Abstract
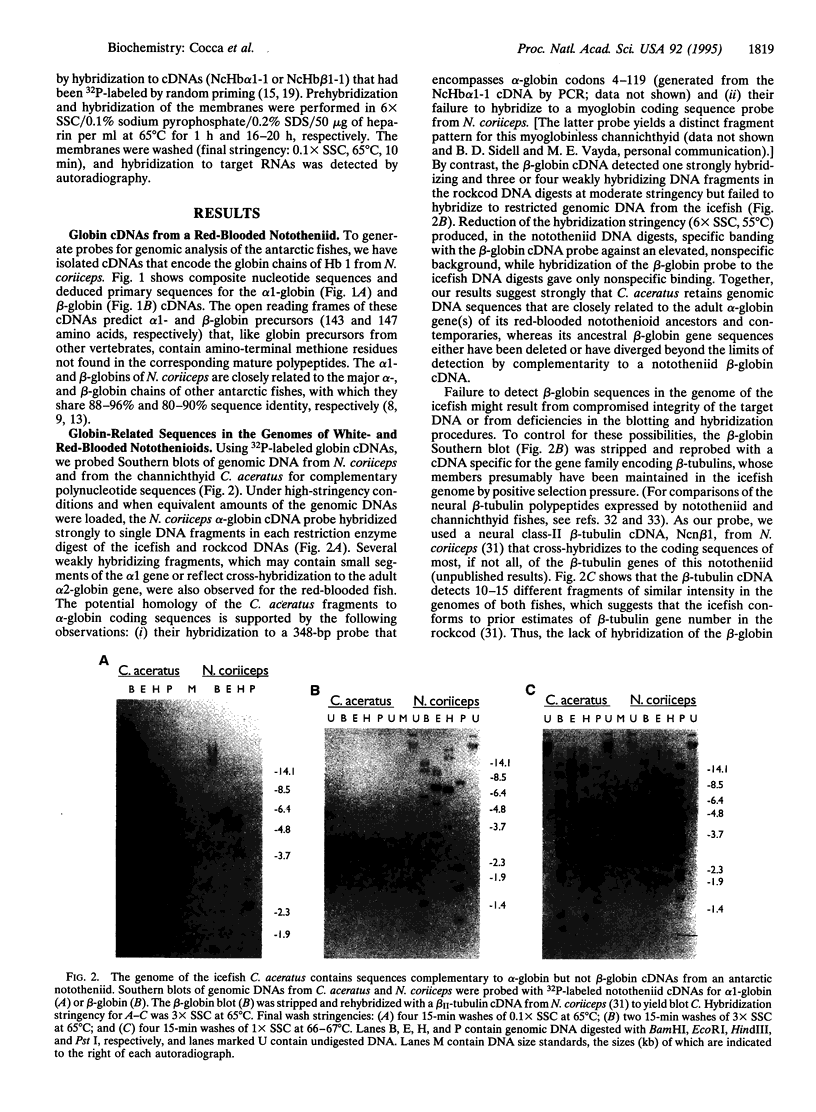
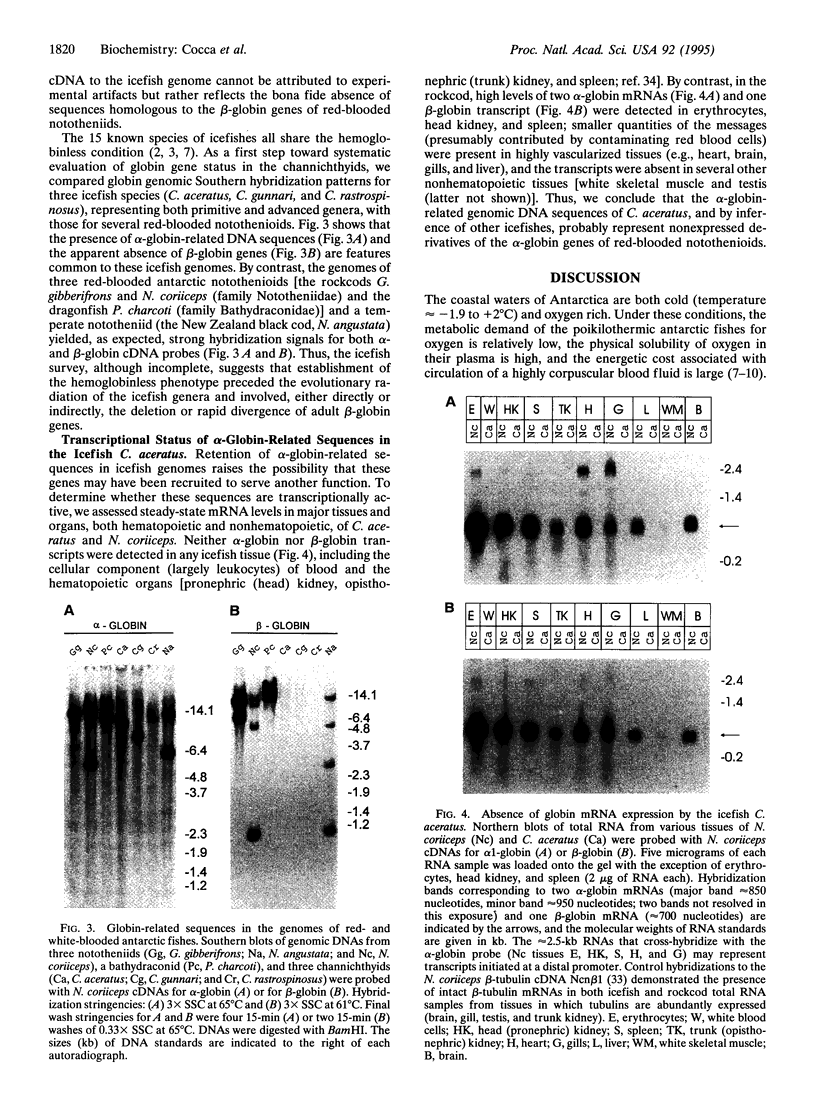
Alone among piscine taxa, the antarctic icefishes (family Channichthyidae, suborder Notothenioidei) have evolved compensatory adaptations that maintain normal metabolic functions in the absence of erythrocytes and the respiratory oxygen transporter hemoglobin. Although the uniquely "colorless" or "white" condition of the blood of icefishes has been recognized since the early 20th century, the status of globin genes in the icefish genomes has, surprisingly, remained unexplored. Using alpha- and beta-globin cDNAs from the antarctic rockcod Notothenia coriiceps (family Nototheniidae, suborder Notothenioidei), we have probed the genomes of three white-blooded icefishes and four red-blooded notothenioid relatives (three antarctic, one temperate) for globin-related DNA sequences. We detect specific, high-stringency hybridization of the alpha-globin probe to genomic DNAs of both white- and red-blooded species, whereas the beta-globin cDNA hybridizes only to the genomes of the red-blooded fishes. Our results suggest that icefishes retain inactive genomic remnants of alpha-globin genes but have lost, either through deletion or through rapid mutation, the gene that encodes beta-globin. We propose that the hemoglobinless phenotype of extant icefishes is the result of deletion of the single adult beta-globin locus prior to the diversification of the clade.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Avino R., Caruso C., Romano M., Camardella L., Rutigliano B., Di Prisco G. Hemoglobin from the Antarctic fish Notothenia coriiceps neglecta. 2. Amino acid sequence of the alpha chain of Hb1. Eur J Biochem. 1989 Feb 15;179(3):707–713. doi: 10.1111/j.1432-1033.1989.tb14604.x. [DOI] [PubMed] [Google Scholar]
