable of producing islet cells, at least in mice. It is still unknown, however, whether all or part of the carbonic anhydrase II-expressing cell population has this developmental potential and whether the differentiation proceeds from PSC or does so as a transdifferentiation process from mature ductal cells.
In contrast, when an exon of Hnf1β (hepatocyte nuclear factor 1β, marking pancreatic ductal cells) was replaced with a Cre-containing transgene, genetic lineage tracing showed that the postnatal Hnf1β+ cells in these transgenic mice did not differentiate into islet cells, under both normal and experimental (including PDL) conditions [82]. The discrepancy between the two studies may arise from the specificity of Cre expression. However, another complication of the latter study is that one copy of Hnf1β was nonfunctional, resulting in halving the production of Hnf1β. Heterozygous Hnf1β mutant mice and humans display pancreatic agenesis [83,84], so haploinsufficiency of this gene in tracing experiments may have compromised the differentiation of ductal progenitors into functional islet cells. Nevertheless, further lineage-tracing studies also demonstrate that adult Sox9+ or Muc+ ductal cells do not give rise to pancreatic endocrine cells after the PDL [85–87]. As the Sox9 and Muc lineage tracings are only labeling a small fraction of adult ductal cells [85–87], better lineage-tracing technology may help address this controversial but important issue.
Interestingly, PDL activates the expression of the adult stem cell marker Lgr5 in the regenerating mouse pancreatic ductal cells [88]. Single sorted ductal cells can be expanded in the form of cystoid colonies in which Lgr5+ stem/progenitor cells are present [88]. Similarly, FACS-isolated human adult ductal cells are able to form self-renewable cystoid epithelial colonies [89]. These studies demonstrate that mouse and human pancreatic ductal cells have a strong expansion capacity. Future studies should concentrate on developing methods for directing these clonal cells toward endocrine lineages.
Are PSCs present in the pancreatic acinar tissue?
In the transplantation clinic, a large population of acinar cells would be discarded after purification of islets from donated pancreas. Identification of potential value for the acinar cells has thus attracted significant interest. After cotransplantation with fetal pancreatic cells under the kidney capsule of immunodeficient mice, these acinar cells gave rise to endocrine cells without evidence of β-cell replication or cell fusion [90]. These experiments suggest the existence of PSC or progenitor cells within the acinar compartment of the adult human pancreas. Consistently, analysis using the Cre/loxP-based tracing system demonstrated that amylase/elastase-expressing acinar cells were able to give rise to insulin-positive cells in a suspension culture [91]. However, because a clonal self-renewal assay of these amylase/elastase-expressing cells and their intermediate steps has not been convincingly demonstrated, this study may simply reveal that mouse and rat pancreatic acinar cells are able to transdifferentiate into surrogate insulin-expressing cells [92,93]. Furthermore, as the donated acinar cells [90] were not purified by flow cytometry, the possibility of contamination by ductal or even islet cells cannot be completely ruled out. Nevertheless, the possibility of transdifferentiation was supported by recent reprogramming studies. Overexpression of either three transcription factor genes, namely, Pdx1, Ngn3, and MafA (musculoaponeurotic fibrosarcoma oncogene family protein A) [94] or transient administration of epidermal growth factor and ciliary neurotrophic factor to diabetic mice [95] directly reprogrammed acinar cells to β-like cells. In contrast, other lineage-tracing experiments in vivo demonstrate that after 70%–80% pancreatectomy, preexisting mouse pancreatic acinar cells do not contribute to the regeneration of islet β cells [96]. These contradictory findings remain to be reconciled.
Are PSCs present in the islets?
There are several pieces of evidence demonstrating that nestin+ cells in the islets are putative PSCs and are hormone− (insulin, glucagon, somatostatin, pancreatic polypeptide, or ghrelin). These nestin+ cells proliferate in culture extensively (∼8 months) and give rise to cells that express liver and acinar pancreas markers, including α-fetoprotein, pancreatic amylase and a ductal/endocrine phenotype with the expression of CK19, neural-specific cell adhesion molecule, insulin, glucagon, and PDX1 [75]. These nestin+ putative progenitor cells may, therefore, participate in the neogenesis of islet endocrine cells [75], mediated at least partially by glucagon-like peptide-1, an incretin hormone processed from proglucagon [97]. Nevertheless, in vivo studies indicate that Nestin+ cells are mostly restricted in nonendodermal-derived cells [98,99]. Hence, whether distinct nestin+ cells in the islets are PSCs has not yet been established.
