Abstract
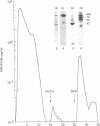
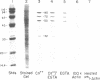
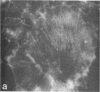
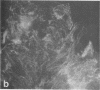
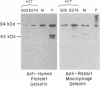
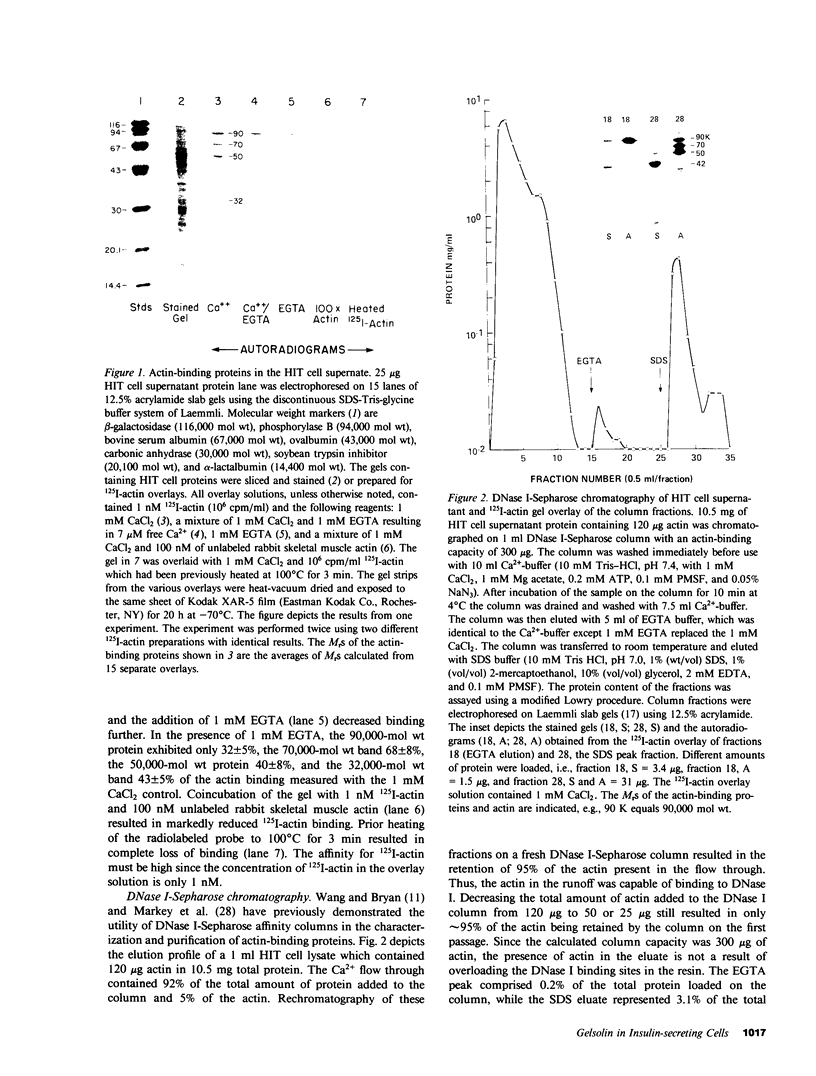
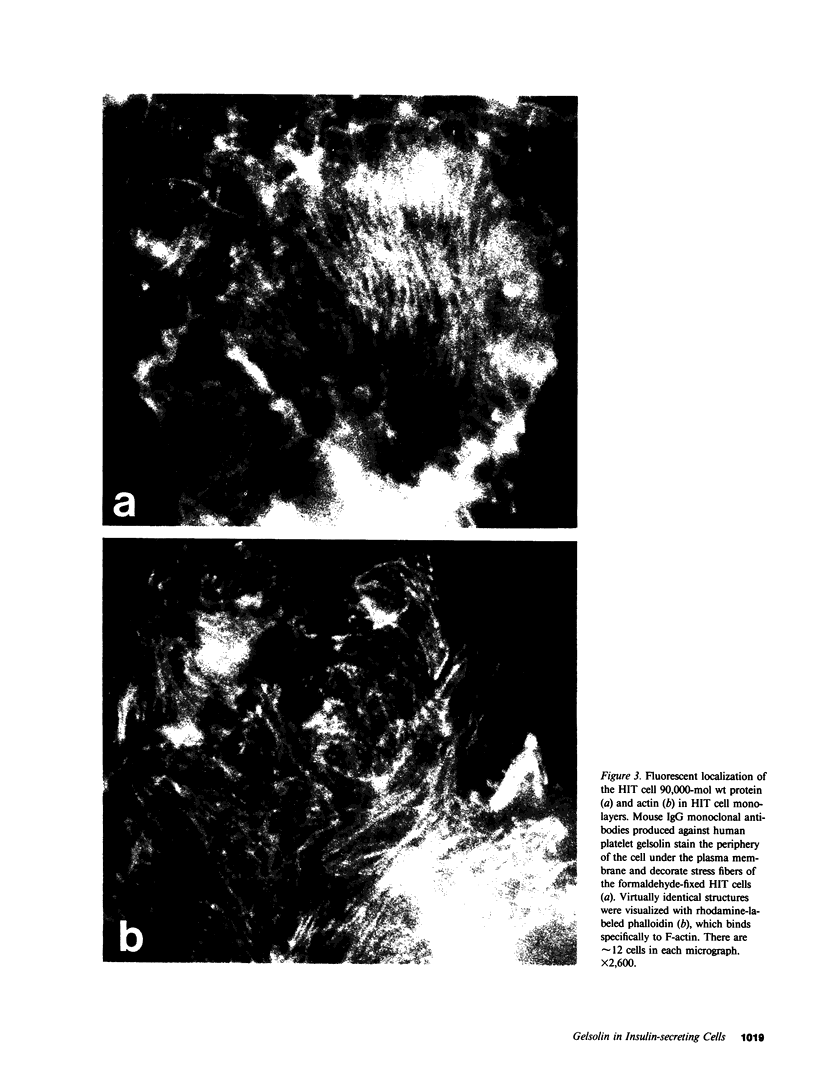
Using a gel overlay technique we have previously described a 90,000-mol wt actin-binding protein in a number of hormone-secreting tissues and tentatively identified this protein as gelsolin. Gelsolin is a protein that cuts or solates cross-linked actin filaments and can also serve as a nucleating site for actin polymerization. The objective of this study was to isolate this protein from a hamster insulin-secreting (HIT) cell line and compare the immunologic properties and peptide maps of purified rabbit macrophage gelsolin, human platelet gelsolin, and the HIT cell 90,000-mol wt protein. DNase I-Sepharose retained the HIT cell actin-binding proteins in 1 mM CaCl2; some of the 90,000-mol wt protein could then be eluted with 1 mM EGTA. The remaining actin-binding proteins were eluted using a buffer containing SDS. The EGTA peak fractions contained two major protein bands of Mr = 90,000 and 42,000, which suggested that a 90,000-mol wt-actin complex was eluted from the DNase I-Sepharose column. Specific antibodies to the human platelet and rabbit macrophage gelsolins bound to the 90,000-mol wt bands in the eluates, but did not crossreact with other actin-binding proteins. Indirect immunofluorescence using an anti-human platelet gelsolin antibody localized the 90,000-mol wt protein to stress fibers that were also stained with phalloidin, which suggested that gelsolin is associated with actin in vivo. Tryptic peptide maps of all three radioiodinated gelsolins were virtually indistinguishable. Thus, gelsolin is a highly conserved gene product found in at least three diverse cell types, an insulin-secreting beta cell line, macrophages, and platelets, and may link a transient increase in Ca2+ cellular levels with changes in actin polymerization and/or the gel-sol state of these cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M., Schrader W. T., O'Malley B. W. Assessment of structural similarities in chick oviduct progesterone receptor subunits by partial proteolysis of photoaffinity-labeled proteins. J Biol Chem. 1983 Jun 25;258(12):7331–7337. [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D., Yin H. L., Stossel T. P. Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature. 1984 Jul 5;310(5972):56–58. doi: 10.1038/310056a0. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Kurth M. C. Actin-gelsolin interactions. Evidence for two actin-binding sites. J Biol Chem. 1984 Jun 25;259(12):7480–7487. [PubMed] [Google Scholar]
