Abstract
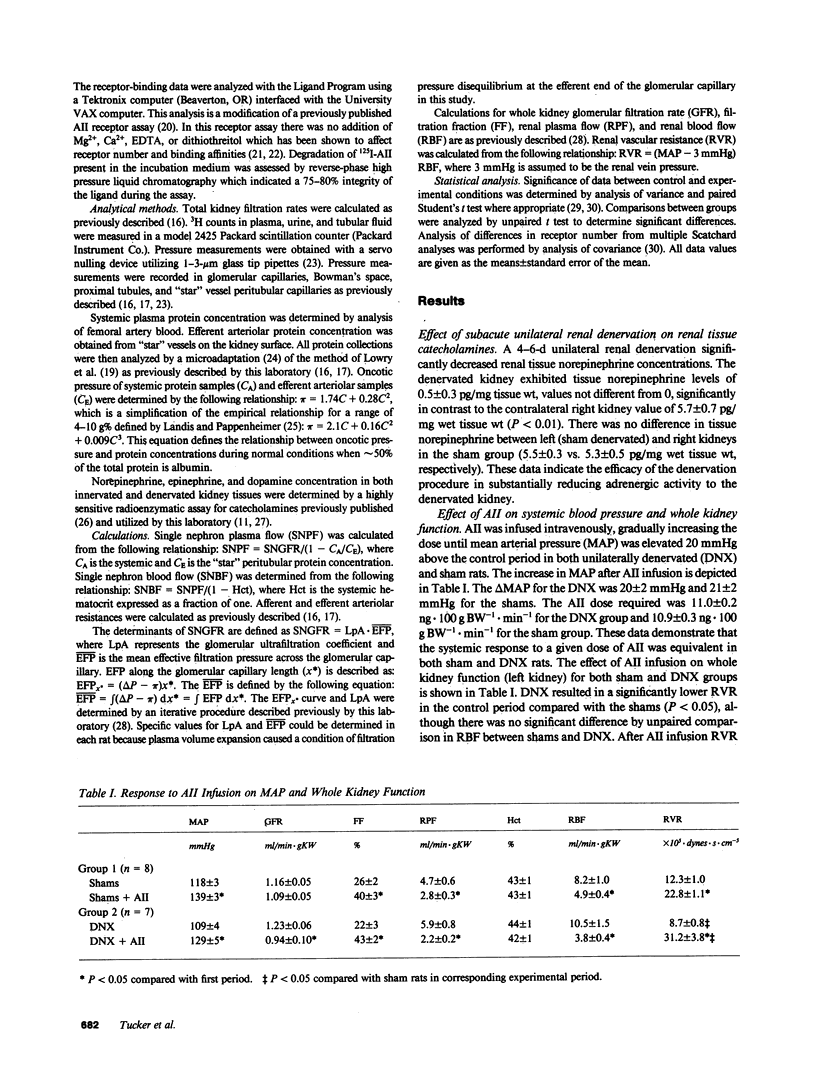
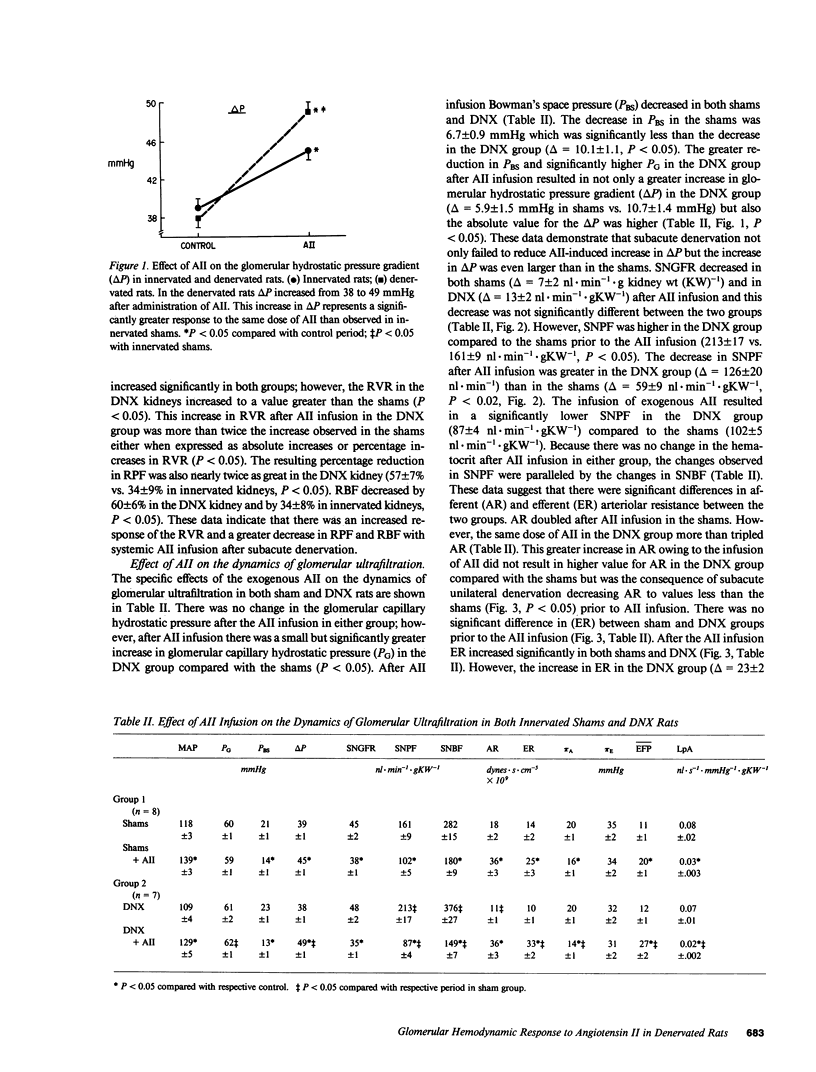
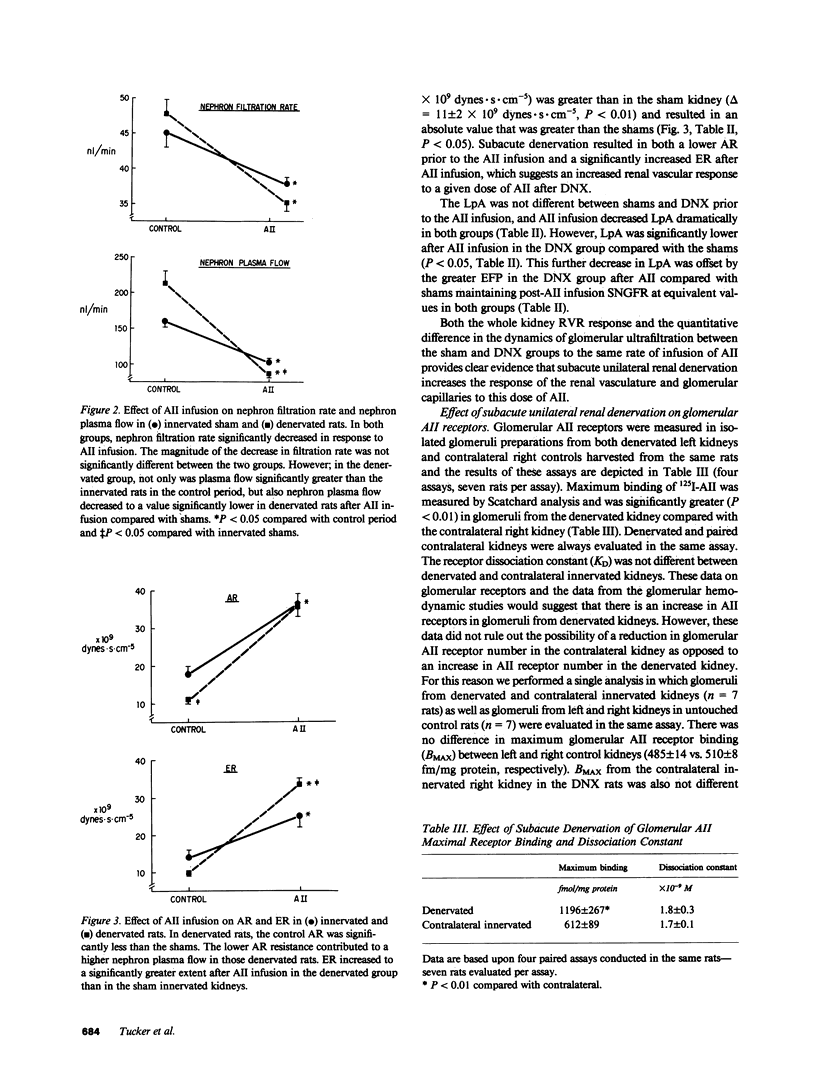
We examined the changes in glomerular hemodynamics produced by angiotensin II (AII) in both normal Munich-Wistar rats and rats which were unilaterally renal denervated (measured kidney) 4-6 d prior to the measurement periods. Measurements of glomerular dynamics were performed in a control period after plasma volume expansion and during infusion of 11 ng X 100 g body wt-1 X min-1 of AII. The glomerular hydrostatic pressure gradient increased from 38 +/- 1 to 49 +/- 1 mmHg in denervated rats compared with a lesser response in controls (from 39 +/- 1 to 45 +/- 1 mmHg, P less than 0.05). Single nephron plasma flow decreased from 213 +/- 17 to 87 +/- 4 nl X min-1 X g kidney wt (KW)-1 in denervated kidneys versus a more modest decrease in control kidneys (from 161 +/- 9 to 102 +/- 5 nl X min X gKW-1). These changes were due to a greater increase in both afferent and efferent arteriolar resistance after AII infusion in denervated compared with control kidneys. Glomerular AII receptor maximum binding was 1,196 +/- 267 fmol/mg protein in denervated kidneys compared with 612 +/- 89 fmol/mg protein (P less than 0.01) in controls with no change in receptor affinity. We conclude the subacute unilateral renal denervation results in renal vasodilation, denervation magnifies the vasoconstrictive effect of AII infusion on glomerular hemodynamics, and the observed increased response to AII after denervation is associated with increases in glomerular AII receptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylis C., Brenner B. M. Modulation by prostaglandin synthesis inhibitors of the action of exogenous angiotensin II on glomerular ultrafiltration in the rat. Circ Res. 1978 Dec;43(6):889–898. doi: 10.1161/01.res.43.6.889. [DOI] [PubMed] [Google Scholar]
- Berl T., Henrich W. L., Erickson A. L., Schrier R. W. Prostaglandins in the beta-adrenergic and baroreceptor-mediated secretion of renin. Am J Physiol. 1979 May;236(5):F472–F477. doi: 10.1152/ajprenal.1979.236.5.F472. [DOI] [PubMed] [Google Scholar]
