Abstract
Past reproductive interactions among incompletely isolated species may leave behind a trail of introgressed alleles, shedding light on historical range movements. Betula pubescens is a widespread native tetraploid tree species in Britain, occupying habitats intermediate to those of its native diploid relatives, B. pendula and B. nana. Genotyping 1134 trees from the three species at 12 microsatellite loci, we found evidence of introgression from both diploid species into B. pubescens, despite the ploidy difference. Surprisingly, introgression from B. nana, a dwarf species whose present range is highly restricted in northern, high-altitude peat bogs, was greater than introgression from B. pendula, which is morphologically similar to B. pubescens and has a substantially overlapping range. A cline of introgression from B. nana was found extending into B. pubescens populations far to the south of the current B. nana range. We suggest that this genetic pattern is a footprint of a historical decline and/or northwards shift in the range of B. nana populations due to climate warming in the Holocene. This is consistent with pollen records that show a broader, more southerly distribution of B. nana in the past. Ecological niche modelling predicts that B. nana is adapted to a larger range than it currently occupies, suggesting additional factors such as grazing and hybridization may have exacerbated its decline. We found very little introgression between B. nana and B. pendula, despite both being diploid, perhaps because their distributions in the past have rarely overlapped. Future conservation of B. nana may partly depend on minimization of hybridization with B. pubescens, and avoidance of planting B. pendula near B. nana populations.
Keywords: climate change, ecological niche modelling, hybridization, introgression, polyploidy
Introduction
Patterns of genetic variation within and among present day species provide evidence about past population dynamics and demographics. However, interpretation of such genetic evidence is difficult, with multiple historical scenarios potentially explaining the same data. A recent example is the observation of Neanderthal-like genetic variants in modern human population of Eurasia. This observation has been variously explained by: a single hybridization event (Green et al. 2010), ancient population structure (Durand et al. 2011; Eriksson & Manica 2012; Sankararaman et al. 2012; Yang et al. 2012) or hybridization at a moving front as modern humans invaded Eurasia (Currat & Excoffier 2011). Such ambiguous situations may be to some extent resolved by additional data sources such as other genetic markers, sample areas, taxa or fossils (Wall et al. 2013). Multiple data sets from exemplar case studies may aid the interpretation of other systems where only a single set of genetic data is available (Buggs 2007).
One major historical influence on patterns of extant genetic variation is past climate change. Gradients of genetic diversity within species in temperate regions, and correlation of gene phylogenies with geography, can be interpreted as legacies of postglacial recolonization with climate warming (Hewitt 1999; Avise 2000; Petit et al. 2003). More detailed evidence about species range shifts in response to climate change may be provided by patterns of genetic exchange between closely related species that meet at hybrid zones (Buggs 2007): specifically, neutral alleles are expected to introgress from a retreating species into an expanding species, leaving behind a molecular footprint of hybrid zone movement (Buggs 2007; Currat et al. 2008; Scriber 2011). Whilst this is a potentially sensitive way of tracing past range shifts, genetic patterns alone may not be sufficient to draw firm conclusions, as illustrated by the case of Neandertals and modern humans mentioned above.
Many tree species hybridize extensively with local relatives, making them good study systems for examining patterns of introgression as a consequence of climate change (Petit et al. 1997). There is much evidence that tree species have shifted their latitudinal and altitudinal ranges in response to climate change (Davis & Shaw 2001), and this process is ongoing as the climate warms (Chen et al. 2011). Evidence for this comes from pollen records (Huntley & Birks 1983), population genetic variability (Petit et al. 2003) and phylogenies (Himes et al. 2008). In areas bounded by inhospitable habitat, some tree species can only respond to climate change by contracting, rather than shifting their ranges leading to the possibility of local extinction (Zhu et al. 2012).
In this study, we set out to test the hypothesis that the decline of a cold-adapted tree species during Holocene climate warming in Britain could be traced in patterns of introgression of its alleles into a closely related tree species that is currently widespread. To aid the interpretation of these introgression patterns, we also analysed patterns of introgression between the widespread species and another close relative with which it is commonly sympatric. We chose a study system with a good fossil record, a well-characterized ecology, and evidence for frequent hybridization. This system is the Betula species of Britain. The genus Betula (birches) consists of wind-pollinated tree species, which frequently hybridize (Nagamitsu et al. 2006; Thórsson et al. 2010).
In Britain, there are three native Betula tree species: tetraploid Betula pubescens and diploids B. pendula and B. nana. Betula pubescens (downy birch) and B. pendula (silver birch) are common, widespread and often sympatric or parapatric, with the former adapted to wetter and colder habitats than the latter (Atkinson 1992). Betula pubescens is thus more concentrated in northern and western parts of Britain, whereas B. pendula is more common in south and east (Gimingham 1984). The two species are hard to distinguish morphologically as there is a continuum of variation between them (Brown & Tuley 1971; Atkinson & Codling 1986). Initially, both were treated as B. alba (Linnaeus 1753) and were split later partly due to the difference in ploidy level (Brown & Aldawoody 1979; Gill & Davy 1983; Brown & Williams 1984). Hybrids between the two are thought to occur in many areas in the British Isles, some of which are fully fertile (Stace 2010). Bidirectional gene flow has occurred between B. pendula and B. pubescens, in Scandinavia and western Russia, but with a bias towards gene flow from B. pendula to B. pubescens (Palmé et al. 2004), perhaps because gene flow is easier from a diploid to a tetraploid than vice versa (Stebbins 1971).
Betula nana (dwarf birch) grows up to only one metre in height and is nationally scarce in Britain, mainly restricted to the Scottish Highlands in fragmented populations (Aston 1984). It is widespread in subarctic tundra and subalpine areas of more northerly countries (DeGroot et al. 1997). In Scotland, B. nana is under active conservation management by organizations such as Trees for Life and Highland Birchwoods. Hybrids between B. nana and B. pubescens have been recorded in the British Isles (Kenworthy et al. 1972; Crawford 2008; Stace 2010). In Iceland, such hybrids have been confirmed using flow cytometry (Anamthawat-Jónsson et al. 2010), morphology (Elkington 1968; Thórsson et al. 2007), cytogenetics (Anamthawat-Jónsson & Thórsson 2003) and genetic markers (Thórsson et al. 2001; Palmé et al. 2004). The morphology of ancient pollen of Betula species in European pollen cores suggested that hybridization between B. nana and B. pubescens has taken place throughout the Holocene (Blackburn 1952; Caseldine 2001). Due to the different cold tolerances of the three Betula species of Britain, we would expect B. nana to be the first colonist of areas coming available after glaciation, followed with climate warming by B. pubescens and finally by B. pendula.
