Abstract
Studies on animal models have documented a role for the water soluble protein fraction of mesenteric lymph as a conduit from hemorrhagic shock to acute lung injury and post-injury multiple organ failure. We hypothesize that mesenteric lymph is not an ultrafiltrate of plasma and contains specific protein mediators that may predispose patients to ALI/MOF. Mesenteric lymph and plasma were collected from critically ill or injured patients and from nine patients with lymphatic injuries, during semi-elective spine reconstruction, or immediately before organ donation. Proteomic analyses were performed through immuno-affinity depletion of the 14 most abundant plasma proteins, and GeLC-MS analyses. Overall, 548 proteins were identified in the patients undergoing semi-elective surgery, of which 155 were uniquely present in the lymph. In addition, the post-shock plasma proteome was characterized by peculiar features, suggesting that only a partial overlap exists between the plasma and mesenteric lymph from trauma patients. Differential proteins between the matched plasma and mesenteric lymph from trauma patients could be related to, coagulopathy and hypercoagulability, cell lysis, pro-inflammatory responses and immune system activation, extracellular matrix remodeling, lymph-specific immunomodulation and vascular hypoactivity/neoangiogenesis, and energy/redox metabolic adaptation to trauma. In conclusion, the proteome of mesenteric lymph is biologically different (in qualitative and quantitative terms) than that of a mere plasma ultrafiltrate.
Keywords: trauma, human, label-free quantitation, mass spectrometry, quantitative changes
Introduction
Despite significant improvements in the field of resuscitation strategies, the management of patients with critical injury and hemorrhagic shock (HS) still represents a challenging task. Leading causes of morbidity and mortality in these patients include the development of post injury multiple organ failure (MOF), as the net result of a dysfunctional immune response to injury characterized by a hyperactive innate system and a suppressed adaptive system (1). Over the years, gut and intestinal barrier dysfunction have been implicated in MOF (2), while mesenteric ischemia reperfusion (IR), subsequent to trauma/hemorrhagic shock (T/HS), is central in the pathogenesis of post-injury organ dysfunction (2-5). In particular, lymphatic diversion prior to T/HS inhibits acute lung injury and attenuates MOF. Thus, the mesenteric lymphatic system is a major conduit for the transport of gut-derived pro-inflammatory mediators to the systemic circulation (6). Animal models have further elucidated how systemic lymph (2) and, in particular, mesenteric lymph (ML) (2,3) triggers pro-inflammatory events leading to MOF, including early post-HS priming of polymorphonuclear leukocytes (PMNs) (3). Targeted investigations on the role of lymph in the development of MOF through activation of innate immunity have implicated a number of mediators which trigger post-shock mesenteric lymph (PSML)-driven PMN priming (3).
The lymphatic system collects extravasated fluid, proteins, and lipids through a filtration process at the interstitial space, and returns them to the blood circulation via the thoracic duct to the subclavian vein (4,5). This filtration process is driven by the hydrostatic pressure in the arterial end of capillaries. Only a small portion of the extravasated fluid re-enters the blood circulatory system in response to intravascular osmotic pressure, while the bulk of the fluid is actually drained into the lymphatics (4,5). The lymphatic system stems from the networking of lymphatic capillaries that are present in every parenchymal organ. Lymphatic capillaries merge into progressively bigger vessels that transport the pre-nodal lymph to nodes disseminated throughout the body (4,5). In lymph nodes >100 kDa molecules and bacteria are either captured or transported through the efferent lymphatic vessels to the next nodal station (6). Smaller molecules (<80-100 kDa) are processed in the conduit system, from which the lymph will further proceed to the high endothelial venules in the nodal sinus. Therefore, the lymphatic system plays a central role in immune and inflammatory responses.
Because lymph is the result of a capillary filtration process, it has been deemed to be compositionally similar to a plasma ultrafiltrate with significant numbers of lymphocytes, macrophages, and plasma proteins, including coagulation factors and albumin (7). In addition, lymph contains a large amount of dietary fats, related lipoproteins and cholesterol absorbed from the intestine, known as chyle (7). The lymph proteome has been characterized employing formal proteomic analyses on PSML collected from animals (rat, ovine, canine) (7-14) and human models of trauma (15,16), because PMN-priming compounds in PSML were present in the soluble fraction (17). Proteomic analyses on the lymph from healthy subjects have been recently reported (18,19), paving the way for the direct comparison of the increasingly disclosed lymph proteome to the thoroughly investigated plasma proteome of healthy (20-22) and trauma patients (23) or animal models (24). Taken together, proteomics demonstrated the partial overlap between the lymph and plasma proteome of healthy patients. Indeed, proteomic analysis revealed the presence of several tissue specific proteins in lymph collected from sites of sterile or pathogen induced inflammation (8-10,25), while lymph from healthy subjects was particularly enriched with soluble peptidome/degradome components in comparison to plasma (26). These results demonstrate that lymph collects proteins and forms an enriched proteome diverse from plasma both from a compositional and quantitative standpoint (18,25,26).
Despite the considerations above, it has yet to be assessed whether PSML is different from post T/HS human plasma, which is relevant because lymph, rather than plasma, is the major culprit in neutrophil priming and MOF (3). Here we hypothesize that mesenteric lymph is not an ultrafiltrate of plasma and contains specific protein mediators that may predispose patients to acute lung injury (ALI)/MOF.
MATERIALS AND METHODS
Study population and data collection
Injured patients admitted to the Rocky Mountain Regional Trauma Center Surgical ICU (SICU) at Denver Health Medical Center (DHMC) and patients undergoing brain-dead organ donation at Colorado hospitals were evaluated for inclusion into a study from 2008 through 2010. The Combined Multiple Institution Review Board approved the study. Patients with various injury mechanisms were included in the study: trauma/hemorrhagic shock, brain death, mesenteric lymphatic injury, and elective spine surgery and all data collection were done per HIPAA regulations. In addition, lymph was also collected from patients undergoing semi-elective spine injury reconstruction; the exposure of the patient's distal thoracic and proximal lumbar vertebral bodies is achieved via a left thoraco-abdominal incision, after obtaining informed consent.
Mesenteric lymph and plasma collection
The cisterna chyli was visualized between the aorta and spine and lymph aspirated using a 27-gauge needle. During the donor operation, before cold preservation, a right medial visceral rotation was performed to expose the vena cava and the aorta just inferiorly to the take off of the superior mesenteric artery. At this level, the left renal vein was easily identified anteriorly crossing the aorta. Running along the retroperitoneal small bowel mesentery and crossing anterior and perpendicular to the left renal vein are typically large distended lymphatic vessels. These were cannulated with a 21-gauge angiocatheter to procure the mesenteric lymph. Patients are NPO (nil per os) overnight in preparation for semi-elective surgery and all nutrition (enteral and parenteral) was discontinued at the time of consent for organ donation. Consent for organ donation as well as lymph collection was obtained by the organ procurement organization, from the deceased (based on a document of gift: donor card, living will, or driver's license) or from the next of kin.
Mesenteric lymph (100ul – 1 mL) was collected and placed into an EDTA containing tube. Samples were centrifuged at 3500 x g for 10 min to remove cellular components, and stored in a freezer at −80 °C. Protein concentration was quantified using the Bradford assay. Patients were categorized based on the predominant illness or mechanism of injury. The Injury Severity Score (ISS), a numerical method to describe the overall magnitude of injury, was calculated for trauma patients.
Blood samples were collected in EDTA vacutainer from each subject. The blood samples were stored upright at 4°C until they were spun at 2500 rpm at 4°C for 15 minutes. The separated plasma was aliquoted and stored at −80°C for further analysis.
