Abstract
Background and Objectives:
Fistula in ano is a common disease seen in the surgical outpatient department. Many procedures are advocated for the treatment of fistula in ano. However, none of the procedures is considered the gold standard. The latest addition to the list of treatment options is video-assisted anal fistula treatment (VAAFT). It is a minimally invasive, sphincter-saving procedure with low morbidity. The aim of our study was to compare the results with a premier study done previously.
Methods:
The procedure involves diagnostic fistuloscopy and visualization of the internal opening, followed by fulguration of the fistulous tract and closure of the internal opening with a stapling device or suture ligation. The video equipment (Karl Storz, Tuttlingen, Germany) was connected to an illuminating source.
Results:
The study was conducted from July 2010 to March 2014. Eighty-two patients with fistula in ano were operated on with VAAFT and were followed up according to the study protocol. The recurrence rate was 15.85%, with recurrences developing in 13 cases. Postoperative pain and discomfort were minimal.
Conclusion:
VAAFT is a minimally invasive procedure performed under direct visualization. It enables visualization of the internal opening and secondary branches or abscess cavities. It is a sphincter-saving procedure and offers many advantages to patients. Our initial results with the procedure are quite encouraging.
Keywords: Fistula in ano, Video-assisted anal fistula treatment (VAAFT), Fistuloscopy, Visual analog score (VAS), Sphincter-saving surgery
INTRODUCTION
Fistula in ano is a common problem in patients presenting to the surgical outpatient department. Various procedures have been advocated for the treatment of fistula in ano, including fistulectomy, fistulotomy, and use of a cutting seton. A considerable risk of recurrence of approximately 6.5% is reported with fistulectomy/fistulotomy for repairing simple fistula.1 The cutting seton is associated with recurrence and incontinence rates of 12% and 18%, respectively. The risk of incontinence is more associated to proximal location of the internal opening in the rectum.2 The major cause behind recurrence is the presence of complex fistula, recurrent fistula, horseshoe extension, failure to identify the secondary branches and the internal opening, and the level of surgeon expertise.3 The high risk of recurrence and incontinence associated with these traditional techniques led to development of various other novel procedures with low morbidity and high patient satisfaction. Some of these procedures that were attempted to treat complex anal fistulas are use of fibrin glues, anal fistula plugs, ligation of intersphincteric fistula tract (LIFT) procedure, and video-assisted anal fistula treatment (VAAFT).4–7
Recently, a new technique of VAAFT is developed by Meinero and Mori.7 The technique comprises fistuloscopy and fulguration of the fistulous tract. The main aims of this technique are identification of the internal opening, secondary tracts, and abscesses with closure of the internal opening of the fistulous tracts. There is no external wound and there is minimal morbidity. We report our experiences with this technique.
MATERIALS AND METHODS
Eighty-two consecutive patients with fistula in ano who were operated by VAAFT were included in the study. The mean age of the patients was 35.4 years (SD 12.3). Of the 82 patients, 66 (80.4%) were male and 16 (19.6%) were female. Patients with Crohn disease, tuberculosis, and malignancy of the tract were excluded from the study. In addition, the patients with anal incontinence were not included in the study. Patients were evaluated clinically, and magnetic resonance imaging scans were done in all patients to evaluate the tract of fistula. Preoperative anal manometry was done and repeated in the postoperative phase at one month and 6 months. The surgery was performed under a subarachnoid block with the patient in the dorsal lithotomy position.
The fistuloscope video equipment (Karl Storz, Tuttlingen Germany) is an 8-degree angled endoscope with optical, working, and irrigation channels. The length of the fistuloscope is 18 cm and the diameter 3.3 × 4.7 mm. It also has a removable handle, which contributes to a successful procedure. The fistuloscope has two channels, one of which is connected to irrigation fluid and the other which introduces the instruments. The instruments used in the procedure are shown in Figures 1 and 2. Figure 1 shows the fistuloscope and the electrical diathermy probe; Figure 2 shows the endobrush and endoscopic grasper. The irrigation fluid used was a glycine-mannitol solution.