Therefore, we considered the Betula species of Britain to be a good study system to rigorously test the hypothesis that the decline of a species with climate warming could be traced in patterns of introgression of its alleles into a closely related species. We surveyed genetic variation at 12 microsatellite loci in 78 populations of B. pubescens and 10 populations of B. nana in Britain, hypothesizing that a trail of alleles from B. nana would be found in B. pubescens populations far south of the current range of B. nana. We expected overall rates of introgression to be low (even if rates of hybridization were high) due to the ploidy difference between the two species, which should result in partial reproductive isolation between them. As a point of comparison in interpreting our results, we also genotyped 32 populations of B. pendula. Because B. pendula is morphologically similar and broadly sympatric with B. pubescens, we hypothesized that more gene flow would have occurred between these two species. We also used ecological niche modelling (ENM) and pollen records to infer the current potential distribution of B. nana and its past distribution, to provide ecological and historical context for the interpretation of our genetic data.
Materials and methods
Sampling and morphological identification
Leaf and twig samples were collected from naturally occurring Betula populations across Britain between April 2010 and August 2013. Samples were pressed and dried in a plant press. Species were identified based on leaf morphology according to the standard guide for UK birch identification (Rich & Jermy 1998), including the Atkinson discriminant function to seek to distinguish between B. pendula and B. pubescens (Atkinson & Codling 1986). In total, 1134 Betula samples were collected from 120 populations. Of these, 120 samples were provisionally identified as B. nana, 169 as B. pendula and 845 as B. pubescens (including some of possible hybrid origin). Figure S1 (Supporting information) shows a representative subset of leaves from the three study species. Three known F1 hybrid individuals were also examined, two B. nana × B. pubescens and one B. nana × B. pendula, which were grown from seed at Queen Mary University of London.
Microsatellite genotyping
Genomic DNA was isolated from dried cambial tissue or leaves following a modified cetyltrimethylammonium bromide (CTAB) protocol (Wang et al. 2013). The isolated DNA was assessed with a Nanovue spectrophotometer (GE Healthcare, UK) and a 1.0% agarose gel. The DNA was diluted to a final concentration of 5–20 ng/μL for subsequent use. A subset of microsatellite loci developed for B. pendula (Kulju et al. 2004) and B. pubescens ssp. tortuosa (Truong et al. 2005) was used (Table S1, Supporting information). The 5′ terminal of forward primers was labelled with FAM, HEX or TAM. Multiplex PCRs were conducted combining four pairs of microsatellites in each multiplex. In each multiplex reaction, two loci with a significant length difference were labelled using the same dye. The final reaction volume was 7.5 μL, including 3.75 μL QIAGEN Multiplex PCR Master Mix, 0.15 μL of primers (10 μm each in initial volume), 1.55 μL H2O and 5–20 ng of DNA dissolved in 1.0 μL TE buffer. Two touchdown PCR programmes (Mellersh & Sampson 1993) were used with differing annealing temperatures according to the primers within each multiplex. For Multiplex 1 and Multiplex 2 (Table S2, Supporting information), an initial denaturation step at 95 °C for 15 min was followed by 28 cycles of denaturation (94 °C for 30 s), annealing (65 °C to 62 °C for 90 s) and extension (72 °C for 60 s) steps, and a final extension step at 60 °C for 30 min. For Multiplex 3 and Multiplex 4 (Table S1, Supporting information), the annealing temperature was from 62 °C to 48 °C, with the remaining steps unchanged. Fragment lengths were determined by capillary gel electrophoresis with capillary sequencer ABI 3730xl (Applied Biosystems). To check the reproducibility of our microsatellite analyses, we selected a subset of 26 individuals, and repeated the microsatellite analyses of these for each individual. The results indicated 100% match in the results, suggesting that our microsatellite analyses are highly reproducible. Alleles were scored using the software genemarker 2.4.0 (Softgenetics) and checked manually.
Three loci with variable flanking regions were genotyped with two sets of primers each to avoid null alleles. One locus, L52, was discarded due to difficulty in reading alleles. Thus, a total of 12 loci were genotyped in our samples. Individuals with more than two missing loci were excluded, resulting in 1134 individuals in the final data set. This data set is available in the Dryad Digital Repository (Wang et al. 2014).
Microsatellite data analysis
Principal coordinates (PCO) analysis of microsatellite data was performed using POLYSAT (Clark & Jasieniuk 2011) implemented in r 2.15.3 (R Core Team 2012), based on pairwise genetic distance calculated by Bruvo's methods (Bruvo et al. 2004). POLYSAT is designed to analyse polyploid microsatellite data by assuming that the allele copy number is always ambiguous in any heterozygotes. POLYSAT was also used to transform the multilocus allele phenotype for each individual into binary arrays of the presence or absence of each allele for each individual, and a further PCO analysis was performed using past 1.7.5 (Hammer et al. 2001) using pairwise Euclidean distances (Kloda et al. 2008).
We also analysed the microsatellite data with a Bayesian clustering approach in structure 2.3.4 (Pritchard et al. 2000) to identify the most likely number of genetic clusters (K), to complement the inference of three disjunct clusters from PCO analysis and taxonomic classification. This implements algorithms accounting for genotypic uncertainty arising from copy number variation when the data include polyploid cytotypes. Individuals are assigned to genetic clusters based on multilocus genotypes. Putative hybrids and admixed individuals could be identified as they have fractions of genomes from different genetic clusters. We performed ten replicates (1 000 000 generations and a burn-in of 100 000 for each run) at each value of K from one to five under the admixture model with the assumption of correlated allele frequencies among populations. Individuals were assigned to clusters based on the highest membership coefficient averaged over the 10 independent runs. The ΔK was calculated based on the rate of change in the log probability of the data between successive K values (Evanno et al. 2005). Replicate runs were grouped based on a symmetric similarity coefficient of >0.9 using the Greedy algorithm in clumpp (Jakobsson & Rosenberg 2007) and visualized in distruct 1.1 (Rosenberg 2004). We chose the optimal value of K based on the PCO analysis and the ΔK analysis of the structure outputs.
The slopes of the latitudinal clines in the admixture proportions (the structure values, logit-transformed) were estimated using a mixed effects model, with slope as a fixed effect and population modelled as a random effect, to allow for genetic drift of each population away from the trend. This analysis was implemented using the lme function in r 2.15.3 (Pinheiro & Bates 2000). Despite logit transformation of the proportions, the residuals were slightly asymmetrical so, as an additional test, the null distribution of slopes was estimated by permuting the latitudes among populations and repeating the analysis, using a custom script in r 2.15.3. Our r scripts are available in the Dryad Digital Repository (Wang et al. 2014).