- Dedman J. R., Welsh M. J., Means A. R. Ca2+-dependent regulator. Production and characterization of a monospecific antibody. J Biol Chem. 1978 Oct 25;253(20):7515–7521. [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Goldstein D. A. Calculation of the concentrations of free cations and cation-ligand complexes in solutions containing multiple divalent cations and ligands. Biophys J. 1979 May;26(2):235–242. doi: 10.1016/S0006-3495(79)85247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kurth M. C., Bryan J. Platelet activation induces the formation of a stable gelsolin-actin complex from monomeric gelsolin. J Biol Chem. 1984 Jun 25;259(12):7473–7479. [PubMed] [Google Scholar]
- Kurth M. C., Wang L. L., Dingus J., Bryan J. Purification and characterization of a gelsolin-actin complex from human platelets. Evidence for Ca2+-insensitive functions. J Biol Chem. 1983 Sep 25;258(18):10895–10903. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., Kowluru A. Calcium-calmodulin-dependent myosin phosphorylation by pancreatic islets. Diabetes. 1982 Jun;31(6 Pt 1):566–570. doi: 10.2337/diab.31.6.566. [DOI] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S., Konrad M., Nowak E. The interaction of bovine pancreatic deoxyribonuclease I and skeletal muscle actin. Eur J Biochem. 1980 Mar;104(2):367–379. doi: 10.1111/j.1432-1033.1980.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Markey F., Persson T., Lindberg U. A 90 000-dalton actin-binding protein from platelets. Comparison with villin and plasma brevin. Biochim Biophys Acta. 1982 Dec 6;709(1):122–133. doi: 10.1016/0167-4838(82)90429-0. [DOI] [PubMed] [Google Scholar]
- Orci L., Gabbay K. H., Malaisse W. J. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972 Mar 10;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Santerre R. F., Cook R. A., Crisel R. M., Sharp J. D., Schmidt R. J., Williams D. C., Wilson C. P. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snabes M. C., Boyd A. E., 3rd, Bryan J. Detection of actin-binding proteins in human platelets by 125I-actin overlay of polyacrylamide gels. J Cell Biol. 1981 Sep;90(3):809–812. doi: 10.1083/jcb.90.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snabes M. C., Boyd A. E., 3rd, Bryan J. Identification of G actin-binding proteins in rat tissues using a gel overlay technique. Exp Cell Res. 1983 Jun;146(1):63–70. doi: 10.1016/0014-4827(83)90324-5. [DOI] [PubMed] [Google Scholar]