Figure 1.
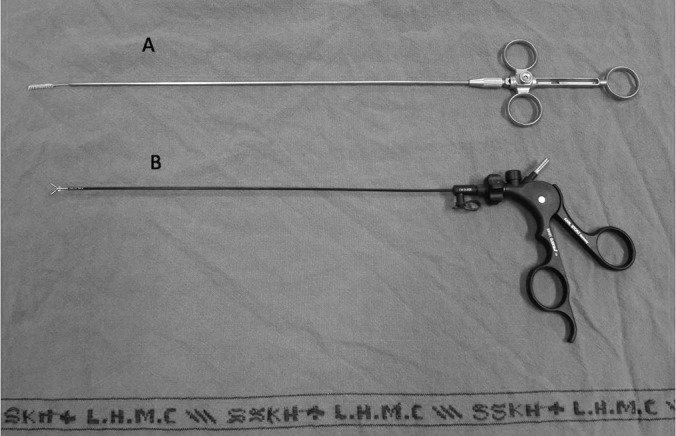
A, Endobrush. B, Endoscopic grasper.
Figure 2.
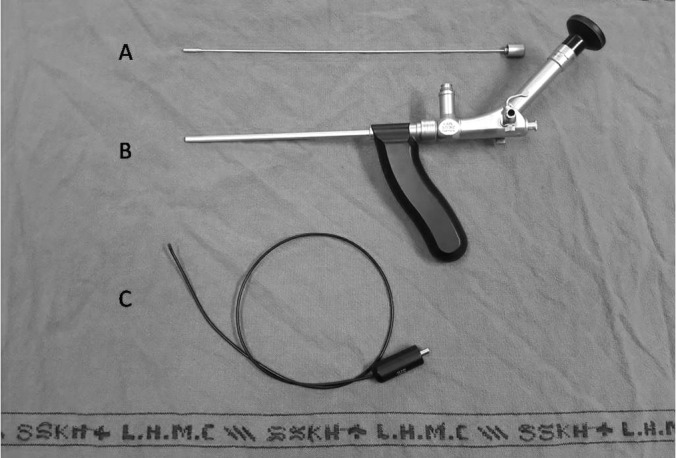
A, Obturator. B, Fistuloscope. C, Diathermy probe.
After proper cleaning and draping of the anal area, an obturator is introduced in the anal canal and fistuloscopy is done to correctly locate the internal opening of the fistulous tract, secondary tracts, and abscess cavity if any. The running glycine-mannitol solution helps to open the fistulous tract. The scope is then advanced forward slowly and the tract is straightened by maneuvering the scope. All of the tracts accommodated the fistuloscope. The next step is visualization of the internal opening, which is identified by the exit of the fistuloscope through it. Narrow openings are identified as a beam of illumination through the rectal mucosa or the exit of irrigating fluid through them, as depicted in Figure 3. Both primary and secondary os and tracts were explored via fistuloscope. After the internal opening was located, absorbable sutures are taken at its site in the rectum or anal canal for applying traction. In the next step, the obturator is removed and the tract is fulgurated with a probe connected to electric diathermy advancing gently from the external opening to the internal opening under direct vision. A fulgurated tract is depicted in Figure 4. The necrotic material is removed with an endobrush and irrigation fluid. The internal opening is then closed by application of absorbable sutures or use of a stapling device.
Figure 3.