- Bichet D., Marc-Aurèle J. Renal intracortical blood flow and renin secretion after denervation by 6-hydroxydopamine. Can J Physiol Pharmacol. 1982 Feb;60(2):184–192. doi: 10.1139/y82-029. [DOI] [PubMed] [Google Scholar]
- Blanc E., Sraer J., Sraer J. D., Baud L., Ardaillou R. Ca+ and Mg2+ dependence of angiotensin II binding to isolated rat renal glomeruli. Biochem Pharmacol. 1978 Feb 15;27(4):517–524. doi: 10.1016/0006-2952(78)90387-8. [DOI] [PubMed] [Google Scholar]
- Blantz R. C. Effect of mannitol on glomerular ultrafiltration in the hydropenic rat. J Clin Invest. 1974 Nov;54(5):1135–1143. doi: 10.1172/JCI107857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976 Feb;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. M., Printz M. P. Chronic estrogen treatment reduces angiotensin II receptors in the anterior pituitary. Endocrinology. 1983 Oct;113(4):1503–1510. doi: 10.1210/endo-113-4-1503. [DOI] [PubMed] [Google Scholar]
- Durrett L. R., Ziegler M. G. A sensitive radioenzymatic assay for catechol drugs. J Neurosci Res. 1980;5(6):587–598. doi: 10.1002/jnr.490050613. [DOI] [PubMed] [Google Scholar]
- Edwards R. M. Segmental effects of norepinephrine and angiotensin II on isolated renal microvessels. Am J Physiol. 1983 May;244(5):F526–F534. doi: 10.1152/ajprenal.1983.244.5.F526. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Guyton A. C., Jackson T. E., Coleman T. G., Lohmeier T. E., Trippodo N. C. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol. 1977 Nov;233(5):F366–F372. doi: 10.1152/ajprenal.1977.233.5.F366. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Guyton A. C., Trippodo N. C., Lohmeier T. E., McCaa R. E., Cowley A. W., Jr Intrarenal control of electrolyte excretion by angiotensin II. Am J Physiol. 1977 Jun;232(6):F538–F544. doi: 10.1152/ajprenal.1977.232.6.F538. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Miele J. F., Brenner B. M. Reversal of renal cortical actions of angiotensin II by verapamil and manganese. Kidney Int. 1979 Aug;16(2):137–147. doi: 10.1038/ki.1979.115. [DOI] [PubMed] [Google Scholar]