The fibroblast-like cells derived from the islets are also proposed as PSCs. These cells proliferate readily and give rise in vitro to hormone-expressing nontypical islet cell aggregates [100]. Studies on human islets transfected with a rat insulin promoter (RIP)-containing transgene demonstrated that RIP-expressing cells were dedifferentiated to fibroblast-like insulin− cells with up to 16 population doublings [101]. In contrast, a rigorous genetic-based lineage tracing in mice under the control of Pdx1 or RIP also demonstrated that PDX1- or RIP-expressing cells did not contribute significantly to these fibroblast-like cells in vitro [102]. Future studies are required to clarify these discrepancies.
Functional β Cells Are Facultative “Stem” Cells
Several pieces of strong evidence demonstrate that islet β cells act as facultative stem cells able to reproduce themselves. Dor et al. used RIP-driven reporter genes for genetic tracing of the fate of insulin-secreting cells [103]. They first revealed that adult mouse pancreatic β cells could be duplicated by RIP-expressing cells within the islets, both physiologically and after partial pancreatectomy. This study assumed that all RIP-expressing cells in adult islets are functional β cells, so it did not preclude the presence and action of PSC. Similarly, use of a transgenic model in which the expression of diphtheria toxin was directed by RIP to β cells resulted in apoptosis of 70%–80% of β cells, destruction of islet architecture, and eventual diabetes. Withdrawal of diphtheria expression led to a significant regeneration of β-cell mass and a spontaneous normalization of blood glucose levels and islet architecture [104]. Simultaneously, RIP-based lineage-tracing analysis indicated that the proliferation of a subset of 20%–30% surviving β cells played a major role in this regeneration and in recovery of euglycemia [104]. These studies suggest that islet β cells can act as facultative stem cells.
Using the sophisticated MADM system in double transgenic mice (designated RIP-CreER; Rosa26GR/Rosa26RG), each RIP-expressing clone was demonstrated to be composed of 5.1±5.4 or 8.2±6.9 cells after 1 or 2 months of chase, respectively [105]. These RIP-expressing clones have been taken as further evidence of regeneration of functional β cells, but this should be balanced in view of fidelity of insulin gene expression, as discussed below. An additional loss-of-function study following knockout of the Hnf4α gene suggested that the β-cell regeneration may involve the Ras/Erk signaling cascade [106] and ultimately be regulated by cycling modulators, including cyclin D2 [107]. Taken together, further identification and characterization of the so-called self-replicative or proliferative RIP-expressing cells both in vivo and in vitro is critical, because these cells may hold a key for a regenerative therapy for T1D.
On the other hand, thymidine-based lineage-tracing experiments showed that β cells were produced within an islet by rare self-renewing cells with a long replication refractory period, but both the identity of these unique cells and the length of the replication refractory period are unknown. The frequency of these self-renewing cells was significantly increased after partial pancreatectomy or during pregnancy [108]. Further studies should determine the molecular signature and biological potential of these replicating self-renewal cells. Due to obvious ethical and technical issues, similar studies cannot be performed in human islets, but such investigation could at least be repeated in larger mammal islets.
The abovementioned investigations of β-cell duplication as a mechanism of islet regeneration have attracted great attention in recent years because of its potential as a regenerative therapy to diabetes. However, a limitation of these studies is whether insulin gene expression (as directed for example by RIP-containing transgenes) is a marker exclusively of functional β cells. There is increasing evidence that it is not. First, the demonstration of insulin+ stem cells [71] precludes insulin as an exclusive marker of functional β cells. Second, we have demonstrated that insulin gene expression starts from the Pdx1+ progenitor stage, progressively increasing through Ngn3+ progenitors and reaching its plateau in mature islet β cells [29,72] (Fig. 2B). Third, insulin protein has been detected in islet progenitors in both humans and mice. In dual fluorescence reporter mouse line, a few Ngn3+ cells in the developing pancreas coexpress insulin [109]. In humans some NGN3+ cells were also detected to coexpress insulin in the fetal pancreas between 10 and 21 weeks [52].