Immunoaffinity Depletion
Multiple Affinity Removal System™ columns (4.6 × 100 mm) designed to deplete 14 abundant proteins (albumin, IgG, antitrypsin, IgA, transferrin, haptoglobin, fibrinogen, alpha2-macroglobulin, alpha1-acid glycoprotein, IgM, apolipoprotein AI, apolipoprotein AII, complement C3, and transthyretin) (21) were purchased from Agilent (Palo Alto, CA). Depletion was performed at room temperature on an AKTAmicro (GE Healthcare Life Sciences) system. Plasma samples were diluted four-fold using the load/wash buffer supplied by the manufacturer, and remaining particulates in the diluted plasma were removed by centrifugation through a 0.22-um spin filter 1 min at 16,000g. After equilibration with the load/wash buffer, the Multiple Affinity Removal System™ column was loaded with 160 uL of the diluted plasma at a low flow rate (0.125 mL min−1) for 4 min. Flow-through fractions, representing depleted plasma were collected and saved. The bound proteins were released with elution buffer at 1.0 mL/min for 10 min. The column was then washed with the load/wash buffer for 11 min at a flow rate of 1 mL/min. Each depletion cycle took 38 min of total run time. Each flow-through portion was individually concentrated using 5000 Da molecular weight cutoff spin concentrators (Agilent Technologies, Palo Alto, CA), followed by buffer exchange with 50 mM NH4HCO3 and protein concentrations were determined by a Bradford protein assay
Plasma Protein Digestion
Proteomics analyses were performed via GeLC-MS (1D gel electrophoresis followed by liquid chromatography coupled online with mass spectrometry), as previously reported (26). In detail, a portion of the sample (30 ug) was diluted into SDS-PAGE sample buffer, heated at 70 °C for 10 min and loaded in a single lane on a 1-mm-thick 4–12% Bis-Tris gel (Invitrogen). After separation, the gel was stained with SimplyBlue SafeStain (Invitrogen). Each lane of the gel was divided into 10 equal-sized bands and proteins in the gel were digested as follows. Bands were destained in 200 ul of 25 mM ammonium bicarbonate in 50 % v/v acetonitrile (CAN) for 15 min, and then 200 ul of 100% ACN was applied for 15 min at room temperature. Dithiothreitol (DTT) was added to a final concentration of 10 mM and incubated at 65 °C for 30 min to reduce the disulfide bonds. Protein cysteines were alkylated with 55 mM iodoacetamide for 30 min at room temperature in the dark. The iodoacetamide was then removed, and washes were performed with 200 ul of distilled water followed by addition of 100 ul of ACN. Then ACN was removed, and 50 ul of the 0.01 ug/ul trypsin solution was added to each plug and allowed to rehydrate the gel plugs at 4 °C for 30 min and then placed at 37 °C and allowed to digest overnight. The tryptic mixtures were acidified with formic acid up to a final concentration of 1%. Peptides were extracted three times from the gel plugs using 50% ACN, 1% FA, concentrated under vacuum (SpeedVac, Savant ThermoFisher) to approximately ~20 uL, and subjected to LC-MS/MS analysis. If necessary, they were stored at −20 °C.
Liquid Chromatography-Tandem Mass Spectrometry
Samples were measured on an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Pittsburgh, PA) coupled to an Eksigent nanoLC-2D system through a nanoelectrospray LC−MS interface. A volume of 8 uL of sample was injected into a 10 uL loop using the autosampler. To desalt the sample, material was flushed out of the loop and loaded onto a trapping column (ZORBAX 300SB-C18, dimensions 5x0.3 mm 5 um - Agilent, Santa Clara, CA) and washed with 0.1% FA at a flow rate of 5 uL/min for 5 minutes. The analytical column was then switched on-line at 600 nl/min over an in house-made 100 um i.d. × 150 mm fused silica capillary packed with 4 um 80Å Synergi Hydro C18 resin (Phenomex; Torrance, CA). After 10 minutes of sample loading, the flow rate was adjusted to 350 nL/min, and each sample was run on a seventy minute gradient from 6% ACN to 40% ACN with 0.1% formic acid to separate the peptides. The mobile phase included water with 0.1% FA (solvent A) and 99.9 % acetonitrile with 0.1% FA (solvent B). Data acquisition was performed using the instrument supplied Xcalibur™ (version 2.1) software. The mass spectrometer was operated in the positive ion mode. Each survey scan of m/z 400–2,000 was followed by collision-assisted dissociation (CAD) MS/MS of twenty most intense precursor ions. Singly charged ions were excluded from CID selection. Normalized collision energies were employed using helium as the collision gas. Lymph and plasma samples were analyzed in duplicate in order to gauge reproducibility and increase protein identification and prediction confidence.
Database searching, protein identification
MS/MS spectra were extracted from raw data files and converted into mgf files using a script (PAVA, UCSF, MSF, San Francisco, CA). These mgf files were then independently searched against SwissProt database using an in-house Mascot™ server (Version 2.2.06, Matrix Science). Mass tolerances were +/− 15ppm for MS peaks, and +/− 0.6 Da for MS/MS fragment ions. Trypsin specificity was used allowing for 1 missed cleavage. Met oxidation, protein N-terminal acetylation, and peptide N-terminal pyroglutamic acid formation were allowed for variable modifications while carbamidomethyl of Cys was set as a fixed modification. Alternative searches were performed indicating semi-trypsin digestion, while maintaining the other search criteria unaltered.
Scaffold (version 4.3.2, Proteome Software, Portland, OR, USA) was used to validate MS/MS based peptide and protein identifications. All mascot DAT files, for each subjects (10 bands each) were loaded together as one “biological sample” within Scaffold. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified unique peptides in the first set of experiments. Subsequently, we performed a second screening of the results by also including proteins identified with 1 single peptide from Semi-trypsin digestion Mascot searches (probability >99% for proteins, >95% per peptide; Mascot score per peptide > 30).
Heat maps and clustering
Quantitative results from Scaffold were exported into .xls files and loaded into GENE-E (v. 3.0.200 - Broad Institute Inc., Cambridge, MA) as to plot heat maps and perform hierarchical clustering analyses (one minus Pearson correlation). Functional annotation for biological functions and cell compartments were performed either with Scaffold or David v. 6.7 (David Bioinformatics services).