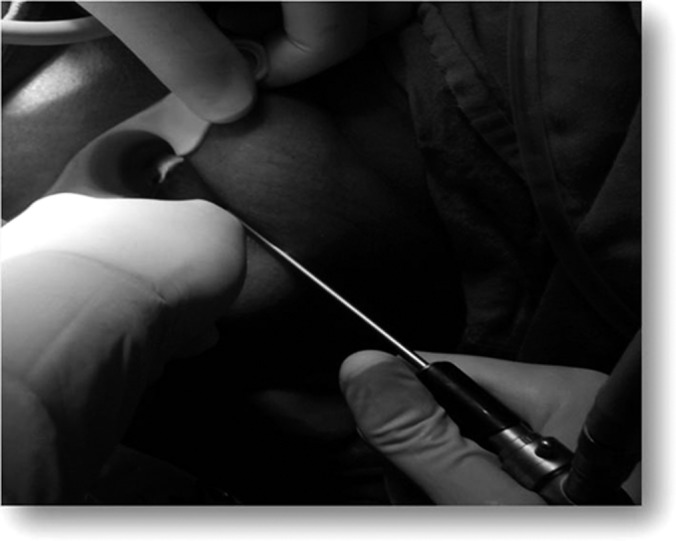
Illuminated internal opening.
Figure 4.
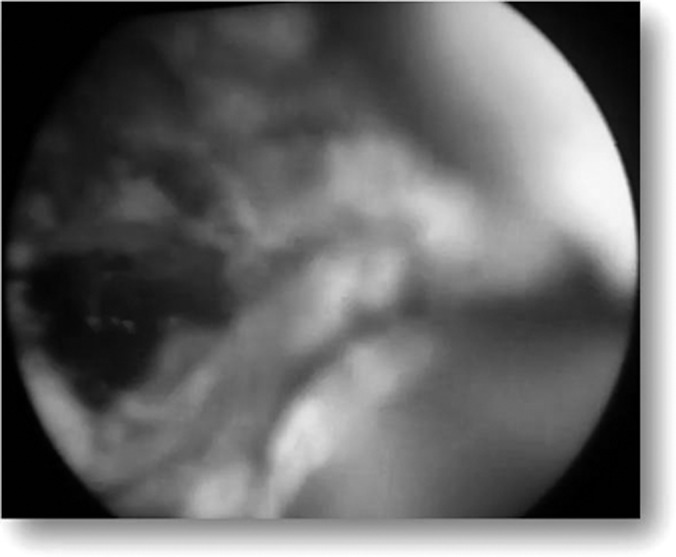
Fulgurated fistulous tract.
Postoperatively, patients were followed up after one month and six months. The parameters considered in the study were:
type of fistula
operative time
blood loss
postoperative visual analog scale (VAS) for pain scoring
time to return to work after surgery
patient acceptability and satisfaction
recurrence rates
anal manometry preoperatively and postoperatively
RESULTS
Of the 82 patients operated on during the duration of the study, 61 (74.39%) had fistulas of the low variety, and the remaining 21 (25.6%) had high fistulas. Of the patients, 62 (80.4%) had a single straight tract, and the remaining 12 (19.6%) patients had a complex tract with two internal openings. The internal opening was in the anal canal in 12 cases (14.63%), at the level of the dentate line in 49 (59.76%), and in the rectum in the remaining 21 (25.6%). In 59 cases (71.95%), the internal opening was located by direct fistuloscopy, and in the remaining 23 (28.05%) by visualization of light in the rectum.
The operating time ranged from 30 to 90 minutes, (mean 45). Blood loss during the surgeries was minimal. Postoperative pain scoring was performed based on a VAS. In the immediate postoperative period, the mean value was 4, which at the end of 24 hours was nil. Twenty-two (26.8%) patients did not require any analgesia in the immediate postoperative period, whereas 44 (53.6%) patients required analgesic on postoperative day 1, and the remaining 16 (19.5%) patients required analgesics for three days. Pain scoring was also repeated at one-week follow-up, at which none of the patients complained of pain.