Overall, insulin gene expression is not a marker exclusively of functional β cells. It is formally possible that β-cell regeneration in adults is derived from both self-duplication of glucose-responsive functional cells and self-renewal and differentiation of PSC. To validate that the multipotent stem cells do exist in the adult islets their self-renewal must be demonstrated at the single cell level, with observations made of the various time points of clonogenesis.
Future Directions for PSC Research
Key goals of the field should be to: define well-agreed general criteria for a PSC; identify specific cell surface markers that can be used to characterize and purify cells that may have PSC potential; establish a simple, effective, and reproducible in vitro assay to examine self-renewal and differentiation potential of purified cells; and to develop in vivo functional assays to determine biological function in both experimental animals and humans.
We propose that PSC should be defined with at least the following criteria:
(1) clonogenesis should be demonstrated at the level of single cells sorted by flow cytometry such as those performed [88,89];
(2) sorted single cells should be tested by in vitro self-renewal assays;
(3) clonogenic cells should give rise in vitro to more than one specialized cell lineage; and
(4) after transplantation, these cells should differentiate in vivo into different functional cell types.
Moreover, investigation of PSC should be particularly encouraged based on two considerations. First, PSC and dedifferentiation or transdifferentiation of non-β cell types in the pancreas may provide an alternative source of surrogate β cells. Second, as there is a significant difference in regeneration capacity between rodent and human islets, it may be wise not to extrapolate directly from regeneration data of rodents to humans.
Unlike previous experiments that were only performed in vivo or in vitro without targeting specific cell types, future PSC work should employ integrated approaches. Identification of cell surface markers and application of flow cytometry would allow fractionation of heterogeneous cell populations for examining in vitro their potential of self-renewal and clonogenesis. Single-cell biology (ie, single-cell transcriptomics and single-cell RNA-seq) [110,111] would dissect their developmental pathways and molecular mechanisms and in combination of genetic tracing examine their lineage contribution and developmental potential in vivo. Finally multidiscipline, multilaboratory, and even multinational collaborations, including the participation of the pharmaceutical sector, will ultimately develop a regenerative therapy for diabetes, particularly T1D.
Acknowledgments
The authors are supported by grants from Juvenile Diabetes Research Foundational International (4-2006-1025), the Diabetes Australia, the Diabetes Research Foundation of Western Australia, the University of Western Australia, National Health and Medical Research Council Program Grant (53000400), and the Medical Research Foundation of Royal Perth Hospital.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Evans MJ. and Kaufman MH. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156 [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78:7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, et al. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199 [DOI] [PubMed] [Google Scholar]
- 4.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195 [DOI] [PubMed] [Google Scholar]
- 5.Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, et al. (1998). Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A 95:13726–13731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, et al. (2004). Generation of pluripotent stem cells from neonatal mouse testis. Cell 119:1001–1012 [DOI] [PubMed] [Google Scholar]
- 7.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, et al. (2006). Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440:1199–1203 [DOI] [PubMed] [Google Scholar]
- 8.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, et al. (2008). Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321:699–702 [DOI] [PubMed] [Google Scholar]
- 9.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, et al. (2008). Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133:250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 11.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, et al. (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146 [DOI] [PubMed] [Google Scholar]
- 12.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, et al. (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 13.Jiang W, Shi Y, Zhao D, Chen S, Yong J, et al. (2007). In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res 17:333–344 [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Bai Z, Zhang D, Shi Y, Yong J, et al. (2008). Differentiation of mouse nuclear transfer embryonic stem cells into functional pancreatic beta cells. Diabetologia 51:1671–1679 [DOI] [PubMed] [Google Scholar]
- 15.Zhang D, Jiang W, Liu M, Sui X, Yin X, et al. (2009). Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19:429–438 [DOI] [PubMed] [Google Scholar]