Results
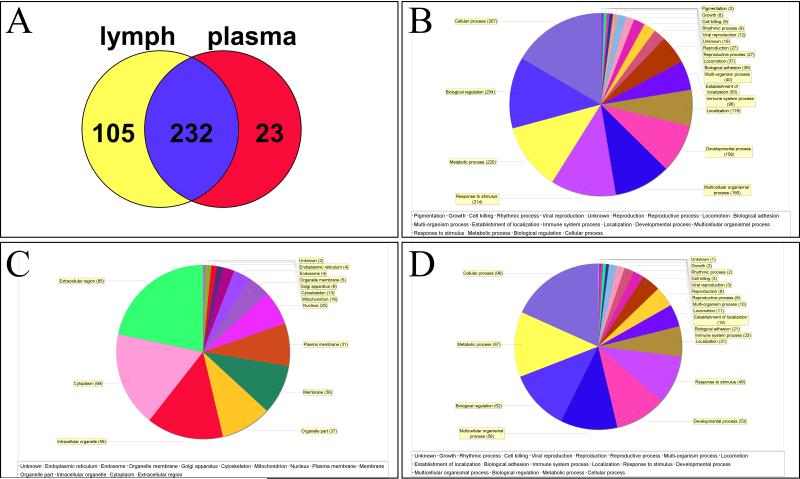
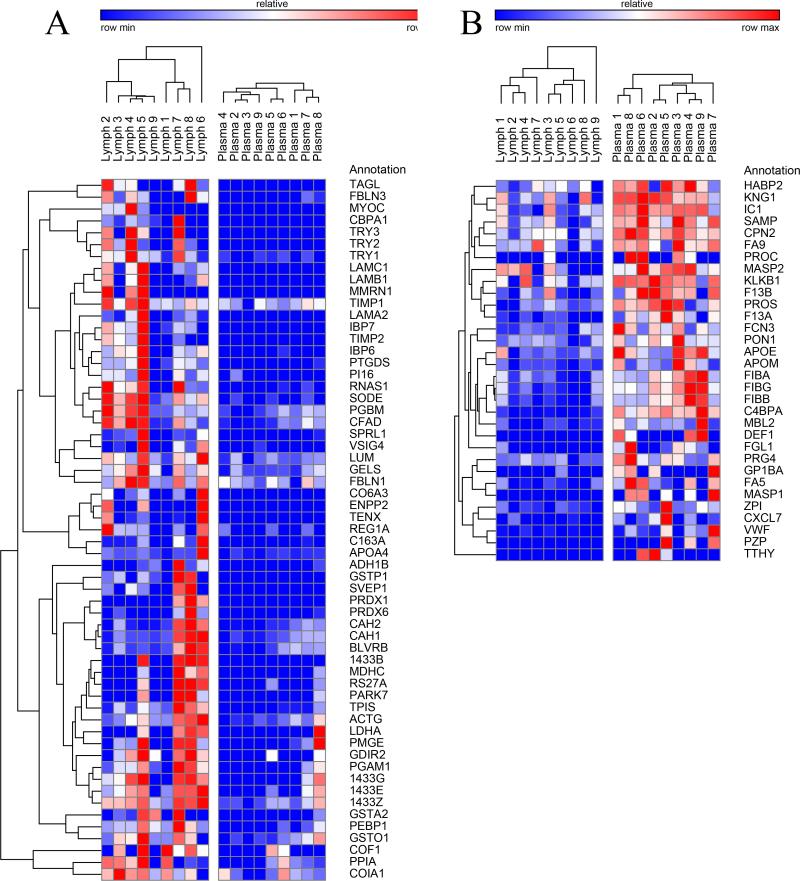
Lymph and plasma samples were collected from nine patients in accordance with local and federal regulations. A summary of the patient profiles are listed in Table 1. The diverse group covers a wide range of ages and medical conditions, with recovery of lymph and plasma during semi-elective spine reconstruction, lymphatic injuries or organ donation. The analysis was carried out using the strategy that combines immuno-affinity depletion of the 14 most abundant proteins for both lymph and plasma and 1D sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) coupled with nanoLC-electrospray tandem mass spectrometry (ESI-MS/MS) analysis, a workflow otherwise referred to as GeLC-MS (27). An average of 5% (w/w) of applied protein was recovered from the Multiple Affinity Removal System™ column. An equal amount (30 ug) of plasma and lymph was fractionated on 4-12% 1D SDS-PAGE to simplify the complexity of the protein mixture, thereby increasing the depth of protein identification. After the electrophoretic run, each track was cut into 10 slices along the migration path, and the proteins content was digested by trypsin and subjected to MS/MS analysis on the LTQ-Orbitrap mass spectrometer. A total of 360 proteins (>99% confidence) with a minimum of 2 distinct peptides were identified, 232 proteins common to lymph and plasma samples, 23 proteins unique to plasma and 105 unique to mesenteric lymph (Figure 1.A). Results are extensively reported in Supplementary Table 1, enlisting protein names together with Uniprot Accession numbers, taxonomy (Homo sapiens), theoretical molecular weight (MW), Fold-change variations and quantitative values from each sample (upon normalization against total spectra for each run in Scaffold) and paired one-tailed T-test-derived p-values. Quantitative values of the detected protein have been exported in GENE-E and mapped as hierarchical clusters (Pearson correlation-1) heat maps, as in Supplementary Figure 1. From this figure it emerges that, although it is possible to cluster PSML and post T/HS plasma samples in the light of their distinctive proteome traits, each group is characterized by intrinsic biological variability. This is evident when considering the single red columns (higher levels) spanning across a handful of proteins that change from subject to subject in both fluids (Supplementary Figure 1).
Table 1.
Patients enrolled in this study
| Patient | Trauma | Death / Survivor | |
|---|---|---|---|
| XK4100 | 48 yo male embolic CVA | N | Death |
| YAY416 | 56 yo female CVA | N | Death |
| YCB241 | 47 yo female ruptured intracranial aneurysm | N | Death |
| XFI466 | 49 yo male ruptured intracranial aneurysm | N | Death |
| YCA333 | 47 yo male motorcycle crash, polytrauma, TBI | Y | Death |
| XIZ039 | 15 yo male GSW to head | Y | Death |
| YAJ151 | 20 yo male GSW to head | Y | Death |
| YCV310 | 43 yo female PEA arrest | N | Death |
| YCX386 | 32 yo male GSW to head | Y | Death |
Figure 1.
Scaffold report of proteome changes in post-shock mesenteric lymph and plasma, as gleaned via HPLC-MS/MS label free peptidomics approaches on nine paired biological replicates per group. In A, a Venn diagram indicates the shared features (232), and those differentially represented only in mesenteric lymph (105) or plasma (23). In B, GO term enrichment for biological functions of the total list of 360 proteins. In C and D, GO term enrichment for cell compartment and biological functions for the 105 features unique to post shock mesenteric lymph, respectively.
In order to cope with this issue, in Table 2 and Figure 2 we highlight the 91 proteins of interest from this analysis showing statistical significance (p-value < 0.05). In particular, in Figure 2 we highlight two main clusters related to proteins On/Up-regulated in PSML (A) or post T/HS plasma (B), respectively. GO term enrichment for biological functions of the detected proteins is presented in Figure 1.B, while in C and D we highlight the results obtained through functional annotations (cell compartment and biological function, respectively) of proteins found only in PSML. These results indicate that most of the PSML specific proteins were of extracellular and cytoplasmic origin (Figure 1.C), and mainly accounted for proteins involved in cellular/metabolic processes (Figure 1.D).
Table 2.
Lymph vs plasma proteome in trauma patients
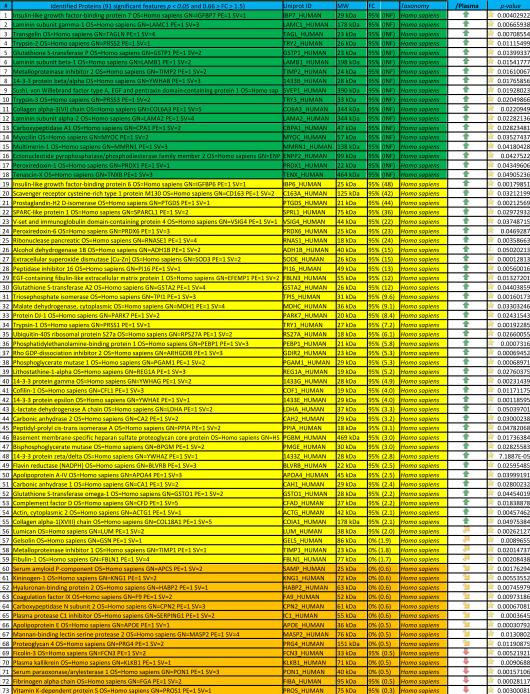
|
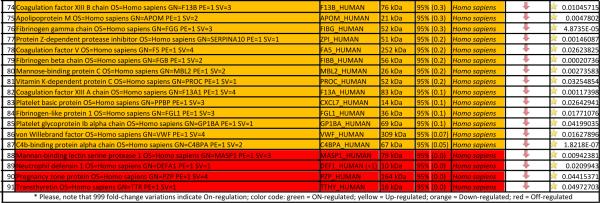
|
Figure 2.
Hierarchical clustering analyses of quantitative dynamic changes of post-shock mesenteric lymph and plasma proteins, either increasing in the former group (A), or in the latter (B), as gleaned by label free quantitative proteomics approaches on paired samples from 9 patients undergoing semi-elective spine injury reconstruction. Quantitative emPAI values were obtained through Mascot search and exported from Scaffold into xls file for heat mapping and hierarchical clustering analysis (Pearson correlation-1) through the software GENE-E. Values increase progressively from blue to red in the color scale, as indicated in the legends on top of each cluster. Proteins are enlisted with their relative UniProt ID names, though the _HUMAN taxonomy specification for Homo sapiens has been deleted as to improve the clarity of the figure. Further details are reported in Table 2 (protein list).
