Abstract
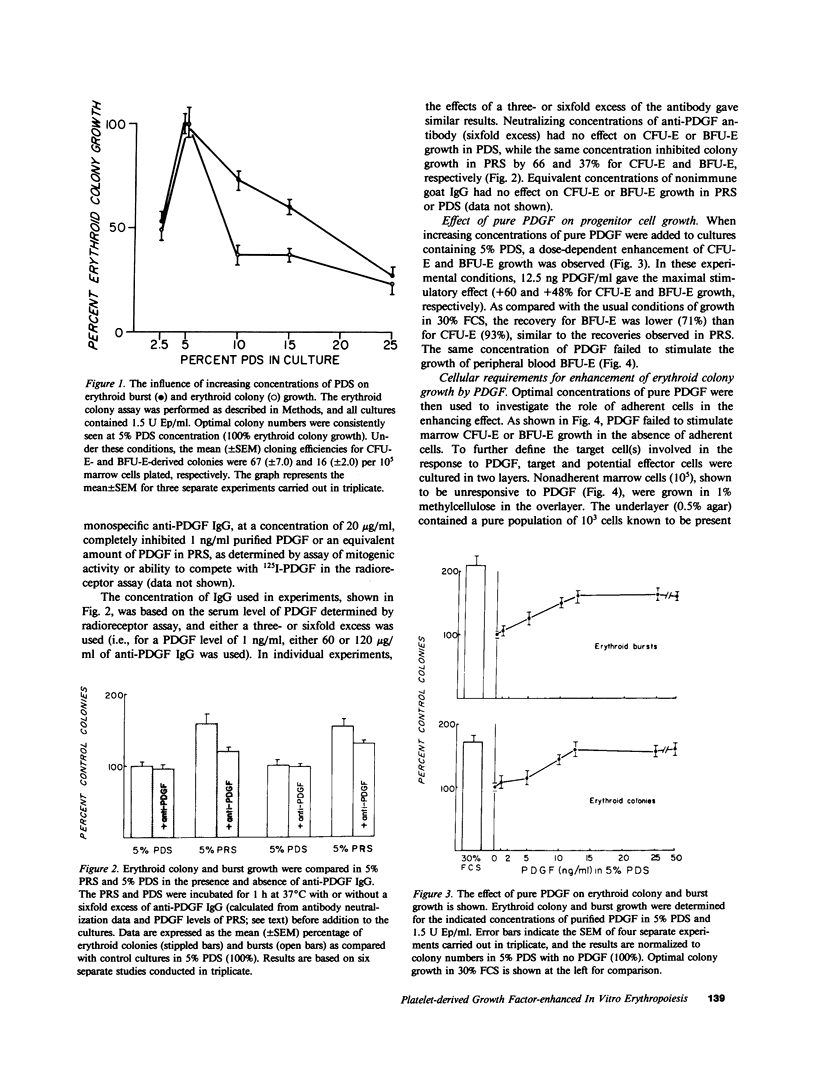
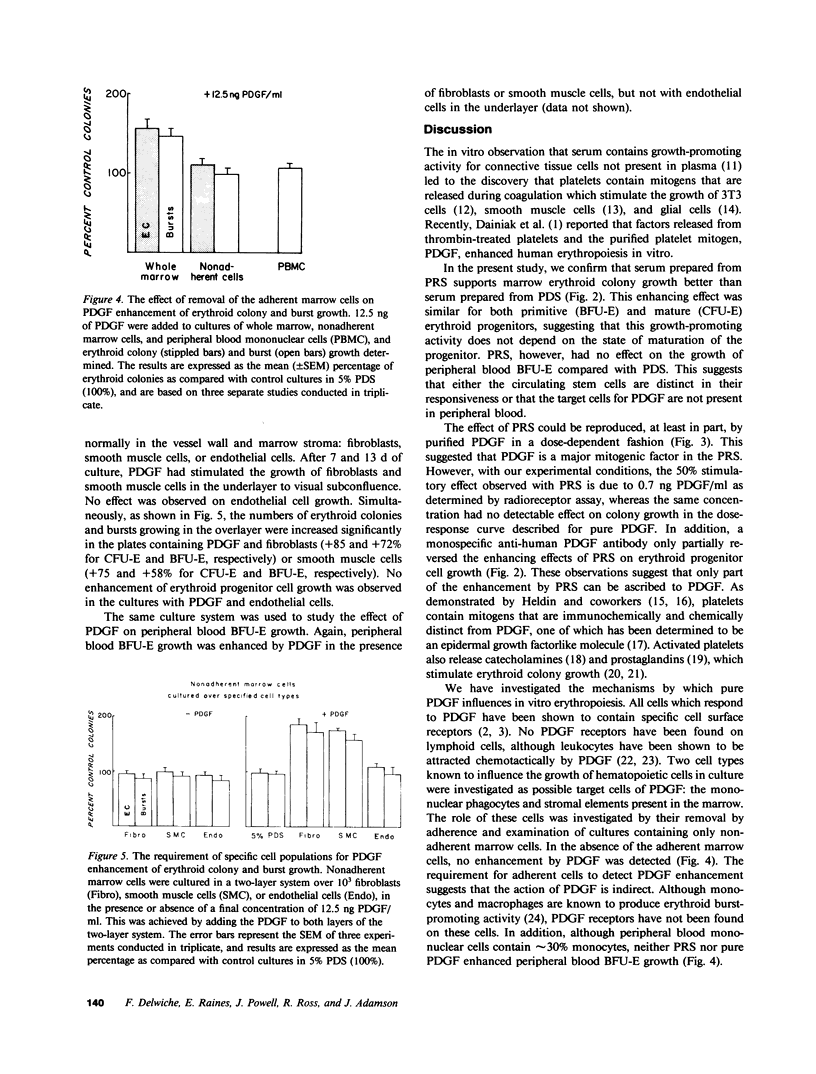
The growth of erythroid colonies (from erythroid colony-forming cells) and erythroid bursts (from burst-forming cells [BFU-E]) is enhanced in the presence of serum as compared with plasma. A significant proportion of the enhanced growth is due to the platelet release product, platelet-derived growth factor (PDGF). Colony growth in cultures of whole marrow cells in platelet-poor plasma-derived serum (PDS) and erythropoietin was enhanced in a dose-dependent fashion by increasing concentrations of purified human PDGF with optimal enhancement at 12.5 ng/ml. However, no effect of platelet-release products or PDGF was observed on nonadherent human marrow cells or peripheral blood BFU-E, suggesting that an accessory cell population was required for the effect of PDGF on hematopoietic progenitors. In a two-layer culture system, pure populations of fibroblasts or smooth muscle cells, known to be present in the marrow microenvironment, restored the response of nonadherent marrow cells in the overlayer to PDGF and also conferred responsiveness to peripheral blood BFU-E. Endothelial cells in the two-layer culture system and macrophages, in contrast, lacked the ability to restore the enhancing effect of PDGF. Because other platelet-release mitogenic products are also found in serum, a monospecific anti-PDGF IgG preparation was added to cultures grown in platelet rich plasma-derived serum. Only partial reduction in colony and burst growth was seen, suggesting that other platelet-release products were acting in this system. These results demonstrate that PDGF enhancement of human hematopoietic progenitor cell growth requires mesenchymal cells, and provide an example and mechanism by which growth factors may influence hematopoietic progenitors via cells of the marrow microenvironment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Adamson J. W. Modulation of in vitro erythropoiesis. The influence of beta-adrenergic agonists on erythroid colony formation. J Clin Invest. 1977 Jul;60(1):70–77. doi: 10.1172/JCI108771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio R., Bianchi G., Löwenberg B., Dicke K. A., Ajmar F. Effects of fibroblasts on the growth of erythroid progenitor cells in vitro. Exp Hematol. 1977 Sep;5(5):341–347. [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Partial purification and characterization of platelet factors stimulating the multiplication of normal human glial cells. Exp Cell Res. 1977 Oct 15;109(2):429–437. doi: 10.1016/0014-4827(77)90023-4. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Chemical and biological properties of a growth factor from human-cultured osteosarcoma cells: resemblance with platelet-derived growth factor. J Cell Physiol. 1980 Nov;105(2):235–246. doi: 10.1002/jcp.1041050207. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Demonstration of an antibody against platelet-derived growth factor. Exp Cell Res. 1981 Dec;136(2):255–261. doi: 10.1016/0014-4827(81)90003-3. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Messner H. A., McCulloch E. A. Interacting cell populations affecting granulopoietic colony formation by normal and leukemic human marrow cells. Blood. 1973 Nov;42(5):701–710. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. J., Pantazis P., Antoniades H. N. Simian sarcoma virus--transformed cells secrete a mitogen identical to platelet-derived growth factor. Science. 1984 Jul 6;225(4657):54–56. doi: 10.1126/science.6328659. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G. B., Migliaccio A. R., Migliaccio G., Lettieri F., Di Rosa M., Peschle C., Mastroberardino G. In vitro interactions of PGE and cAMP with murine and human erythroid precursors. Blood. 1980 Jul;56(1):74–79. [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Formation of prostaglandins during the aggregation of human blood platelets. J Clin Invest. 1973 Apr;52(4):965–969. doi: 10.1172/JCI107262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Quadracci L. J., Striker G. E. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol. 1978 Aug;96(2):203–213. doi: 10.1002/jcp.1040960209. [DOI] [PubMed] [Google Scholar]
- Westermark B., Wasteson A. A platelet factor stimulating human normal glial cells. Exp Cell Res. 1976 Mar 1;98(1):170–174. doi: 10.1016/0014-4827(76)90476-6. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Antoniades H. N., Goetzl E. J. Platelet-derived growth factor stimulates mouse 3T3 cell mitogenesis and leukocyte chemotaxis through different structural determinants. J Clin Invest. 1983 Nov;72(5):1759–1763. doi: 10.1172/JCI111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S. Human erythroid burst-forming units. Growth in vitro is dependent on monocytes, but not T lymphocytes. J Clin Invest. 1981 Mar;67(3):702–709. doi: 10.1172/JCI110086. [DOI] [PMC free article] [PubMed] [Google Scholar]