All patients returned to work by the end of postoperative day 5. Patient acceptability and discomfort were assessed on the basis of discharge from the wound and perianal discomfort. Twenty-eight (34.15%) patients had discharge from the wound one week after surgery, and 13 patients (15.85%) patients had discharge at one month that then continued at six months. These thirteen patients developed recurrence and a patent fistulous tract was discovered on magnetic resonance imaging scan. The recurrence rate in our study was 15.85%, which is less than that reported in the premier study done by Meinero and Mori.7 Patients who underwent a redo VAAFT currently are under observation. Anal manometry was done preoperatively and then repeated postoperatively after one month from surgery. None of the patients complained of flatulence, or loose stools. Anal manometry provided the objective evidence of the sphincteric function. There were no statistically significant changes in mean resting anal pressures and mean squeeze pressure when preoperative values were compared with postoperative values. Sixty-nine (84.15%) patients accepted the procedure well with a high level of satisfaction, and the remaining 13 (15.85%) patients were dissatisfied with the procedure after index surgery in view of recurrence.
DISCUSSION
The most widely used classification for fistula in ano is the Parks Classification, which distinguishes four kinds of fistula: intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric.8 Fistulae are also be classified as low fistula and high fistula, depending on the level of the internal opening below or above the anorectal ring.9
There are many surgical procedures advocated for fistula in ano, ranging from simply laying open the tract to colostomy. Fistulectomy and fistulotomy are the most widely accepted procedures performed for the management of simple fistula in ano with minimal involvement of the anal sphincter. The recurrence rate approaches 6.5%, the majority of which is caused by failure to identify the internal opening at the time of surgery. The rest may be a result of the failure to recognize the secondary branches or of early closure of the surgical wound.1 The treatment of complex fistulas is very cumbersome because of the high risk of postoperative complications such as incontinence. Fistulotomy has recently been advocated as a good technique for complex fistulas, with a success rate of 96% and acceptable objective anal parameters.10 The treatment of fistula in ano is directed at identification of the fistulous tract and internal opening, excision of the fistulous tract, and preservation of the continence mechanism.
The use of fibrin glue injection for the treatment of fistula in ano is described in the literature. The encouraging factors regarding this technique are its noninvasiveness, its simplicity, and its reproducibility. There is no sphincteric injury, so continence is preserved. The long-term success rates range from 14% to 69%.9,11–13 An anal fistula plug is a simple procedure that does not compromise the external sphincter. Thus, it has become a promising and cost-effective technique for the management of complex fistula in which there is a considerable risk of recurrence and incontinence. It involves the closure of the internal opening with a biological fistula plug. The success rates reported in the literature range from 34% to 77%.14–16
The transanal rectal advancement flap technique is described for the treatment of complex perianal and rectovaginal fistula. This procedure is intended to preserve the continence mechanism by avoiding sphincteric injury. It is a complex procedure with florid recurrence rates that range from 6% to 41% in various studies.17–19 The presence of Crohn disease was a significant predictor of outcome in some studies,17 whereas the previous attempts at fistula repair were the only significant variables of outcome in other studies.18,19 The poor outcome of these techniques is a result of local tissue ischemia after mobilization of local structures and the tendency of flaps to retract or dehisce. The rate of postoperative anal incontinence from these techniques is reported to be around 9% to 38%.18,19
A recent addition to the armamentarium of surgical procedure is LIFT. It comprises ligation and division of the fistulous tract in the intersphincteric space, curettage of the tract, and suture ligation of the external opening. The procedure is especially useful for high and complex fistula because it effectively preserves the continence. The success rate reported in various studies range from 57% to 94.5%. There was no reported morbidity in the form of fecal incontinence.6,20–22
Direct closure of the internal fistula opening without advancement flaps for the treatment of fistula in ano has been proposed by Athanasiadis et al. It comprises a three-layered, nonstaggered closure of the mucosa, submucosa, and internal and external sphincter after excision of the entire tract along with the internal and external openings.23 The major drawback of the procedure is the risk of suture line dehiscence, leading to persistence or recurrence of the fistula. It was reported in approximately 22.5% of the cases. Another study using this procedure reported the success rate as 59%.24
Another procedure advocated recently is fistula tract laser closure, which consists of an initial procedure of draining the abscess and placing the seton. This is later followed by closure of the internal opening using a flap and fulguration of the fistulous tract with a radial emitting laser probe (FiLaC, Biolitec, Jena, Germany). The authors reported a success rate of 81.8%.25
The use of stem cells derived from adipose tissue in conjunction with the fibrin plug for the treatment of complex anal fistula has also been described, with a reported success rate of 71%.26 The advantages of the technique are (1) no requirement to resect the fistulous tract and (2) no injury to the sphincteric mechanism. However, the limitations are the procedure's high cost and its technically demanding nature, which involves closure of the internal opening and obliteration of the tract with the cell suspension.