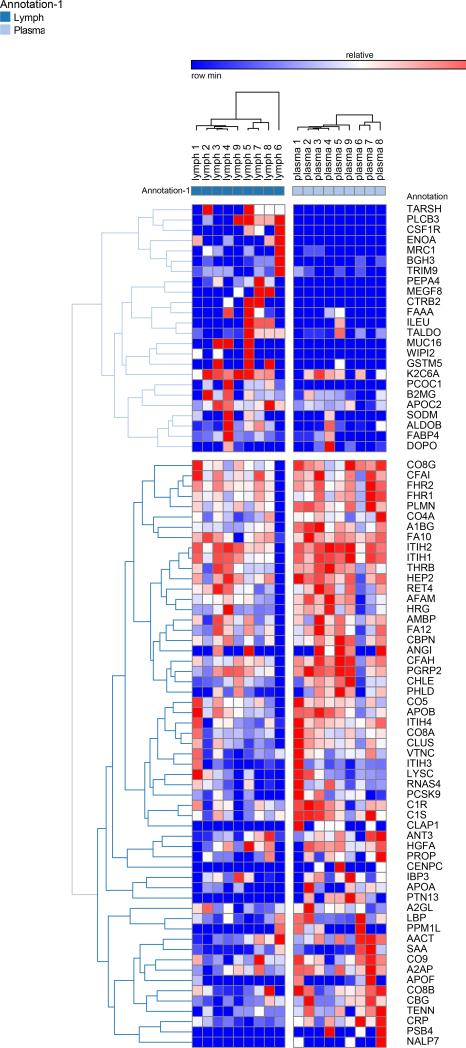
A second post hoc elaboration of the results was performed as to include also proteins identified with 1 single peptide (probability score > 95%, Mascot score > 30) from Semi-tryptic digestion Mascot search. This search was thought as to include only proteins deriving from (full or partial) non-tryptic cleavage (or tryptic miscleavage), since protease activation is a distinguishing feature of post T/HS activation of clotting and immune (e.g. complement) cascades in plasma and lymph, and might result in the accumulation of potential self-antigens of critical importance in the modulation of immune responses (18). Results of this analysis are extensively reported in Supplementary Table 2, in analogy to the template established for Supplementary Table 1. Statistically significant results (p < 0.05 one-tailed paired T-test) from this second analysis have been thus exported in Table 3. This table was manually curated as to include only those proteins showing significant (p<0.05) fold change variation (0.6>FC>1.6) that were not present in Table 2. Overall, we observed an additional 81 proteins, 58 of which being up/on-regulated in post T/HS plasma (Table 3). Quantitative results for these proteins were thus graphed and hierarchically clustered via the software GENE-E, as in Figure 3.
Table 3.
Significantly regulated proteins identified in trauma plasma and lymph from 9 different individuals (Semi-trypsin, 1 peptide >95%).
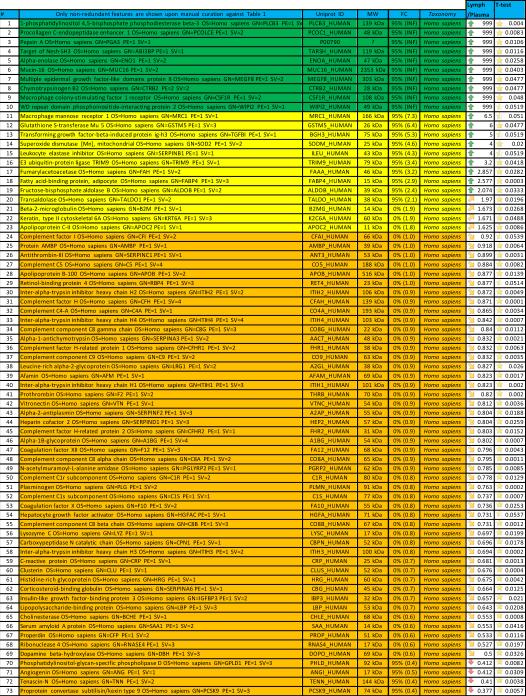
|
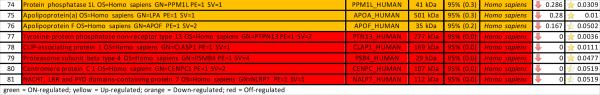
|
Figure 3.
Hierarchical clustering analyses of quantitative dynamic changes of post-shock mesenteric lymph and plasma proteins, either increasing in the upper cluster, or decreasing in the lower on, as a result of a Mascot search including semitryptic peptides and at least one peptide (>95% probability, Mascot score>30) for a positive identification. Only proteins non-redundant with Figure 1 were plotted. Quantitative information was gleaned through label free quantitative proteomics analyses on paired samples from 9 patients undergoing semi-elective spine injury reconstruction. Quantitative emPAI values were obtained through Mascot search and exported from Scaffold into xls file for heat mapping and hierarchical clustering analysis (Pearson correlation-1) through the software GENE-E. Values increase progressively from blue to red in the color scale, as indicated in the legends on top of each cluster. Proteins are enlisted with their relative UniProt ID names, though the _HUMAN taxonomy specification for Homo sapiens has been deleted as to improve the clarity of the figure. Further details are reported in Table 3 (protein list).
Notably, 155 On-regulated proteins were found uniquely in PSML albeit not in plasma (Supplementary Table 2), out of which 25 have gone hitherto undetected in plasma (Table 4), as gleaned upon a direct search in the most recently updated version of the Human Plasma Proteome Project online database (20). The peptides identified in the search including Semi-trypsin digestion have been enlisted in Supplementary Table 3, and have been divided into four categories including (i) fully miscleaved, (ii) N-ter miscleaved, (iii) C-ter miscleaved and (iv) tryptic peptides.
Table 4.