- 16.Hebrok M. (2012). Generating beta cells from stem cells-the story so far. Cold Spring Harb Perspect Med 2:a007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagliuca FW. and Melton DA. (2013). How to make a functional beta-cell. Development 140:2472–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, et al. (2008). Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- 19.Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, et al. (2011). Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol 29:750–756 [DOI] [PubMed] [Google Scholar]
- 20.Xie R, Everett LJ, Lim HW, Patel NA, Schug J, et al. (2013). Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell 12:224–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, et al. (2009). A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5:258–265 [DOI] [PubMed] [Google Scholar]
- 22.Chetty S, Pagliuca FW, Honore C, Kweudjeu A, Rezania A, et al. (2013). A simple tool to improve pluripotent stem cell differentiation. Nat Methods 10:553–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sneddon JB, Borowiak M. and Melton DA. (2012). Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature 491:765–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, et al. (2012). The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia 55:358–371 [DOI] [PubMed] [Google Scholar]
- 25.Cheng X, Ying L, Lu L, Galvao AM, Mills JA, et al. (2012). Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell 10:371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hrvatin S, O'Donnell CW, Deng F, Millman JR, Pagliuca FW, et al. (2014). Differentiated human stem cells resemble fetal, not adult, beta cells. Proc Natl Acad Sci U S A 111:3038–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, et al. (2000). Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 6:278–282 [DOI] [PubMed] [Google Scholar]
- 28.Bouwens L, Houbracken I. and Mfopou JK. (2013). The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat Rev Endocrinol 9:598–606 [DOI] [PubMed] [Google Scholar]
- 29.Jiang FX. and Morahan G. (2012). Pancreatic stem cells: from possible to probable. Stem Cell Rev 8:647–657 [DOI] [PubMed] [Google Scholar]
- 30.Pictet RL, Clark WR, Williams RH. and Rutter WJ. (1972). An ultrastructural analysis of the developing embryonic pancreas. Dev Biol 29:436–467 [DOI] [PubMed] [Google Scholar]
- 31.Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, et al. (2004). Beta cell differentiation during early human pancreas development. J Endocrinol 181:11–23 [DOI] [PubMed] [Google Scholar]
- 32.Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, et al. (1991). Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development 113:1257–1265 [DOI] [PubMed] [Google Scholar]
- 33.Richardson MK, Hanken J, Gooneratne ML, Pieau C, Raynaud A, et al. (1997). There is no highly conserved embryonic stage in the vertebrates: implications for current theories of evolution and development. Anat Embryol (Berl) 196:91–106 [DOI] [PubMed] [Google Scholar]
- 34.Fougerousse F, Bullen P, Herasse M, Lindsay S, Richard I, et al. (2000). Human-mouse differences in the embryonic expression patterns of developmental control genes and disease genes. Hum Mol Genet 9:165–173 [DOI] [PubMed] [Google Scholar]
- 35.Lukinius A, Ericsson JL, Grimelius L. and Korsgren O. (1992). Ultrastructural studies of the ontogeny of fetal human and porcine endocrine pancreas, with special reference to colocalization of the four major islet hormones. Dev Biol 153:376–385 [DOI] [PubMed] [Google Scholar]
- 36.Polak M, Bouchareb-Banaei L, Scharfmann R. and Czernichow P. (2000). Early pattern of differentiation in the human pancreas. Diabetes 49:225–232 [DOI] [PubMed] [Google Scholar]
- 37.Pan FC. and Brissova M. (2014). Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes 21:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Krijger RR, Aanstoot HJ, Kranenburg G, Reinhard M, Visser WJ, et al. (1992). The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol 153:368–375 [DOI] [PubMed] [Google Scholar]
- 39.Li K, Zhu S, Russ HA, Xu S, Xu T, et al. (2014). Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell 14:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonsson J, Carlsson L, Edlund T. and Edlund H. (1994). Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371:606–609 [DOI] [PubMed] [Google Scholar]
- 41.Ohneda K, Mirmira RG, Wang J, Johnson JD. and German MS. (2000). The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol 20:900–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao T, Mc Kenna B, Li C, Reichert M, Nguyen J, et al. (2014). Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab 19:259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu G, Dubauskaite J. and Melton DA. (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, et al. (2007). A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13:103–114 [DOI] [PubMed] [Google Scholar]
- 45.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, et al. (2007). SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A 104:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang FX, Stanley EG, Gonez LJ. and Harrison LC. (2002). Bone morphogenetic proteins promote development of fetal pancreas epithelial colonies containing insulin-positive cells. J Cell Sci 115:753–760 [DOI] [PubMed] [Google Scholar]
- 47.Jiang FX. and Harrison LC. (2005). Convergence of bone morphogenetic protein and laminin-1 signaling pathways promotes proliferation and colony formation by fetal mouse pancreatic cells. Exp Cell Res 308:114–122 [DOI] [PubMed] [Google Scholar]