The essence of VAAFT is the visualization of the fistulous tract and its internal opening. It allows real-time visualization of the tract, precise identification of the anatomy by fistuloscopy, and fulguration of the tract under direct vision. The branching tracts and abscess cavities, which preclude the successful treatment of fistula in ano, can also be identified and dealt with appropriately. Moreover, the internal opening can be adequately managed either by suturing or using a stapling device. Adoption of fistuloscopy along with closure of the internal opening either by suturing or stapling device allows an effective treatment of complex anal fistulas with preservation of the anal sphincters. The recurrence rate reported by Meinero and Mori was approximately 26.5%. The recurrent cases were treated with either repeat VAAFT or injection of cyanoacrylate glue.7
Despite the recurrence rate of 26.5% in the study by Meinero and Mori, we still performed our study with VAAFT because the morbidity associated with the procedure is minimal. There was minimal discharge and pain at the surgical site, no raw area, and an early return to work for the patient, which led to a high level of patient satisfaction. Our results are better than that of Meinero and Mori because most the patients in our study had simple fistula, whereas their study was performed only in patients with complex fistula (ie, recurrent fistula, high fistula, and branching tracts). The main advantage of VAAFT is that it is associated with minimal morbidity. Although it is associated with recurrence, it should be more acceptable to the patients as the treatment modality because of its low morbidity.
CONCLUSIONS
VAAFT is a novel technique in the management of fistula in ano. It offers many advantages compared with the conventional procedures. It is performed as outpatient surgery; it avoids the morbidities of conventional procedures such as open wounds in the perianal region and postoperative sphincteric disturbances; it is a less invasive procedure; it is a cost-effective procedure that requires less workup preoperatively; it offers visualization of the internal opening; the instrument is reusable after high-level disinfection; and it offers economic benefit to the patient because it is an outpatient procedure, has a short recovery period, which encourages an short return-to-work time. Our initial experiences with the technique are encouraging. In our institution we perform this procedure in every case of fistula in ano, and the patients are analyzed regularly in follow-up.
Contributor Information
Gaurav Kochhar, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
Sudipta Saha, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
Manoj Andley, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
Ashok Kumar, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
Gyan Saurabh, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
Rahul Pusuluri, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
Vikas Bhise, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
Ajay Kumar, Department of Surgery, Lady Hardinge Medical College, New Delhi, India..