List of mesenteric lymph proteins hitherto unreported in the Human Plasma Proteome (HPP) database
| # | Protein name | Uniprot AC | MW | Taxonomy |
|---|---|---|---|---|
| 1 | Ankyrin repeat domain-containing protein 53 OS=Homo sapiens GN=ANKRD53 PE=2 SV=2 | ANR53_HUMAN | 60 kDa | Homo sapiens |
| 2 | Carboxypeptidase A2 OS=Homo sapiens GN=CPA2 PE=1 SV=3 | CBPA2_HUMAN | 47 kDa | Homo sapiens |
| 3 | Coiled-coil domain-containing protein 106 OS=Homo sapiens GN=CCDC106 PE=1 SV=1 | CC106_HUMAN | 32 kDa | Homo sapiens |
| 4 | Protein capicua homolog OS=Homo sapiens GN=CIC PE=1 SV=2 | CIC_HUMAN | 164 kDa | Homo sapiens |
| 5 | Chymotrypsinogen B2 OS=Homo sapiens GN=CTRB2 PE=2 SV=2 | CTRB2_HUMAN | 28 kDa | Homo sapiens |
| 6 | G2/M phase-specific E3 ubiquitin-protein ligase OS=Homo sapiens GN=G2E3 PE=1 SV=1 | G2E3_HUMAN | 81 kDa | Homo sapiens |
| 7 | =lap endonuclease GEN homolog 1 OS=Homo sapiens GN=GEN1 PE=1 SV=2 | GEN_HUMAN | 103 kDa | Homo sapiens |
| 8 | g heavy chain V-I region HG3 OS=Homo sapiens PE=4 SV=1 | HV102_HUMAN | 13 kDa | Homo sapiens |
| 9 | ntraflagellar transport protein 46 homolog OS=Homo sapiens GN=IFT46 PE=2 SV=1 | IFT46_HUMAN | 34 kDa | Homo sapiens |
| 10 | g gamma-2 chain C region OS=Homo sapiens GN=IGHG2 PE=1 SV=2 | IGHG2_HUMAN | 36 kDa | Homo sapiens |
| 11 | g kappa chain C region OS=Homo sapiens GN=IGKC PE=1 SV=1 | IGKC_HUMAN | 12 kDa | Homo sapiens |
| 12 | Protein KRBA1 OS=Homo sapiens GN=KRBA1 PE=2 SV=2 | KRBA1_HUMAN | 108 kDa | Homo sapiens |
| 13 | g kappa chain V-I region Roy OS=Homo sapiens PE=1 SV=1 | KV116_HUMAN | 12 kDa | Homo sapiens |
| 14 | .eft-right determination factor 2 OS=Homo sapiens GN=LEFTY2 PE=1 SV=2 | LFTY2_HUMAN | 41 kDa | Homo sapiens |
| 15 | .atexin OS=Homo sapiens GN=LXN PE=1 SV=2 | LXN_HUMAN | 26 kDa | Homo sapiens |
| 16 | Mucin-4 OS=Homo sapiens GN=MUC4 PE=1 SV=4 | MUC4_HUMAN | 232 kDa | Homo sapiens |
| 17 | Jbiquitin thioesterase OTU1 OS=Homo sapiens GN=YOD1 PE=1 SV=1 | OTU1_HUMAN | 38 kDa | Homo sapiens |
| 18 | Pepsin A OS=Homo sapiens GN=PGA3 PE=1 SV=1 | P00790_HUMAN | 42 kDa | Homo sapiens |
| 19 | .ithostathine-1-beta OS=Homo sapiens GN=REG1B PE=1 SV=1 | REG1B_HUMAN | 19 kDa | Homo sapiens |
| 20 | Soluble scavenger receptor cysteine-rich domain-containing protein SSC5D OS=Homo sapiens GN=SSC5D PE=2 SV= | SRCRL_HUMAN | 166 kDa | Homo sapiens |
| 21 | Transcriptional adapter 2-beta OS=Homo sapiens GN=TADA2B PE=1 SV=2 | TAD2B_HUMAN | 48 kDa | Homo sapiens |
| 22 | Protein TBRG4 OS=Homo sapiens GN=TBRG4 PE=1 SV=1 | TBRG4_HUMAN | 71 kDa | Homo sapiens |
| 23 | E3 ubiquitin-protein ligase TRIM68 OS=Homo sapiens GN=TRIM68 PE=1 SV=1 | TRI68_HUMAN | 56 kDa | Homo sapiens |
| 24 | WD repeat domain phosphoinositide-interacting protein 2 OS=Homo sapiens GN=WIPI2 PE=1 SV=1 | WIPI2_HUMAN | 49 kDa | Homo sapiens |
| 25 | Skin-specific protein 32 OS=Homo sapiens GN=XP32 PE=1 SV=1 | XP32_HUMAN | 26 kDa | Homo sapiens |
Finally, the 91 (Table 2, Figure 2) and 81 features (Table 3, Figure 3) showing statistically significant quantitative fluctuations (Up/On and Down/Off-regulation) in PSML in comparison to post T/HS plasma have been exported for functional GO term annotation in the software David, as summarized in Supplementary Table 4. In addition, most of the Down/Off and Up/On-regulated proteins in PSML in comparison to post T/HS plasma were annotated as part of the secreted extracellular fraction (GO:0044421 and GO:0005576 – Supplementary Table 4).
Coagulation factors and clotting cascades
In the present study we observed that coagulation factors (GO:0007596) were over-represented in post T/HS plasma samples (including pro-coagulation factors II, V, IX, X, XII, XIIIA and B fibrinogen alpha, beta and gamma chains, apolipoprotein A, von Willebrand Factor, plasma kallikrein, platelet glycoprotein Ib alpha chain; and anti-coagulation components such a heparin cofactor 2 – SERPIND2, vitamin K-dependent protein C, plasma protease C1 inhibitor – SERPING1, Kininogen-1, plasminogen, antithrombin-III – SERPINC1, Vitamin K-dependent protein S – Supplementary Table 4).
Acute phase inflammatory responses and immunity
Analogously, post T/HS plasma was enriched in proteins involved in responses to wounding (GO:0009611) and acute inflammatory responses (GO:0002526) (Supplementary Table 4). This group included inter-alpha-trypsin inhibitor heavy chain H4, lipopolysaccharide-binding protein, Complement component C1r, C1s C4b-binding protein alpha chain, 4A, 5, C8 alpha, beta and gamma chain, 9, and factor H, I, P; clusterin, c-reactive protein, alpha-2-antiplasmin (SERPINF2), SERPINA10, ficolin-3, lysozyme C, mannan-binding lectin serine protease 1 and 2 (Tables 2 and 3, Supplementary Table 4). As noted above, several complement components were over-represented in post T/HS plasma (GO:0006958).
On the other hand, PSML was characterized by higher levels of proteins known to act in immune effector processes (GO:0002252), including complement factor D, peroxiredoxin 1, 14-3-3 protein zeta/delta, and V-set and immunoglobulin domain-containing protein 4 (Supplementary Table 4). Cell adhesion molecules were enriched in PSML (GO:0007155 – Supplementary Table 4) , including Sushi von Willebrand factor type A, EGF and pentraxin domain-containing protein 1, multimerin-1, Tenascin-X, Laminin subunits alpha 2, beta-1, and gamma 1, mucin 16, collagen alpha-3(VI) chain and alpha-1(XVIII) chain, Basement membrane-specific heparan sulfate proteoglycan core protein, transforming growth factor-beta-induced protein ig-h3.
Extracellular matrix proteins
ECM-related proteins were over-represented in PSML (GO:0031012), also including, in addition to those mentioned in the previous paragraph, trypsin 2, metalloproteinase inhibitor 1 and 2, EGF-containing fibulin-like extracellular matrix protein 1, lumican, SPARC-like protein 1, extracellular superoxide dismutase (Cu-Zn), and fibulin-1 (Tables 2 and 3, Supplementary Table 4). Markers of actin cytoskeleton reorganization (GO:0030036) were over-represented in PSML in comparison to plasma (Rho GDP-dissociation inhibitor 2, cofilin-1, actin cytoplasmic 2, gelsolin, – Supplementary Table 4).
Intracellular components as markers of red cell hemolysis and tissue damage
PSML appeared to scavenge traces of proteins from different tissues, including pancreatic proteins (GO:0007586), such as chymotrypsinogen B2, trypsin 1, 2 and 3, apolipoprotein A4, ribonuclease pancreatic, lithostathine-1-alpha. Moreover, enrichment of cytoplasmic proteins in PSML is further reflected by GO annotations (Figure 1.C, Supplementary Table 4) and is consistent with previous observations from our group (15). In addition, some of these proteins might origin in platelets (GO:0031093 - platelet alpha granule lumen – Supplementary Table 4), such as complement factor D, multimerin 1 (further cleaved into Platelet glycoprotein Ia and 155 kDa platelet multimerin), and metalloproteinase inhibitor 1. However, previous proteomics observations on human mesenteric lymph have documented the accumulation of red blood cell-derived soluble protein components in PSML (15). Consistently, we hereby observed the significant accumulation in PSML of carbonic anhydrases (1 and 2 – Table 2), hemoglobin (alpha, beta, delta and gamma-1 chains), haptoglobin (fold-change increase >2.5-3.0, p = 0.07 ANOVA) and flavin (NADPH) reductase. Lastly, extracellular proteases were enriched in PSML (GO:0006508 – Supplementary Table 4).
Oxidative Stress
Accumulation of anti-oxidant proteins (GO:0006800) was observed in PSML, a group enlisting peroxiredoxin 1 and 6, superoxide dismutase (Cu-Zn) (extracellular) and (Mn) (mitochondrial), DJ-1/Park-7 (Supplementary Table 4). Interestingly, increased levels of glutathione transferases were also detected in PSML (GO:0004364), including prostaglandin-H2 D-isomerase, Glutathione S-transferase A2, Mu 5, omega-1 and P (Tables 2 and 3).