- 48.Jiang FX. and Harrison LC. (2005). Laminin-1 and epidermal growth factor family members co-stimulate fetal pancreas cell proliferation and colony formation. Differentiation 73:45–49 [DOI] [PubMed] [Google Scholar]
- 49.Jin L, Feng T, Shih HP, Zerda R, Luo A, et al. (2013). Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A 110:3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugiyama T, Benitez CM, Ghodasara A, Liu L, McLean GW, et al. (2013). Reconstituting pancreas development from purified progenitor cells reveals genes essential for islet differentiation. Proc Natl Acad Sci U S A 110:12691–12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holland AM, Gonez LJ, Naselli G, Macdonald RJ. and Harrison LC. (2005). Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes 54:2586–2595 [DOI] [PubMed] [Google Scholar]
- 52.Lyttle BM, Li J, Krishnamurthy M, Fellows F, Wheeler MB, et al. (2008). Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia 51:1169–1180 [DOI] [PubMed] [Google Scholar]
- 53.Jeon J, Correa-Medina M, Ricordi C, Edlund H. and Diez JA. (2009). Endocrine cell clustering during human pancreas development. J Histochem Cytochem 57:811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakano D, Shiraki N, Kikawa K, Yamazoe T, Kataoka M, et al. (2014). VMAT2 identified as a regulator of late-stage beta-cell differentiation. Nat Chem Biol 10:141–148 [DOI] [PubMed] [Google Scholar]
- 55.Gradwohl G, Dierich A, Le Meur M. and Guillemot F. (2000). neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, et al. (2008). Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132:197–207 [DOI] [PubMed] [Google Scholar]
- 57.Cai Q, Bonfanti P, Sambathkumar R, Vanuytsel K, Vanhove J, et al. (2014). Prospectively isolated NGN3-expressing progenitors from human embryonic stem cells give rise to pancreatic endocrine cells. Stem Cells Transl Med 3:489–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, et al. (2000). Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127:3533–3542 [DOI] [PubMed] [Google Scholar]
- 59.Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, et al. (2000). Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes 49:163–176 [DOI] [PubMed] [Google Scholar]
- 60.Oliver-Krasinski JM, Kasner MT, Yang J, Crutchlow MF, Rustgi AK, et al. (2009). The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. J Clin Invest 119:1888–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desgraz R. and Herrera PL. (2009). Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development 136:3567–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyatsuka T, Kosaka Y, Kim H. and German MS. (2011). Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc Natl Acad Sci U S A 108:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dezaki K. (2013). Ghrelin function in insulin release and glucose metabolism. Endocr Dev 25:135–143 [DOI] [PubMed] [Google Scholar]
- 64.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, et al. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- 65.Wierup N, Sundler F. and Heller RS. (2014). The islet ghrelin cell. J Mol Endocrinol 52:R35–R49 [DOI] [PubMed] [Google Scholar]
- 66.Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, et al. (2003). Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 17:2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B. and Sussel L. (2004). Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A 101:2924–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill JT, Mastracci TL, Vinton C, Doyle ML, Anderson KR, et al. (2009). Ghrelin is dispensable for embryonic pancreatic islet development and differentiation. Regul Pept 157:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arnes L, Hill JT, Gross S, Magnuson MA. and Sussel L. (2012). Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS One 7:e52026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alpert S, Hanahan D. and Teitelman G. (1988). Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell 53:295–308 [DOI] [PubMed] [Google Scholar]
- 71.Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, et al. (2011). The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8:281–293 [DOI] [PubMed] [Google Scholar]
- 72.Jiang FX, Mehta M. and Morahan G. (2010). Quantification of insulin gene expression during development of pancreatic islet cells. Pancreas 39:201–208 [DOI] [PubMed] [Google Scholar]
- 73.Cornelius JG, Tchernev V, Kao KJ. and Peck AB. (1997). In vitro-generation of islets in long-term cultures of pluripotent stem cells from adult mouse pancreas. Horm Metab Res 29:271–277 [DOI] [PubMed] [Google Scholar]
- 74.Suzuki A, Oyama K, Fukao K, Nakauchi H. and Taniguchi H. (2002). Establishment of clonal colony-forming assay system for pancreatic stem/progenitor cells. Cell Transplant 11:451–453 [PubMed] [Google Scholar]
- 75.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, et al. (2001). Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 50:521–533 [DOI] [PubMed] [Google Scholar]
- 76.Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, et al. (2004). Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 22:1115–1124 [DOI] [PubMed] [Google Scholar]