Population genetic parameters were calculated for the selected 55 populations with at least eight individuals from each population. These include six B. nana populations, 39 B. pubescens populations and 10 B. pendula populations (Table S3, Supporting information). Pairwise FST tests based on allele frequency were conducted for these populations in POLYSAT. A matrix of geographical distance was generated based on latitude and longitude in r package ‘fields’ (Furrer et al. 2011). A Mantel test with 9999 permutations was conducted in r package ‘ade4’ to test for a significant signal of isolation by distance (Dray & Dufour 2007).
Distribution range modelling
To model the potential distribution range of the three Betula species in Britain, all available occurrence records for the three species were organized into a single database from a number of sources (Botanical Society of the British Isles, National Biodiversity Network, Highland Birchwoods and Scottish Natural Heritage), resulting in 48 164 records. The data were filtered to include only complete records with a spatial resolution <1 km and dated post-1950 to remain consistent with available environmental data; this resulted in 11 879 records. Twenty-two bioclimatic variables were considered as possible predictors for Betula species distribution. These included 19 bioclimatic variable layers obtained from WorldClim (http://www.worldclim.org) (Hijmans et al. 2005); elevation data, also obtained from WorldClim; and soil type and peat depth (where >2 m) variables (categorical) obtained from the european soil database version 2 (http://eusoils.jrc.ec.europa.eu). All layers were resampled to 1 km resolution and clipped to include only the British Isles using Environmental Systems Research Institute's arcgis version 10. Modelling was conducted in maxent version 3.3 (Phillips et al. 2004, 2006), a maximum entropy-based machine-learning programme that estimates the probability distribution for species occurrence, based on environmental predictors and presence-only data. We ran maxent under default settings, with 10 subsampled replicated runs, a limit of 5000 iterations and 25% of the data partitioned for testing of the model. maxent was used to calculate the area under the curve (AUC) averaged over the replicate runs, to allow comparison of model performance between the study species. Resulting values range from 0.5 (random) to 1.0 (exact match). The resulting potential species distribution map was then opened and manipulated in arcgis. Thresholds probabilities for species presence are unknown, thus the resulting values ranging from 0 to 0.88 and were arbitrarily regrouped into six classes: 0–0.15, 0.16–0.30, 0.31–0.45, 0.46–0.60, 0.61–0.75 and 0.76–0.90.
Niche overlap between species was measured using Schoener's D (Schoener 1968), and the I statistic (Warren et al. 2008), calculated in enmtools version 1.4.3 (Warren et al. 2010). Similarly, species range overlap was also tested in enmtools version 1.4.3, over a range of manually defined presence probability thresholds to explore the characteristics of the data. We chose a conservative value of 0.45, although we note that the comparative relationships between the three species remain consistent over a broad range.
Pollen record gathering
To build a picture of the past distribution of these species in the UK, we examined pollen records of Betula species in the European Pollen Database (EPD, http://www.europeanpollendatabase.net/data/). For some pollen cores, palaeobotanists have identified pollen type to the species level, whereas, others are identified at the genus level only. We mapped these pollen sites using coordinates given in the EPD. For eight pollen sites, coordinates are not given in the EPD, so we mapped the sites according to the geographical descriptions given in the original literature. The detailed pollen records are listed in Table S2 (Supporting information).
Results
Microsatellite analysis
Broad characterization of genetic diversity among the three Betula species was conducted with principal coordinates (PCO) analysis. The Bruvo's genetic distances of all 1134 individuals were calculated and scaled. The first axis separated B. pendula from a cluster of B. pubescens and B. nana, and the second axis separated B. nana from B. pubescens and B. pendula. Thus, three distinct clusters corresponded to B. nana,B. pubescens and B. pendula (Fig.1). The PCO analysis of these individuals based on pairwise Euclidean distances showed a similar pattern (Fig. S2, Supporting information).
Figure 1.
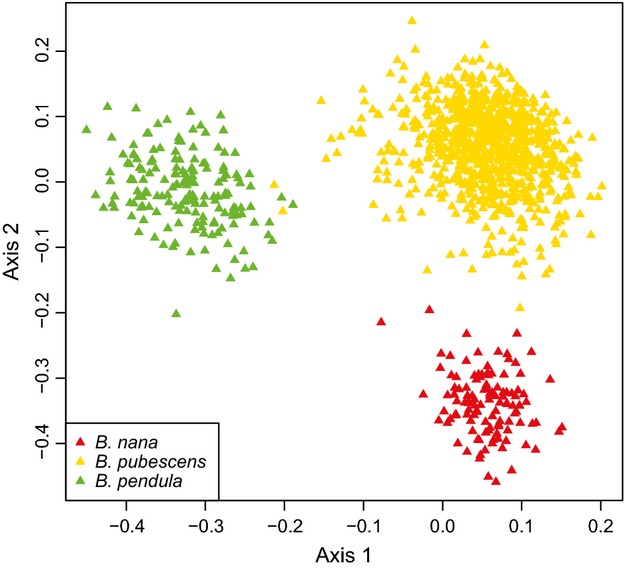
Principal coordinates (PCO) analysis of microsatellite genotypes in B. nana,B. pubescens and B. pendula populations sampled, based on Bruvo's genetic distance.
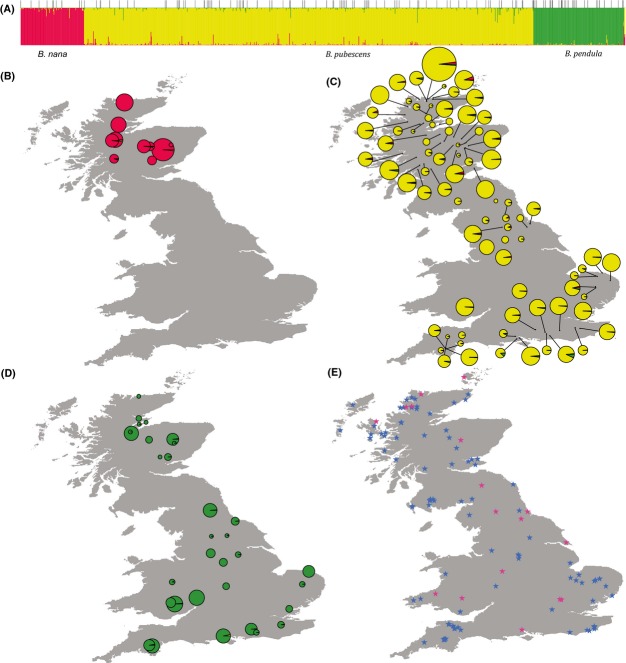
Genetic admixture among species within individuals was examined with Bayesian analysis using structure under the admixture model. Analysis was conducted assuming three populations (K = 3) based on clear clustering in the PCO distribution, corroborated by the ΔK criterion (Fig. S5A, Supporting information). The estimated admixture between B. pendula and B. nana was negligible (Fig.2), but admixture was inferred between B. pubescens and B. nana and also between B. pubescens and B. pendula (Fig.2). Higher levels of admixture from B. nana to B. pubescens were found in the north than in the south of Britain. The cline of B. nana admixture in B. pubescens populations was positively correlated with latitude (Fig.3A, P = 0.0045). Conversely, the cline of B. pendula admixture in B. pubescens was negatively correlated with latitude (Fig.3B, P = 0.0166).