Metabolism
We detected post T/HS plasma accumulation of proteins related to responses to glucocorticoid stimuli (GO:0051384), including insulin-like growth factor-binding protein 7, phosphatidylethanolamine-binding protein 1, adipocyte fatty acid-binding protein, and fructosebisphosphate aldolase B (Supplementary Table 4). Increased levels of plasma lipoprotein particle proteins post T/HS were observed as well (GO:0034358 – Apolipoproteins B, E, F, J, M; serum amyloid protein; Serum paraoxonase/arylesterase 1). The enzymes involved in glucose catabolic processes (GO:0006007) were enriched in PSML (Supplementary Table 4): transaldolase, malate dehydrogenase cytoplasmic, fructose-bisphosphate aldolase B triosephosphate isomerase, bisphosphoglycerate mutase, phosphoglycerate mutase 1, alpha-enolase, lactate dehydrogenase A.
Discussion
The principles in management of T/HS include stopping hemorrhage, prompt identification and treatment of injuries, and fluid resuscitation in order to restore tissue perfusion and oxygen balance (1). Trauma patients are frequently coagulopathic early after injury and become hypercoagulable within days of injury. The causes underlying hyperfibrinolysis and trauma-induced coagulopathy are associated with depletion of coagulation factors secondary to blood loss, dilution and consumption, as well as an interaction between proteases and anti-proteases involved in this pathway (28,29).
In the present study, post-T/HS plasma samples were enriched for coagulation factors and pro-coagulant proteins. From a biological standpoint it is worth noting two main points:
Post T/HS responses involve alterations in the levels of proteases/anti-proteases involved in clotting cascades (especially serine protease and the relative inhibitors, SERPINs (29);
Although coagulation factors and protease inhibitors can be both found in plasma and lymph samples from healthy subjects (26) and trauma patients (15,23), the direct comparison of the two fluids we performed in the present study helped determining semi-quantitative alterations in the levels of the proteins involved in homeostatic responses.
Post T/HS activation of the cytokine (30) and complement pathway is known to play a key role in the adverse immune consequences of hemorrhagic trauma with subsequent shock and resuscitation (31), and has been previously associated with lactic acidosis, endotoxemia, and possibly other, as of yet undisclosed, ischemia-related tissue alterations.
Release of complement component 5a in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury resulted in neutrophil priming during the first 4h from reperfusion of myocardium (31). In the present study, minor decreases of complement component 5 were observed in PSML in comparison to post T/HS plasma (Supplementary Table 1). Though these data only provide a cursory view of the complement system and further investigations are mandatory, the enrichment of complement factor D in lymph is suggestive of the up-regulation of complement activation cascades via the alternative pathway, likely inhibited in plasma by the up-regulation of inhibitory factor H and I. On the other hand, in plasma classical and lectin pathways are likely to be the eligible choice in post T/HS complement activation. Such pathways are triggered by pathogen binding antibodies and in turn trigger activation of immune responses. In this respect, increased levels of cell adhesion molecules in PSML might mediate immune cell migration and activation. These proteins act at the cross-roads between immunity and acute phase responses (32), and are involved in collagen-cell interactions. In particular, increased levels of laminins in lymph in comparison to plasma had already been documented in healthy subjects (23), and pointed to the likely involvement of extracellular matrix components in the PSML-modulated PMN priming.
Rat models of hemorrhagic shock and resuscitation have helped suggest a role for extracellular matrix (ECM) modulation in the onset of neutrophil priming responses (31). Matrix metalloproteinase (MMPs, especially 2, 8, 9 and 13) activity on ECM proteins has been recently associated with increased neutrophil activation, likely mediated by MMPs-dependent release of bioactive peptides (such as acetyl-PGP) which are coupled to CXCRs as to promote chemoattraction albeit not adhesion (32). ECM-related proteins were over-represented in PSML. It is worth noting that the list would be dramatically expanded (by ~20 components – data not shown) if we also included all the Up and On-regulated proteins in PSML instead of considering only those reaching significance (p-values<0.05). This statement is further underpinned by pondering the effect of biological variability on the statistical output of the study, whereas some ECM-related proteins did not achieve statistical significance while showing On-regulation in at least one PSML sample (for example MMP2 detected only in one samples – Supplementary Table 1). The presence of those components suggests tissue damage either by the trauma itself or by degradation of other matrix by activated proteases.
Actin cytoskeleton reorganization markers have been found to be over-represented in PSML in comparison to plasma. Increased levels of gelsolin in PSML in comparison to post T/HS plasma are interesting in that previous gel-based approaches in animal models had documented decreases in the levels of this protein in response to T/HS (10). On the other hand, HPLC-MS-based (iTRAQ) investigations on animal models had observed a post T/HS increase of gelsolin (13). The present study is methodologically consistent and the results are in agreement with the latter. Despite the considerations above, increased detection of actin/cytoskeleton-related proteins in PSML might also be interpreted in the light of T/HS-induced tissue damage and cell lysis.
PSML was significantly enriched with proteins from other tissues, including several pancreatic proteins usually involved in digestion. Altered post T/HS plasma levels of gastrointestinal regulatory peptides had already been reported in the literature, while increases in pancreatic proteins in PSML had been documented through proteomics experiments in rat models (13,16). Furthermore, PSML was also enriched with cytosolic proteins of platelet or RBC origin, including hemoglobins and haptoglobin. It is worth recalling that haptoglobin binds free hemoglobin with high avidity during hemolysis, thus protecting organs from iron-generated reactive oxygen species. Consistently, increases in the levels of flavin reductase (NADPH) are relevant in the light of the role of this protein in heme catabolism. Anemia secondary to hemolysis in response to T/HS represents an additional burden to the oxygen transport capacity in these patients. Such phenomenon might aggravate endothelial cell or macrophage responses to hypoxia by triggering nitric oxide-mediated nitrosative stress to promote vasodilatory responses. Besides, red cell hemolysis would provide free iron in the extracellular environment to fuel reactive oxygen species (ROS)-generating Haber Weiss and Fenton reactions and oxidative stress that might further aggravate pro-inflammatory responses and drive damage in susceptible organs, such as the kidneys and the liver.
Since T/HS challenges the circulatory homeostasis, this translates into impaired oxygen transport and nutrient delivery in peripheral tissue. Nutrient depletion might represent one leading cause driving gut bacteria translocation, an event secondary to T/HS that has recently come into the spotlight owing to its potential correlation to the accumulation of factors mediating MOF either in the protein or metabolic fraction. Temporary nutrient deprivation subsequent to T/HS has been reported to dramatically alter the metabolism of the patients, through the activation of a series of events with the utter goal to rapidly replenish energy reservoirs, including fatty acid mobilization and lipolysis, enhanced glycolysis and incomplete lactate oxidation resulting in the promotion of lactate/pyruvate utilization to sustain gluconeogenesis in the liver, and proteolysis (32). These phenomena phenotypically result in increased oxygen debt and systemic acidosis, culminating in organ injury. Moreover such events are driven by neuroendocrine factors, involving hormone dysregulation of the insulin/glucagon axis (32). Consistently with the literature, we hereby observed a post T/HS accumulation of proteins related to responses to glucocorticoid stimuli.
Lipid mobilization is another distinctive trait of metabolic alterations subsequent to T/HS, and plasma lipoproteins were particularly enriched in post T/HS plasma samples. Glucose catabolism-related enzymes were also enriched in PSML samples. It is worth noting that although massive glucose utilization via glycolysis and active gluconeogenesis are expected in traumatized patients (32), the present results might reflect the scavenging of cell lysates by PSML, as a conduit to get rid of these proteins by channeling towards proteolytic degradation in lymph nodes or by increased extracellular proteases. Bisphosphoglycerate mutase, a red blood cell protein that plays a major role in regulating hemoglobin oxygen affinity by controlling the levels of its allosteric effector 2,3-bisphosphoglycerate, as it mediates its interconversion to the glycolytic 1,3 isomer through the Rapoport-Luebering shunt. Taken together, our data encourage further investigations on the metabolome of PSML and post T/HS plasma.