- 77.Suzuki A, Nakauchi H. and Taniguchi H. (2004). Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes 53:2143–2152 [DOI] [PubMed] [Google Scholar]
- 78.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, et al. (2000). In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 97:7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seeberger KL, Dufour JM, Shapiro AM, Lakey JR, Rajotte RV, et al. (2006). Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest 86:141–153 [DOI] [PubMed] [Google Scholar]
- 80.Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, et al. (2008). Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans 36:353–356 [DOI] [PubMed] [Google Scholar]
- 81.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, et al. (2008). Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, et al. (2009). Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 17:849–860 [DOI] [PubMed] [Google Scholar]
- 83.Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, et al. (2005). Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A 102:1490–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haumaitre C, Fabre M, Cormier S, Baumann C, Delezoide AL, et al. (2006). Severe pancreas hypoplasia and multicystic renal dysplasia in two human fetuses carrying novel HNF1beta/MODY5 mutations. Hum Mol Genet 15:2363–2375 [DOI] [PubMed] [Google Scholar]
- 85.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, et al. (2011). Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43:34–41 [DOI] [PubMed] [Google Scholar]
- 86.Kopinke D. and Murtaugh LC. (2010). Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, et al. (2011). Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138:653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, et al. (2013). Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32:2708–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee J, Sugiyama T, Liu Y, Wang J, Gu X, et al. (2013). Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife 2:e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, et al. (2006). Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med 12:310–316 [DOI] [PubMed] [Google Scholar]
- 91.Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, et al. (2005). Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci U S A 102:15116–15121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, et al. (2005). In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 48:49–57 [DOI] [PubMed] [Google Scholar]
- 93.Minami K, Okano H, Okumachi A. and Seino S. (2008). Role of cadherin-mediated cell-cell adhesion in pancreatic exocrine-to-endocrine transdifferentiation. J Biol Chem 283:13753–13761 [DOI] [PubMed] [Google Scholar]
- 94.Zhou Q, Brown J, Kanarek A, Rajagopal J. and Melton DA. (2008). In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, et al. (2014). Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol 32:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, et al. (2007). Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 117:971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abraham EJ, Leech CA, Lin JC, Zulewski H. and Habener JF. (2002). Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology 143:3152–3161 [DOI] [PubMed] [Google Scholar]
- 98.Lardon J, Rooman I. and Bouwens L. (2002). Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol 117:535–540 [DOI] [PubMed] [Google Scholar]
- 99.Selander L. and Edlund H. (2002). Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev 113:189–192 [DOI] [PubMed] [Google Scholar]
- 100.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, et al. (2004). Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science 306:2261–2264 [DOI] [PubMed] [Google Scholar]
- 101.Russ HA, Bar Y, Ravassard P. and Efrat S. (2008). In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes 57:1575–1583 [DOI] [PubMed] [Google Scholar]
- 102.Chase LG, Ulloa-Montoya F, Kidder BL. and Verfaillie CM. (2007). Islet-derived fibroblast-like cells are not derived via epithelial-mesenchymal transition from Pdx-1 or insulin-positive cells. Diabetes 56:3–7 [DOI] [PubMed] [Google Scholar]
- 103.Dor Y, Brown J, Martinez OI. and Melton DA. (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- 104.Nir T, Melton DA. and Dor Y. (2007). Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brennand K, Huangfu D. and Melton D. (2007). All beta cells contribute equally to islet growth and maintenance. PLoS Biol 5:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta RK, Gao N, Gorski RK, White P, Hardy OT, et al. (2007). Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev 21:756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Georgia S. and Bhushan A. (2004). Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teta M, Rankin MM, Long SY, Stein GM. and Kushner JA. (2007). Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12:817–826 [DOI] [PubMed] [Google Scholar]
- 109.Hara M, Dizon RF, Glick BS, Lee CS, Kaestner KH, et al. (2006). Imaging pancreatic beta-cells in the intact pancreas. Am J Physiol Endocrinol Metab 290:E1041–E1047 [DOI] [PubMed] [Google Scholar]
- 110.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, et al. (2014). The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shapiro E, Biezuner T. and Linnarsson S. (2013). Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet 14:618–630 [DOI] [PubMed] [Google Scholar]