- Intaglietta M., Pawula R. F., Tompkins W. R. Pressure measurements in the mammalian microvasculature. Microvasc Res. 1970 Apr;2(2):212–220. doi: 10.1016/0026-2862(70)90009-9. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I. Contractile properties of the glomerular mesangium. Fed Proc. 1983 Nov;42(14):3053–3057. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Brenner B. M. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res. 1975 Jul;37(1):101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- Osborn J. L., Holdaas H., Thames M. D., DiBona G. F. Renal adrenoceptor mediation of antinatriuretic and renin secretion responses to low frequency renal nerve stimulation in the dog. Circ Res. 1983 Sep;53(3):298–305. doi: 10.1161/01.res.53.3.298. [DOI] [PubMed] [Google Scholar]
- Pelayo J. C., Blantz R. C. Analysis of renal denervation in the hydropenic rat: interactions with angiotensin II. Am J Physiol. 1984 Jan;246(1 Pt 2):F87–F95. doi: 10.1152/ajprenal.1984.246.1.F87. [DOI] [PubMed] [Google Scholar]
- Pelayo J. C., Ziegler M. G., Blantz R. C. Angiotensin II in adrenergic-induced alterations in glomerular hemodynamics. Am J Physiol. 1984 Nov;247(5 Pt 2):F799–F807. doi: 10.1152/ajprenal.1984.247.5.F799. [DOI] [PubMed] [Google Scholar]
- Pelayo J. C., Ziegler M. G., Jose P. A., Blantz R. C. Renal denervation in the rat: analysis of glomerular and proximal tubular function. Am J Physiol. 1983 Jan;244(1):F70–F77. doi: 10.1152/ajprenal.1983.244.1.F70. [DOI] [PubMed] [Google Scholar]
- Ploth D. W., Navar L. G. Intrarenal effects of the renin-angiotensin system. Fed Proc. 1979 Aug;38(9):2280–2285. [PubMed] [Google Scholar]
- Reid I. A., Schrier R. W., Earley L. E. An effect of extrarenal beta adrenergic stimulation on the release of renin. J Clin Invest. 1972 Jul;51(7):1861–1869. doi: 10.1172/JCI106988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogenes P. R., Gottschalk C. W. Renal function in conscious rats with chronic unilateral renal denervation. Am J Physiol. 1982 Feb;242(2):F140–F148. doi: 10.1152/ajprenal.1982.242.2.F140. [DOI] [PubMed] [Google Scholar]