Figure 2.
Genetic admixture among the three native Betula species in Britain, with locations of populations tested, and pollen fossil sites. (A) Sharing of microsatellite alleles among the three species B. nana,B. pubescens and B. pendula shown as a structure plot with K = 3 corresponding with the three species. Within each species grouping, populations are ordered by latitude, with more northerly populations to the left-hand side. Thin vertical lines above the structure plot indicate population divisions. Three known F1 hybrid seedlings are shown on the far right: B. nana × B. pendula,B. nana × B. pubescens and B. nana × B. pubescens, respectively. (B–D) The locations of the sampled populations of B. nana,B. pubescens and B. pendula, respectively: pie charts show the mean proportion of individual genotypes in each population assigned to a particular lineage by structure, and pie chart size is proportional to the sample size for each population. The centre of pie charts represents approximately its sampling locality unless the pie chart is connected to its sampling locality by a straight line. (E) Pollen sites of Betula species across Britain. Red stars represent the pollen sites of B. nana and B. nana, and blue stars represent the pollen sites of Betula likely to be B. pubescens and B. pendula.
Figure 3.
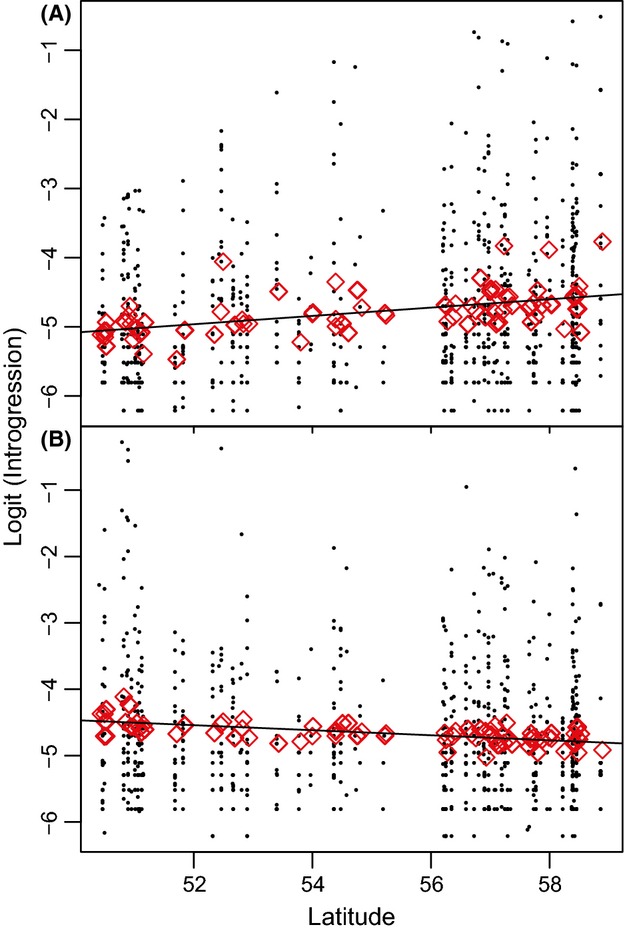
Clines of B. nana and B. pendula admixture in B. pubescens populations. The latitude of each sample populations is shown on the horizontal axis, and logit-transformed structure admixture proportions for each B. pubescens individual are shown as circles. Red diamonds represent the value for each B. pubescens population fitted by the mixed effects model. (A) The cline of B. nana admixture into B. pubescens populations, which showed a significant positive correlation with latitude (P = 0.0045). (B) The cline of B. pendula admixture into B. pubescens populations, which showed a significant negative correlation with latitude (P = 0.0166).
Significant isolation-with-distance was detected among B. nana populations (Fig. S3, Supporting information, Mantel test, r = 0.7035, P = 0.0086) and among B. pubescens populations (Fig. S3, Supporting information, Mantel test, r = 0.1384, P = 0.0093), but not among B. pendula populations (Fig. S3, Supporting information, Mantel test, r = −0.0418, P = 0.5709). Genetic differentiation between B. nana and B. pendula was higher than between B. nana and B. pubescens, and between B. pubescens and B. pendula (Fig. S4, Supporting information). Genetic structure was detected among B. nana populations, but not among either B. pubescens or B. pendula populations when the three species were analysed independently with the admixture model (Fig. S6, Supporting information).
Model-based prediction of past distribution ranges
Ecological niche models constructed with maxent from species occurrence records, performed well for B. nana (AUC = 0.959, SD = 0.018) and were satisfactory for B. pendula (0.723 ± 0.009) and B. pubescens (0.645 ± 0.008). The most important environmental predictors were soil type and annual mean temperature, with the exception of B. nana for which altitude was of primary importance. The results suggest that suitable habitats for B. nana may currently exist in large areas in the Scottish Highlands, SW England, Wales, middle and North England (Fig.4): an area larger than the area currently occupied by B. nana. Suitable habitat for B. pubescens and B. pendula appears widespread in Britain, the most suitable habitat for B. pendula being towards the south and east, and suitable habitat for B. pubescens being widespread. Analysis of pairwise niche overlap revealed considerable similarity between B. pubescens and B. pendula niches (Schoener's D = 0.82, I = 0.97). There was substantially less overlap when comparing B. nana with B. pubescens (D = 0.25, I = 0.58) and B. pendula (D = 0.18, I = 0.48). Range overlap analysis at a conservative occurrence probability threshold (0.45) identified extensive overlap between B. pubescens and B. pendula (73%) and small overlap between B. pubescens and B. nana (5%), but no range overlap between B. nana and B. pendula. Suitable habitats for B. nana either overlap with or are surrounded by suitable habitats for B. pubescens (Fig.4).
Figure 4.
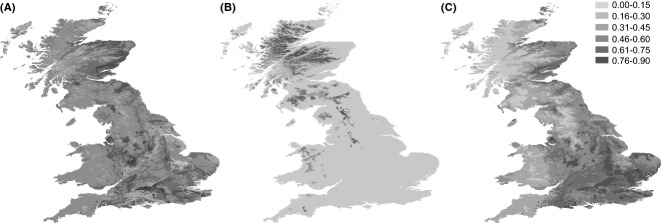
Ecological niche model predicted distribution British ranges for (A) B. pubescens (B) B. nana and (C) B. pendula. At an occurrence probability threshold of 0.45, range overlap is as follows: B. pubescens and B. pendula (73%); B. pubescens and B. nana (5%); B. nana and B. pendula (0%).