The accumulation of metabolic enzymes in biological fluids in response to trauma can be alternatively interpreted in light of the so-called “moonlightning proteins” hypothesis (33), which posits that single proteins might have dual but related functions in intracellular and extracellular microenvironments. In this view, enzymes enriched in PSML might either preserve their metabolic activity, or rather display alternative functions that could be related with or represent key drivers of the major untoward events following T/HS.
Out of the proteins unique to PSML, several have been never reported in the Human Plasma Proteome Database (22). Some of the observed PSML peculiar proteins have been already discussed above and might deserve further targeted investigations in the future, including proteases chymotrypsinogen and pepsin. The latter in particular might play a role in mediating albumin N-ter cleavage at acidic pH, resulting in the generation of a candidate biomarker for T/HS (34).
However, the remaining differences might stem from the incomplete annotation/coverage of the database (whose continuous expansion still represents one of the ambitious goals in the HPPP ambitious agenda (20,22). Conversely, while some of the proteins are indeed absent in the database list, isoforms to those proteins have been actually annotated (such as in the case of immunoglobulins, carboxypeptidase 2, mucin 4, and so on and so forth). The question here is whether we are looking for something that is uniquely present in mesenteric lymph, or we might rather be interested in an evident marker, shared with plasma/serum, that might be characterized by significant post T/HS fold change fluctuations and could correlate with the incidence of organ injury and patients outcome. If this is the case, proteomics investigations such as those presented hereby offer the opportunity to improve our understanding of the biological mechanisms underlying post T/HS responses, as described above, other than to suggest a limited list of potential candidates showing statistically significant fluctuations in response to trauma in a fluid-specific fashion (Table 2 and 3).
Previous studies have documented the existence of a vast array of lymph-circulating peptides originating from a variety of processing pathways including caspases, cathepsins, MMPs, ADAMs, kallikreins, calpains, and granzymes, among others (18,19).These self-peptides might play a role in central and peripheral tolerance, as they can be directly loaded on circulatory dendritic cells (18,19). Through bioinformatics elaboration of the present dataset, we hereby highlighted the presence of non-tryptic digestion peptides in post-T/HS samples. While such a bionformatic elaboration has the potential to result in increased FDRs the inclusion of non-tryptic peptides, as reported above, and paves the way for more targeted analyses in the future. Although it is beyond the scope of the present paper to further characterize this fraction, it is interesting to note that some cleavage sites for specific proteins such as Aspartate76 in hemoglobin alpha chain (KVADALTNAVAHVDDM – C-ter miscleavage Mascot score 117 – Supplementary Table 3) were already annotated in the Merops database (35). This cleavage site can be attacked by two different proteases, cathepsin L1 of falcipain-2, or rather represent an artifact induced by CID activation (36). On the other hand, the list of miscleaved peptides is evidently enriched with complement components, while the observed cleavage sites are not either annotated in currently available databases (36), nor they have been associated to technical caveats. In the light of the key role of complement components in mediating immune and inflammatory responses, this area of investigation deserves further investigation efforts in the future.
In conclusion, in the present study we compared the post T/HS plasma and PSML proteomes. As a result, we identified a list of approximately 155 proteins being uniquely represented in the latter fluid. However, when including also semi-tryptically digested peptides in the search, post T/HS proteins specific to plasma were observed as well. These results are consistent with PSML not being a mere plasma ultrafiltrate, even in response to T/HS, a condition that is known to promote fluid extravasation and thus ML enrichment with plasma proteins.
Unique proteins in both fluids were related to previous observations on PSML in animal models and human healthy subjects or trauma patients, while expanding currently available knowledge by covering a larger portion of the proteome in comparison to the existing literature in the field. These results were fostered by the pre-analytical simplification of the fluid proteomes through the targeted depletion of the 14 most abundant proteins.
Although proteomics technologies serve to highlight post T/HS plasma or lymph specific proteins, it should be also pointed out that such a high-throughput analytical approach is partially biased by technical and biological issues. From a technical standpoint, the analytical workflow is time-consuming, dependent on processing/analytical strategies, and might result in the depletion of low abundance proteins at the immunodepletion step. Indeed, high abundance proteins like albumin are known to promote the so-called “sponge effect” (adsorption of low abundance proteins) (37), and their selective removal would as well result in the partial removal of less represented proteins.
From a biological standpoint, results might be influenced by the small set of samples assayed and their intrinsic biological variability, arising for example from the different groups of clinical patients enrolled in this study. However, although conclusions cannot be overgeneralized, the present results indicate that, from a proteomics standpoint, lymph is more than a simple plasma ultrafiltrate.
The hereby documented observations allowed us to establish a plausible role for acute phase response proteins and innate immunity in mediating PSMLs role as a systemic conduit for clearance of damaged tissue debris and cell lysates secondary to T/HS. Protein enrichment in PSML might in turn be related to immune-modulatory responses (acute phase responses and PMN priming), utterly thus promoting ALI/MOF. At the same time, we highlighted the deregulation of proteases/anti-proteases components in post T/HS plasma, a phenomenon that is likely related to trauma-induced coagulopathy and late hypercoagulability.
Increased levels of ECM-related components suggest a protease dependent remodeling that provides a cross-talk between the lymphatic and the immune system. Hypovolemic shock-dependent hypooxygenation could in turn promote endothelial cells and macrophage-triggered generation of nitric oxide radicals, while red cell lysis would contribute to the onset of oxidative stress in the PSML. Other than redox metabolism, energy metabolism is apparently compromised, as gleaned through the over-representation of lipid mobilization-associated proteins in post T/HS plasma and glycolysis-related enzymes in PSML, downstream to the up-regulation of the insulin/glucagon-axis. Post-hoc analyses on trypsin miscleaved peptides are suggestive that future research efforts should take into account the low molecular weight component of the post T/HS plasma and PSML, including the peptidome (and its interactions with immunity and inflammation) and the metabolome, the shortest link between phenotype and stress.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by grants from the National Institutes of Health, National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM049222, National Center for Research Resources (Grant Number S10RR023015), and University of Colorado Comprehensive Cancer Center Core Support (P30 CA046934-17).
List of abbreviations
- DB
Database
- ESI
Electrospray ionization
- GeLC-MS
1D-SDS Gel electrophoresis coupled with Liquid Chromatography – Mass Spectrometry
- HS
Hemorrhagic shock
- ML
Mesenteric lymph
- nLC
Nano-flow liquid chromatography
- LTQ
Linear ion trap mass spectrometer
- MOF
Multiple Organ Failure
- MS
Mass spectrometry
- MS/MS
Tandem mass spectrometry
- PSML
Post-shock mesenteric lymph
- RP
Reversed Phase
Footnotes
Conflict of interest The authors disclose no conflict of interest.