- SMYTHE C. M., NICKEL J. F., BRADLEY S. E. The effect of epinephrine (USP), l-epinephrine, and l-norepinephrine on glomerular filtration rate, renal plasma flow, and the urinary excretion of sodium, potassium, and water in normal man. J Clin Invest. 1952 May;31(5):499–506. doi: 10.1172/JCI102634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwietzer G., Gertz K. H. Changes of hemodynamics and glomerular ultrafiltration in renal hypertension of rats. Kidney Int. 1979 Feb;15(2):134–143. doi: 10.1038/ki.1979.19. [DOI] [PubMed] [Google Scholar]
- Skorecki K. L., Ballermann B. J., Rennke H. G., Brenner B. M. Angiotensin II receptor regulation in isolated renal glomeruli. Fed Proc. 1983 Nov;42(14):3064–3070. [PubMed] [Google Scholar]
- Sraer J., Baud L., Cosyns J. P., Verroust P., Nivez M. P., Ardaillou R. High affinity binding of 125I-angiotensin II to rat glomerular basement membranes. J Clin Invest. 1977 Jan;59(1):69–81. doi: 10.1172/JCI108623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner R. W., Blantz R. C. Acute reversal by saralasin of multiple intrarenal effects of angiotensin II. Am J Physiol. 1979 Nov;237(5):F386–F391. doi: 10.1152/ajprenal.1979.237.5.F386. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Bowen P. D., Parma R., Osgood R. W., Bowman R. L., Stein J. H. Measurement of renal cortical and medullary blood flow by laser-Doppler spectroscopy in the rat. Am J Physiol. 1979 Jan;236(1):F80–F87. doi: 10.1152/ajprenal.1979.236.1.F80. [DOI] [PubMed] [Google Scholar]
- Taher M. S., McLain L. G., McDonald K. M., Schrier R. W., Gilbert L. K., Aisenbrey G. A., McCool A. L. Effect of beta adrenergic blockade on renin response to renal nerve stimulation. J Clin Invest. 1976 Feb;57(2):459–465. doi: 10.1172/JCI108297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Factors determining superficial nephron filtration in the mature, growing rat. Am J Physiol. 1977 Feb;232(2):F97–104. doi: 10.1152/ajprenal.1977.232.2.F97. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Peterson O. W., Ziegler M. G., Blantz R. C. Analysis of adrenergic effects of the anesthetics Inactin and alpha-chloralose. Am J Physiol. 1982 Sep;243(3):F253–F259. doi: 10.1152/ajprenal.1982.243.3.F253. [DOI] [PubMed] [Google Scholar]
- Vander A. J. Effect of catecholamines and the renal nerves on renin secretion in anesthetized dogs. Am J Physiol. 1965 Sep;209(3):659–662. doi: 10.1152/ajplegacy.1965.209.3.659. [DOI] [PubMed] [Google Scholar]