Pollen records
Betula ssp. pollen was found recorded for 112 British sites of preserved pollen in the European Pollen Database. The majority most likely represent B. pubescens and B. pendula, which produce abundant pollen, but 13 sites contained pollen identified as B. nana pollen and four contained pollen identified as ‘B. nana’ (Fig.2E; Table S2, Supporting information). These included sites in the south that are outside the current range of B. nana suggesting a much more southerly distribution of B. nana in the past. These pollen records provide us with a longer-term view of the past distribution ranges of B. nana than herbarium collections.
Discussion
Allele sharing among closely related species may occur for a variety of reasons: (A) shared alleles may have been inherited from a polymorphic common ancestor due to incomplete lineage sorting, (B) convergent mutations may have caused the same alleles to have arisen independently in different species, (C) alleles may have moved from one species to another via introgressive hybridization within a framework of stable species' ranges, perhaps assisted by selection, (D) alleles may have moved via introgressive hybridization where neutral gene flow has been increased by the retreat of the range of one species and the concomitant expansion of the range of the species with which it can hybridize. We consider that in the present study, the balance of evidence points towards (D).
Although incomplete lineage sorting (A) may be frequent among tree species due to their large effective population sizes and long generation times (Bouillé & Bousquet 2005; Chen et al. 2010), this seems an unlikely explanation for the patterns of allele sharing observed between B. nana and B. pubescens, because we find a gradient of B. nana alleles that increases closer to the current location of B. nana populations in the north. If the loci in the study were neutral with respect to selection, which is expected of microsatellite alleles, then incomplete lineage sorting would not be expected to give a geographic signal (Barton 2001).
Convergent mutations (B) also seem an improbable explanation. Convergence is intrinsically unlikely at neutral loci, and if it did occur, it would be expected to yield symmetric allele sharing between B. pubescens and B. nana. However, we observe a pattern of asymmetric allele sharing between B. pubescens and B. nana (Fig.2).
The pattern we observe is therefore likely to be caused by hybridization. Betula nana and B. pubescens currently have parapatric distributions and often occur close together in natural environments. Several putative hybrids have been noted by taxonomists in Scotland (Kenworthy et al. 1972), and extensive hybridization and gene flow have been shown to occur between the two species in Iceland (Anamthawat-Jónsson & Thórsson 2003; Maliouchenko et al. 2007), Scandinavia and Russia (Maliouchenko et al. 2007). However, the pattern of introgression that we observe is unlikely to have been caused (C) simply by spread of alleles from the current distribution range of B. nana. High genetic differentiation and significant isolation-with-distance (Fig. S3, Supporting information) among B. nana populations suggest that B. nana has a low capacity for gene flow, as is to be expected for a dwarf tree producing small amounts of pollen and seed compared to its larger tree relatives (Bradshaw 1981). Also, because microsatellites markers are expected to be neutral to selection, the presence of B. nana alleles in occasional B. pubescens populations far from the present range of B. nana in the middle of Britain is unlikely to have been caused by natural selection.
The observed level of introgression from B. nana to B. pubescens is not less than the level of introgression we observe from B. pendula to B. pubescens (Student's t-test, t = 0.082, P = 0.934). This is surprising given that B. pendula is a tree that disperses more pollen than B. nana and frequently occurs in sympatry with B. pubescens in much of its British range (Atkinson 1992). Given that B. nana and B. pendula are diploid with the same chromosome number, they are unlikely to differ in chromosomal postzygotic reproductive isolation with tetraploid B. pubescens. Hybrids between B. pendula and B. pubescens have been recorded in the UK (Brown et al. 1982), and a study of chloroplast introgression in Scandinavia and western Russia found higher rates of introgression between B. pendula and B. pubescens than between B. nana and B. pubescens (Palmé et al. 2004). The fact, therefore, that we find similar introgression from B. nana to B. pubescens and from B. pendula to B. pubescens requires an explanation.
The most likely explanation of the pattern observed in this study is (D) that we are seeing a trail of introgression resulting from past retreat of the range of B. nana accompanied by the northwards expansion of the range of B. pubescens. This could explain the high level of introgression found relative to B. pendula–B. pubescens introgression and the geographic pattern of introgression observed. This hypothesis fits with the fact that fossils of B. nana and B. nana pollen are distributed across Britain (Fig.2E) showing a larger and more southerly range in the past. Both genetic and fossil evidence therefore point to the northwards movement of the range of B. pubescens in the UK, at the expense of B. nana, with some hybridization occurring between them during this expansion/retreat, leaving a molecular footprint.
What caused this expansion of B. pubescens at the expense of B. nana? The fact that B. nana pollen is found outside the current environmental niche range of B. nana suggests that past climate change has played a major role in the species' decline, specifically climate warming in the Holocene after the last glacial maximum. But the fact that B. nana is currently more restricted in its range than the area that it is adapted to according to the ENM suggests that other factors may also have contributed to the decline of B. nana, such as overgrazing by sheep and deer (Tanentzap et al. 2013), and burning of moorland for grouse shooting (DeGroot et al. 1997). Our study suggests a further contributing factor may be pollen swamping of B. nana by B. pubescens, reducing the production of fertile B. nana offspring in B. nana populations. The low levels of introgression found in this study support the pollen-swamping hypothesis: due to the ploidy difference between B. nana and B. pubescens, we expect most hybrids to be sterile, so only a minority of hybrids formed will be capable of contributing to introgression between the two species. Therefore, the small amount of introgression we observe between B. nana and B. pubescens suggests that large numbers of hybrids have been formed, as has been found in Icelandic populations of B. nana and B. pubscens where up to 10% of trees may be hybrids (Anamthawat-Jónsson & Tómasson 1999; Anamthawat-Jónsson & Thórsson 2003). Furthermore, the asymmetric pattern of gene flow that we observe suggests that on the rare occasions when hybrids are capable of backcrossing, they do so mainly with B. pubescens, rather than B. nana. This, and the fact that B. pubescens is a tree with far greater pollen dispersal ability than B. nana, suggests that B. nana ovules may be frequently fertilized by B. pubescens pollen. Thus, reproduction of B. nana may be reduced by the production of (mainly sterile and nonbackcrossing) hybrids with B. pubescens. Such a dynamic has been shown to occur in a hybrid zone between diploid and hexaploid Mercurialis annua, where the hexaploid form is apparently being eliminated by the diploid form due to pollen swamping and the production of sterile hybrids (Buggs & Pannell 2006). Even when hybrids are not mainly sterile, pollen swamping can still contribute to the advance of one species' range at the expense of another, for example, pollen swamping of Quercus robur by Q. petraea seems to assist the latter in invading the range of the former (Petit et al. 2004).