References
- 1.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, O'Keefe GE, Cohen MJ, Moldawer LL, Tompkins RG, Maier RV. Inflammation and the Host Response to Injury Collaborative Research Program. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med. 2012;40(4):1129–35. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228(4):518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Johnson JL, Silliman CC. Mesenteric lymph is responsible for post-hemorrhagic shock systemic neutrophil priming. J Trauma. 2001;51(6):1069–72. doi: 10.1097/00005373-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(1–2):3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 5.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 6.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Meng Z, Veenstra TD. Proteomic analysis of serum, plasma, and lymph for the identification of biomarkers. Proteomics Clin Appl. 2007;1(8):747–57. doi: 10.1002/prca.200700243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittal A, Middleditch M, Ruggiero K, Buchanan CM, Jullig M, Loveday B, Cooper GJS, Windsor JA, Phillips ARJ. The proteome of rodent mesenteric lymph. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G895–G903. doi: 10.1152/ajpgi.90378.2008. [DOI] [PubMed] [Google Scholar]
- 9.Leak LV, Liotta LA, Krutzsch H, Jones M, Fusaroa VA, Ross SJ, Zhao Y., III EFP Proteomic analysis of lymph. Proteomics. 2004;4(3):753–765. doi: 10.1002/pmic.200300573. [DOI] [PubMed] [Google Scholar]
- 10.Peltz ED, Moore EE, Zurawel AA, Jordan JR, Damle SS, Redzic JS, Masuno T, Eun J, Hansen KC, Banerjee A. Proteome and system ontology of hemorrhagic shock: exploring early constitutive changes in postshock mesenteric lymph. Surgery. 2009;146(2):347–57. doi: 10.1016/j.surg.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zurawel A, Moore EE, Peltz ED, Jordan JR, Damle S, Dzieciatkowska M, Banerjee A, Hansen KC. Proteomic profiling of the mesenteric lymph after hemorrhagic shock: Differential gel electrophoresis and mass spectrometry analysis. Clin Proteomics. 2010;8(1):1. doi: 10.1186/1559-0275-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang JF, Shih LY, Yuan KC, Fang KY, Hwang TL, Hsieh SY. Proteomic analysis of post-hemorrhagic shock mesenteric lymph. Shock. 2010;34(3):291–298. doi: 10.1097/SHK.0b013e3181ceef5e. [DOI] [PubMed] [Google Scholar]
- 13.Mittal A, Middleditch M, Ruggiero K, Loveday B, Delahunt B, Jüllig M, Cooper GJ, Windsor JA, Phillips AR. Changes in the mesenteric lymph proteome induced by hemorrhagic shock. Shock. 2010;34(2):140–9. doi: 10.1097/SHK.0b013e3181cd8631. [DOI] [PubMed] [Google Scholar]
- 14.Diebel LN, Liberati DM, Ledgerwood AM, Lucas CE. Changes in lymph proteome induced by hemorrhagic shock: the appearance of damage-associated molecular patterns. J Trauma Acute Care Surg. 2012;73(1):41–50. doi: 10.1097/TA.0b013e31825e8b32. [DOI] [PubMed] [Google Scholar]
- 15.Dzieciatkowska M, Wohlauer MV, Moore EE, Damle S, Peltz E, Campsen J, Kelher M, Silliman C, Banerjee A, Hansen KC. Proteomic analysis of human mesenteric lymph. Shock. 2011;35(4):331–8. doi: 10.1097/SHK.0b013e318206f654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal A, Phillips ARJ, Middleditch M, Ruggiero K, Loveday B, Delahunt B, Cooper GJS, Windsor JA. The proteome of mesenteric lymph during acute pancreatitis and implications for treatment. JOP. 2009;10(2):130–142. [PubMed] [Google Scholar]
- 17.Jordan JR, Moore EE, Sarin EL, Damle SS, Kashuk SB, Silliman CC, Banerjee A. Arachidonic acid in post shock mesenteric lymph induces pulmonary synthesis of leukotriene B4. J Appl Physiol. 2008;104(4):1161–1166. doi: 10.1152/japplphysiol.00022.2007. [DOI] [PubMed] [Google Scholar]
- 18.Clement CC, Santambrogio L. The Lymph Self-Antigen Repertoire. Front Immunol. Dec 16. 2013;4:424. doi: 10.3389/fimmu.2013.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santambrogio L, Stern LJ. Carrying yourself: self antigen composition of the lymphatic fluid. Lymphat Res Biol. 2013;11(3):149–54. doi: 10.1089/lrb.2013.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omenn GS, States DJ, Adamski M, Blackwell TW, Menon R, Hermjakob H, et al. Overview of the HUPO Plasma Proteome Project: results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics. 2005;13:3226–45. doi: 10.1002/pmic.200500358. [DOI] [PubMed] [Google Scholar]
- 21.Echan LA, Tang HY, Li-Khan N, Lee K, Speicher DW. Depletion of multiple high-abundance proteins improves protein profiling capacities of human serum and plasma. Proteomics. 2005;13:3292–303. doi: 10.1002/pmic.200401228. [DOI] [PubMed] [Google Scholar]
- 22.Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, Bletz JA, et al. A high confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics. 2011;10(9):6353–14. doi: 10.1074/mcp.M110.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Qian WJ, Gritsenko MA, Xiao W, Moldawer LL, Kaushal A, et al. High dynamic range characterization of the trauma patient plasma proteome. Mol Cell Proteomics. 2006;5:1899–913. doi: 10.1074/mcp.M600068-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiao J, Gao M, Zhang H, Wang N, Xiao Z, Liu K, Yang M, Wang K, Xiao X. Identification of potential biomarkers by serum proteomics analysis in rats with sepsis. Shock. 2014;42:75–81. doi: 10.1097/SHK.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 25.Yuan KC, Fang JF, Hsieh SY, Shih HN. Comparative proteomic analysis of rodent plasma and mesenteric lymph. Chin J Physiol. 2013;56(3):163–73. doi: 10.4077/CJP.2013.BAB116. [DOI] [PubMed] [Google Scholar]
- 26.Clement CC, Aphkhazava D, Nieves E, Callaway M, Olszewski W, Rotzschke O, Santambrogio L. Protein expression profiles of human lymph and plasma mapped by 2D-DIGE and 1D SDS-PAGE coupled with nanoLC-ESI-MS/MS bottom-up proteomics. J Proteomics. 2013;78:172–87. doi: 10.1016/j.jprot.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzieciatkowska M, Hill R, Hansen KC. GeLC-MS/MS analysis of complex protein mixtures. Methods Mol Biol. 2014;1156:53–66. doi: 10.1007/978-1-4939-0685-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58(3):475–80. doi: 10.1097/01.ta.0000153938.77777.26. [DOI] [PubMed] [Google Scholar]
- 29.Pike RN, Buckle AM, le Bonniec BF, Church FC. Control of the coagulation system by serpins. Getting by with a little help from glycosaminoglycans. FEBS J. 2005;272(19):4842–51. doi: 10.1111/j.1742-4658.2005.04880.x. [DOI] [PubMed] [Google Scholar]
- 30.Cuschieri J, Bulger E, Schaeffer V, Sakr S, Nathens AB, Hennessy L, Minei J, Moore EE, et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34:346–351. doi: 10.1097/SHK.0b013e3181d8e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dreyer WJ, Michael LH, Nguyen T, Smith CW, Anderson DC, Entman ML, Rossen RD. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ Res. 1992;71(6):1518–24. doi: 10.1161/01.res.71.6.1518. [DOI] [PubMed] [Google Scholar]
- 32.Wohlauer M, Moore EE, Silliman CC, Fragoso M, Gamboni F, Harr J, Accurso F, Wright F, Haenel J, Fullerton D, Banerjee A. Nebulized hypertonic saline attenuates acute lung injury following trauma and hemorrhagic shock via inhibition of matrix metalloproteinase-13. Crit Care Med. 2012;40(9):2647–53. doi: 10.1097/CCM.0b013e3182592006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huberts DH, van der Klei IJ. Moonlighting proteins: an intriguing mode of multitasking. Biochim Biophys Acta. 2010;1803(4):520–5. doi: 10.1016/j.bbamcr.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser VL, Sifri ZC, Senthil M, Dikdan GS, Lu Q, Xu DZ, Deitch EA. Albumin peptide: A molecular marker for trauma/hemorrhagic-shock in rat mesenteric lymph. Peptides. 2005;26:2491–2499. doi: 10.1016/j.peptides.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Rawlings ND, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012;40:D343–50. doi: 10.1093/nar/gkr987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mekecha TT, Amunugama R, McLuckey SA. Ion trap collision-induced dissociation of human hemoglobin alpha-chain cations. J Am Soc Mass Spectrom. 2006;17(7):923–31. doi: 10.1016/j.jasms.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Liumbruno G, D'Alessandro A, Grazzini G, Zolla L. Blood-related proteomics. J Proteomics. 2010;73(3):483–507. doi: 10.1016/j.jprot.2009.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.