- Snabes M. C., Boyd A. E., 3rd Increased filamentous actin in islets of Langerhans from fasted hamsters. Biochem Biophys Res Commun. 1982 Jan 15;104(1):207–211. doi: 10.1016/0006-291x(82)91960-x. [DOI] [PubMed] [Google Scholar]
- Snabes M. C., Boyd A. E., 3rd, Pardue R. L., Bryan J. A DNase I binding/immunoprecipitation assay for actin. J Biol Chem. 1981 Jun 25;256(12):6291–6295. [PubMed] [Google Scholar]
- Southwick F. S., Hartwig J. H. Acumentin, a protein in macrophages which caps the "pointed" end of action filaments. Nature. 1982 May 27;297(5864):303–307. doi: 10.1038/297303a0. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., Stossel T. P. Isolation of an inhibitor of actin polymerization from human polymorphonuclear leukocytes. J Biol Chem. 1981 Mar 25;256(6):3030–3036. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L., Bryan J. Isolation of calcium-dependent platelet proteins that interact with actin. Cell. 1981 Sep;25(3):637–649. doi: 10.1016/0092-8674(81)90171-9. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Osborn M., Weber K. Visualization of the same PtK2 cytoskeletons by both immunofluorescence and low power electron microscopy. Exp Cell Res. 1978 Nov;117(1):47–61. doi: 10.1016/0014-4827(78)90426-3. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. The roles of intracellular and extracellular Ca++ in glucose-stimulated biphasic insulin release by rat islets. J Clin Invest. 1978 Aug;62(2):451–458. doi: 10.1172/JCI109146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Albrecht J. H., Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Purification and structural properties of gelsolin, a Ca2+-activated regulatory protein of macrophages. J Biol Chem. 1980 Oct 10;255(19):9490–9493. [PubMed] [Google Scholar]
- Yin H. L., Zaner K. S., Stossel T. P. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980 Oct 10;255(19):9494–9500. [PubMed] [Google Scholar]
- de Lanerolle P., Adelstein R. S., Feramisco J. R., Burridge K. Characterization of antibodies to smooth muscle myosin kinase and their use in localizing myosin kinase in nonmuscle cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4738–4742. doi: 10.1073/pnas.78.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]