References:
- 1. Sangwan YP, Rosen L, Riether RD, et al. Is simple fistula-in-ano simple? Dis Colon Rectum. 1994;37:885–889. [DOI] [PubMed] [Google Scholar]
- 2. Ritchie RD, Sackier JM, Hodde JP. Incontinence rates after cutting seton treatment for anal fistula. Colorectal Dis. 2009;11:564–571. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Aguilar J, Belmonte C, Wong WD, et al. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–729. [DOI] [PubMed] [Google Scholar]
- 4. Cirocchi R, Santoro A, Trastulli S, et al. Meta-analysis of fibrin glue versus surgery for treatment of fistula-in-ano. Ann Ital Chir. 2010;81:349–356. [PubMed] [Google Scholar]
- 5. Lupinacci RM, Vallet C, Parc Y, et al. Treatment of fistula-in-ano with the Surgisis(®) AFP(™) anal fistula plug. Gastroenterol Clin Biol. 2010;34:549–553. [DOI] [PubMed] [Google Scholar]
- 6. Rojanasakul A. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol. 2009;13:237–240. [DOI] [PubMed] [Google Scholar]
- 7. Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol. 2011;15(4):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. [DOI] [PubMed] [Google Scholar]
- 9. Mishra A, Shah S, Nar AS, Bawa A. The role of fibrin glue in the treatment of high and low fistula in ano. J Clinic Diagn Res. 2013;7(5):876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atkin GK, Martins J, Tozer P, et al. For many high anal fistulas, lay open is still a good option. Tech Coloproctol. 2011;15:143–150. [DOI] [PubMed] [Google Scholar]
- 11. Buchanan GN, Bartram CI, Phillips RKS, et al. Efficacy of fibrin sealant in the management of complex anal fistula: a prospective trial. Dis Colon Rectum. 2003;46:1167–1174. [DOI] [PubMed] [Google Scholar]
- 12. Gisbertz SS, Sosef MN, Festen S, et al. Treatment of fistulas in ano with fibrin glue. Dig Surg. 2005;22:91–94. [DOI] [PubMed] [Google Scholar]
- 13. Sentovich SM. Fibrin glue for anal fistulas: long-term results. Dis Colon Rectum. 2003;46:498–502. [DOI] [PubMed] [Google Scholar]
- 14. Adamina M, Hoch JS, Burnstein MJ. To plug or not to plug: a cost-effectiveness analysis for complex anal fistula. Surgery. 2010;147:72–78. [DOI] [PubMed] [Google Scholar]
- 15. Wang JY, Garcia-Aguilar J, Sternberg JA, et al. Treatment of transsphincteric anal fistulas: are fistula plugs an acceptable alternative? Dis Colon Rectum. 2009;52:692–697. [DOI] [PubMed] [Google Scholar]
- 16. Johnson EK, Gaw JU, Armstrong DN. Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum. 2006;49:371–376. [DOI] [PubMed] [Google Scholar]
- 17. Sonoda T, Hull T, Piedmonte MR, Fazio VW. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum. 2002;45:1622–1628. [DOI] [PubMed] [Google Scholar]
- 18. Schouten WR, Zimmermann DD, Briel JW. Transanal advancement flap repair of transsphincteric fistulas. Dis Colon Rectum. 1999;42:1419–1423. [DOI] [PubMed] [Google Scholar]
- 19. Mizrahi N, Wexner SD, Zmora O, et al. Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum 2002;45:1616–1621. [DOI] [PubMed] [Google Scholar]
- 20. Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano: the ligation of intersphincteric fistula tract. J Med Asso Thai. 2007;90:581–586. [PubMed] [Google Scholar]
- 21. Bleier JI, Moloo H, Goldberg SM. Ligation of the intersphincteric fistula tract: an effective new technique for complex fistulas. Dis Colon Rectum. 2010;53:43–46. [DOI] [PubMed] [Google Scholar]
- 22. Shanwani A, Nor AM, Amri N. Ligation of the intersphincteric fistula tract (LIFT): a sphincter-saving technique for fistula-in-ano. Dis Colon Rectum. 2010;53:39–42. [DOI] [PubMed] [Google Scholar]
- 23. Athanasiadis S, Helmes C, Yazigi R, Kohler A. The direct closure of the internal fistula opening without advancement flap for transsphincteric fistulas-in-ano. Dis Colon Rectum. 2004;47:1174–1180. [DOI] [PubMed] [Google Scholar]
- 24. Thomson WH, Fowler AL. Direct appositional (no flap) closure of deep anal fistula. Colorectal Dis. 2004;6:32–36. [DOI] [PubMed] [Google Scholar]
- 25. Wilhelm A. New technique for anal fistula repair using a novel radial emitting laser probe (FILAC™) Tech Coloproctol. 2011;15:445–449. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. [DOI] [PubMed] [Google Scholar]