We find very little introgression between B. nana and B. pendula, despite the fact that a reproductive barrier due to ploidy does not separate them. While we do not know whether other reproductive barriers separate them, we have found diploid hybrids when growing up seeds collected from B. nana populations in Scotland, in an area recently planted with B. pendula in afforestation, suggesting that B. nana—B. pendula hybrids do form in Scotland. The most probably explanation for the lack of introgression between the two species in our study is the disjunct nature of their natural distributions: the environmental niches of the two rarely overlap (Fig.4). Betula nana is adapted to cold and wet habitats (DeGroot et al. 1997), whereas B. pendula prefers warm and dry habitats (Gimingham 1984). Betula nana commonly grows above the treeline, whereas B. pendula grows in regions with low altitude usually below a few hundred metres (Gimingham 1984). A 6-year study in Sweden showed the germination rates of B. pendula seeds to decrease strongly with altitude (Holm 1994). Maintenance of the geographical separation between B. nana and B. pendula may be a key to preventing future hybridization between them.
We conclude that a balance of evidence from both genetic data and fossils suggests that a zone of hybridization between B. nana and B. pubescens moved northwards through the UK since the last glacial maximum, leaving behind a footprint of introgressed genes in the genome of B. pubescens. Though likely to have been mainly driven by climate change, the decline of B. nana may have been exacerbated by hybridization with B. pubescens. Today, B. nana is nationally scarce in Britain and under active conservation management. Successful conservation of B. nana may partly depend on minimization of future gene flow from B. pubescens. However, a bigger threat may be hybridization with B. pendula; although there appears to have been little hybridization between B. nana and B. pendula in the past, this may be due to ecological separation rather than reproductive incompatibility (Wilsey et al. 1998), and planting of B. pendula saplings in areas where B. pendula could not establish from seeds could cause a new anthropogenic threat to the reproduction of B. nana.
Acknowledgments
This work was funded by NERC Fellowship NE/G01504X/1 to Richard Buggs, and NERC CASE studentship NE/J017388/1 in collaboration with Trees for Life and Highland Birchwoods. Nian Wang is funded by the Chinese Scholarship Council. We thank landowners for birch twigs, and Jasmin Zohren, Hao-Chih Kuo and Xiu-Guang Mao for helpful discussions.
N.W. and R.J.A.B. designed research; N.W., J.S.B., A.K., W.J.A.B. and R.J.A.B. performed research; N.W., J.S.B. and R.A.N. analysed data; and N.W., J.S.B and R.J.A.B. wrote the paper.
Data accessibility
Microsatellite data and R scripts used in data analyses are available in the Dryad Digital Repository, doi:10.5061/dryad.mt5sj. Herbarium sheets of specimens will be deposited in the Natural History Museum Herbarium, London. The species occurrence records data used in the distribution range modelling were from: Botanical Society of the British Isles (http://www.bsbi.org.uk), National Biodiversity Network (https://data.nbn.org.uk/), Highland Birchwoods (http://www.highlandbirchwoods.co.uk) and Scottish Natural Heritage (http://www.snh.gov.uk). The bioclimatic and elevation data used in the distribution range modelling are available from WorldClim (http://www.worldclim.org) and the soil data from the European Soil Database version 2 (http://eusoils.jrc.ec.europa.eu). The pollen records used from the European Pollen Database (http://www.europeanpollendatabase.net) and published studies are shown in Table S2 (Supporting information). The population sample site locations from this study are listed in Table S3 (Supporting information).
Supporting Information
Additional supporting information may be found in the online version of this article.
Summary of the 16 polymorphic microsatellite loci multiplexed for all samples.
Table S2 Summary of published pollen records of Betula species in the UK.
Table S3 Genetic diversity of 55 populations with over seven samples of the three Betula species, based on microsatellites.
Fig. S1 Pairs of leaves from a representative sample of trees used in the present study, showing upper and lower sides.
Fig. S2PCO analysis based on Euclidean distance.
Fig. S3 Pairwise genetic differentiation (FST) within species.
Fig. S4 Pairwise genetic differentiation (FST) between B. nana,B. pubescens and B. pendula.
Fig. S5 (A) The log-likelihood values of each K and ΔK values. (B) The structure output at K = 2, 3 and 4.
Fig. S6 The structure output of B. nana,B. pubescens and B. pendula separately, at K = 2, 3 and 4, based on the admixture model.
References
- Anamthawat-Jónsson K, Thórsson AT. Natural hybridisation in birch: triploid hybrids between Betula nana and B. pubescens. Plant Cell Tissue and Organ Culture. 2003;75:99–107. [Google Scholar]
- Anamthawat-Jónsson K, Tómasson T. High frequency of triploid birch hybrid by Betula nana seed parent. Hereditas. 1999;130:191–193. [Google Scholar]
- Anamthawat-Jónsson K, Thórsson AT, Temsch EM, Greilhuber J. Icelandic birch polyploids-the case of perfect fit in genome size. Journal of Botany. 2010;2010:347254. [Google Scholar]
- Aston D. Betula nana L., a note on its status in the United Kingdom. Proceedings of the Royal Society of Edinburgh Section B-Biological Sciences. 1984;85:43–47. [Google Scholar]
- Atkinson MD. Betula pendula Roth (B. verrucosa Ehrh) and B. pubescens Ehrh. Journal of Ecology. 1992;80:837–870. [Google Scholar]
- Atkinson MD, Codling AN. A reliable method for distinguishing between Betula pendula and B. pubescens. Watsonia. 1986;7:5–76. [Google Scholar]
- Avise JC. Phylogeography: The History and Formation of Species. Cambridge, Massachusetts: Harvard University Press; 2000. [Google Scholar]
- Barton NH. The role of hybridisation in evolution. Molecular Ecology. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. [DOI] [PubMed] [Google Scholar]
- Blackburn KB. The dating of a deposit containing an elk skeleton found at Neasham, near Darlington, County Durham. New Phytologist. 1952;51:364. [Google Scholar]
- Bouillé M, Bousquet J. Trans-species shared polymorphisms at orthologous nuclear gene loci among distant species in the conifer Picea (Pinaceae): implications for the long-term maintenance of genetic diversity in trees. American Journal of Botany. 2005;92:63–73. doi: 10.3732/ajb.92.1.63. [DOI] [PubMed] [Google Scholar]
- Bradshaw RHW. Modern pollen-representation factors for woods in south-east England. Journal of Ecology. 1981;69:45–70. [Google Scholar]
- Brown IR, Aldawoody D. Observations on meiosis in three cytotypes of Betula alba L. New Phytologist. 1979;83:801–811. [Google Scholar]
- Brown IR, Tuley G. A study of a population of birches in Glen Gairn. Botanical Journal of Scotland. 1971;41:231–245. [Google Scholar]
- Brown IR, Williams DA. Cytology of Betula alba L. complex. Proceedings of the Royal Society of Edinburgh Section B-Biological Sciences. 1984;85:49–64. [Google Scholar]
- Brown IR, Kennedy D, Williams DA. The occurrence of natural hybrids between Betula pendula Roth and B. pubescens Ehrh. Watsonia. 1982;14:133–145. [Google Scholar]
- Bruvo R, Michiels NK, D'Souza TG, Schulenburg H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Molecular Ecology. 2004;13:2101–2106. doi: 10.1111/j.1365-294X.2004.02209.x. [DOI] [PubMed] [Google Scholar]
- Buggs RJA. Empirical study of hybrid zone movement. Heredity. 2007;99:301–312. doi: 10.1038/sj.hdy.6800997. [DOI] [PubMed] [Google Scholar]
- Buggs RJA, Pannell JR. Rapid displacement of a monoecious plant lineage is due to pollen swamping by a dioecious relative. Current Biology. 2006;16:996–1000. doi: 10.1016/j.cub.2006.03.093. [DOI] [PubMed] [Google Scholar]
- Caseldine C. Changes in Betula in the Holocene record from Iceland—a palaeoclimatic record or evidence for early Holocene hybridisation? Review of Palaeobotany and Palynology. 2001;117:139–152. [Google Scholar]
- Chen J, Kallman T, Gyllenstrand N, Lascoux M. New insights on the speciation history and nucleotide diversity of three boreal spruce species and a Tertiary relict. Heredity. 2010;104:3–14. doi: 10.1038/hdy.2009.88. [DOI] [PubMed] [Google Scholar]
- Chen IC, Hill JK, Ohlemuller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- Clark LV, Jasieniuk M. POLYSAT: an R package for polyploid microsatellite analysis. Molecular Ecology Resources. 2011;11:562–566. doi: 10.1111/j.1755-0998.2011.02985.x. [DOI] [PubMed] [Google Scholar]
- Crawford RMM. Plants at the Margin: Ecological Limits and Climate Change. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- Currat M, Excoffier L. Strong reproductive isolation between humans and Neanderthals inferred from observed patterns of introgression. Proceedings of the National cademy of Sciences of the United States of America. 2011;108:15129–15134. doi: 10.1073/pnas.1107450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currat M, Ruedi M, Petit RJ, Excoffier L. The hidden side of invasions: massive introgression by local genes. Evolution. 2008;62:1908–1920. doi: 10.1111/j.1558-5646.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- Davis MB, Shaw RG. Range shifts and adaptive responses to Quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- DeGroot WJ, Thomas PA, Wein RW. Betula nana L. and Betula glandulosa Michx. Journal of Ecology. 1997;85:241–264. [Google Scholar]
- Dray S, Dufour AB. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software. 2007;22:1–20. [Google Scholar]
- Durand EY, Patterson N, Reich D, Slatkin M. Testing for ancient admixture between closely related populations. Molecular Biology and Evolution. 2011;28:2239–2252. doi: 10.1093/molbev/msr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington TT. Introgressive hybridization between Betula nana L. and B. pubescens Ehrh. in North-West Iceland. New Phytologist. 1968;67:109–118. [Google Scholar]
- Eriksson A, Manica A. Effect of ancient population structure on the degree of polymorphism shared between modern human populations and ancient hominins. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13956–13960. doi: 10.1073/pnas.1200567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Furrer R, Nychka D, Sain S. Fields: Tools for Spatial Data. 2011. Available from http://cran.r-project.org/package=fields. [Google Scholar]
- Gill JA, Davy AJ. Variation and polyploidy within lowland populations of the Betula pendulaBetula pubescens complex. New Phytologist. 1983;94:433–451. [Google Scholar]
- Gimingham CH. Ecological aspects of birch. Proceedings of the Royal Society of Edinburgh Section B-Biological Sciences. 1984;85B:65–72. [Google Scholar]
- Green RE, Krause J, Briggs AW, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Vol. 4. Paleontologia Electronica; 2001. pp. 1–9. Available from http://palaeo-electronica.org/2001_1/past/issue1_01.htm. [Google Scholar]
- Hewitt GM. Post-glacial re-colonization of European biota. Biological Journal of the Linnean Society. 1999;68:87–112. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Himes CMT, Gallardo MH, Kenagy GJ. Historical biogeography and post-glacial recolonization of South American temperate rain forest by the relictual marsupial Dromiciops gliroides. Journal of Biogeography. 2008;35:1415–1424. [Google Scholar]
- Holm SO. Reproductive patterns of Betula pendula and B. pubescens Coll along a regional altitudinal gradient in Northern Sweden. Ecography. 1994;17:60–72. [Google Scholar]
- Huntley B, Birks HJB. An Atlas of Past and Present Pollen Maps for Europe: 0-13000 Years Ago. Cambridge, UK: Cambridge University Press; 1983. [Google Scholar]
- Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Kenworthy JB, Aston D, Bucknall SA. A study of hybrids between Betula pubescens Ehrh. and Betula nana L. from Sutherland—an integrated approach. Transactions of the Botanical Society of Edinburgh. 1972;41:517–539. [Google Scholar]
- Kloda JM, Dean PDG, Maddren C, MacDonald DW, Mayes S. Using principle component analysis to compare genetic diversity across polyploidy levels within plant complexes: an example from British Restharrows (Ononis spinosa and Ononis repens) Heredity. 2008;100:253–260. doi: 10.1038/sj.hdy.6801044. [DOI] [PubMed] [Google Scholar]
- Kulju KKM, Pekkinen M, Varvio S. Twenty-three microsatellite primer pairs for Betula pendula (Betulaceae) Molecular Ecology Notes. 2004;4:471–473. [Google Scholar]
- Linnaeus C. Species Plantarum. Stockholm: Laurentius Salvius; 1753. [Google Scholar]
- Maliouchenko O, Palmé AE, Buonamici A, Vendramin GG, Lascoux M. Comparative phylogeography and population structure of European Betula species, with particular focus on B. pendula and B. pubescens. Journal of Biogeography. 2007;34:1601–1610. [Google Scholar]
- Mellersh C, Sampson J. Simplifying detection of microsatellite length polymorphisms. BioTechniques. 1993;15:582–584. [PubMed] [Google Scholar]
- Nagamitsu T, Kawahara T, Kanazashi A. Endemic dwarf birch Betula apoiensis (Betulaceae) is a hybrid that originated from Betula ermanii and Betula ovalifolia. Plant Species Biology. 2006;21:19–29. [Google Scholar]
- Palmé AE, Su Q, Pálsson S, Lascoux M. Extensive sharing of chloroplast haplotypes among European birches indicates hybridization among Betula pendulaB. pubescens and B. nana. Molecular Ecology. 2004;13:167–178. doi: 10.1046/j.1365-294x.2003.02034.x. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Pineau E, Demesure B, et al. Chloroplast DNA footprints of postglacial recolonization by oaks. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9996–10001. doi: 10.1073/pnas.94.18.9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit RJ, Aguinagalde I, de Beaulieu JL, et al. Glacial refugia: hotspots but not melting pots of genetic diversity. Science. 2003;300:1563–1565. doi: 10.1126/science.1083264. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Bodénès C, Ducousso A, Roussel G, Kremer A. Hybridization as a mechanism of invasion in oaks. New Phytologist. 2004;161:151–164. [Google Scholar]
- Phillips SJ, Dudík M, Schapire RE. A maximum entropy approach to species distribution modeling. ACM International Proceedings Series. 2004;69:655. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. [Google Scholar]
- Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ( http://www.R-project.org ) [Google Scholar]
- Rich TCG, Jermy AC. Plant Crib. London: BSBI; 1998. [Google Scholar]
- Rosenberg NA. DISTRUCT: a program for the graphical display of population structure. Molecular Ecology Notes. 2004;4:137–138. [Google Scholar]
- Sankararaman S, Patterson N, Li H, Paabo S, Reich D. The date of Interbreeding between Neandertals and modern humans. PloS Genetics. 2012;8:e1002947. doi: 10.1371/journal.pgen.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoener TW. The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology. 1968;49:704–726. [Google Scholar]
- Scriber JM. Impacts of climate warming on hybrid zone movement: geographically diffuse and biologically porous “species borders”. Insect Science. 2011;18:121–159. [Google Scholar]
- Stace CA. New Flora of the British Isles. 3rd edn. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- Stebbins GL. Chromosomal Evolution in Higher Plants. London: Edward Arnold; 1971. [Google Scholar]
- Tanentzap AJ, Zou J, Coomes DA. Getting the biggest birch for the bang: restoring and expanding upland birchwoods in the Scottish Highlands by managing red deer. Ecology and Evolution. 2013;3:1890–1901. doi: 10.1002/ece3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thórsson TH, Salmela E, Anamthawat-Jónsson K. Morphological, cytogenetic, and molecular evidence for introgressive hybridization in birch. Journal of Heredity. 2001;92:404–408. doi: 10.1093/jhered/92.5.404. [DOI] [PubMed] [Google Scholar]
- Thórsson TH, Pálsson S, Sigurgeirsson A, Anamthawat-Jónsson K. Morphological variation among Betula nana (diploid), B. pubescens (tetraploid) and their triploid hybrids in Iceland. Annals of Botany. 2007;99:1183–1193. doi: 10.1093/aob/mcm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thórsson TH, Pálsson S, Lascoux M, Anamthawat-Jónsson K. Introgression and phylogeography of Betula nana (diploid), B. pubescens (tetraploid) and their triploid hybrids in Iceland inferred from cpDNA haplotype variation. Journal of Biogeography. 2010;37:2098–2110. [Google Scholar]
- Truong C, Palmé AE, Felber F, Naciri-Graven Y. Isolation and characterization of microsatellite markers in the tetraploid birch, Betula pubescens ssp. tortuosa. Molecular Ecology Notes. 2005;5:96–98. [Google Scholar]
- Wall JD, Yang MA, Jay F, et al. Higher levels of Neanderthal ancestry in East Asians than in Europeans. Genetics. 2013;194:199–209. doi: 10.1534/genetics.112.148213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Thomson M, Bodles WJA, et al. Genome sequence of dwarf birch (Betula nana) and cross-species RAD markers. Molecular Ecology. 2013;22:3098–3111. doi: 10.1111/mec.12131. [DOI] [PubMed] [Google Scholar]
- Wang N, Borrell J, Bodles W, Kuttapitiya A, Nicholes RA, Buggs RJA. Data from: molecular footprints of the Holocene retreat of dwarf birch in Britain. Dryad Digital Repository. 2014 doi: 10.1111/mec.12768. doi:10.5061/dryad.mt5sj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution. 2008;62:2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- Warren DL, Glor RE, Turelli M. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography. 2010;33:607–611. [Google Scholar]
- Wilsey BJ, Haukioja E, Koricheva J, Sulkinoja M. Leaf fluctuating asymmetry increases with hybridization and elevation in tree-line birches. Ecology. 1998;79:2092–2099. [Google Scholar]
- Yang MA, Malaspinas AS, Durand EY, Slatkin M. Ancient structure in Africa unlikely to explain Neanderthal and non-African genetic similarity. Molecular Biology and Evolution. 2012;29:2987–2995. doi: 10.1093/molbev/mss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Woodall CW, Clark JS. Failure to migrate: lack of tree range expansion in response to climate change. Global Change Biology. 2012;18:1042–1052. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the 16 polymorphic microsatellite loci multiplexed for all samples.
Table S2 Summary of published pollen records of Betula species in the UK.
Table S3 Genetic diversity of 55 populations with over seven samples of the three Betula species, based on microsatellites.
Fig. S1 Pairs of leaves from a representative sample of trees used in the present study, showing upper and lower sides.
Fig. S2PCO analysis based on Euclidean distance.
Fig. S3 Pairwise genetic differentiation (FST) within species.
Fig. S4 Pairwise genetic differentiation (FST) between B. nana,B. pubescens and B. pendula.
Fig. S5 (A) The log-likelihood values of each K and ΔK values. (B) The structure output at K = 2, 3 and 4.
Fig. S6 The structure output of B. nana,B. pubescens and B. pendula separately, at K = 2, 3 and 4, based on the admixture model.
Data Availability Statement
Microsatellite data and R scripts used in data analyses are available in the Dryad Digital Repository, doi:10.5061/dryad.mt5sj. Herbarium sheets of specimens will be deposited in the Natural History Museum Herbarium, London. The species occurrence records data used in the distribution range modelling were from: Botanical Society of the British Isles (http://www.bsbi.org.uk), National Biodiversity Network (https://data.nbn.org.uk/), Highland Birchwoods (http://www.highlandbirchwoods.co.uk) and Scottish Natural Heritage (http://www.snh.gov.uk). The bioclimatic and elevation data used in the distribution range modelling are available from WorldClim (http://www.worldclim.org) and the soil data from the European Soil Database version 2 (http://eusoils.jrc.ec.europa.eu). The pollen records used from the European Pollen Database (http://www.europeanpollendatabase.net) and published studies are shown in Table S2 (Supporting information). The population sample site locations from this study are listed in Table S3 (Supporting information).