- D'Avino R., Caruso C., Schininà M. E., Rutigliano B., Romano M., Camardella L., Bossa F., Barra D., di Prisco G. The amino acid sequence of the alpha- and beta-chains of the two hemoglobins of the Antarctic fish Notothenia coriiceps neglecta. FEBS Lett. 1989 Jun 19;250(1):53–56. doi: 10.1016/0014-5793(89)80683-0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Overton S. A. Heterogeneity and structure of brain tubulins from cold-adapted Antarctic fishes. Comparison to brain tubulins from a temperate fish and a mammal. J Biol Chem. 1986 Aug 15;261(23):10922–10930. [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Parker S. K. Divergent neural beta tubulin from the Antarctic fish Notothenia coriiceps neglecta: potential sequence contributions to cold adaptation of microtubule assembly. Cell Motil Cytoskeleton. 1993;24(3):156–166. doi: 10.1002/cm.970240303. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Prasad V., Ludueña R. F. Cold-stable microtubules from Antarctic fishes contain unique alpha tubulins. J Biol Chem. 1987 Jun 15;262(17):8360–8366. [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fago A., D'Avino R., Di Prisco G. The hemoglobins of Notothenia angustata, a temperate fish belonging to a family largely endemic to the Antarctic Ocean. Eur J Biochem. 1992 Dec 15;210(3):963–970. doi: 10.1111/j.1432-1033.1992.tb17501.x. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kay R. M., Harris R., Patient R. K., Williams J. G. Complete nucleotide sequence of a cloned cDNA derived from the major adult alpha-globin mRNA of X. laevis. Nucleic Acids Res. 1983 Mar 11;11(5):1537–1542. doi: 10.1093/nar/11.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. Poly(A) signals. Cell. 1991 Feb 22;64(4):671–674. doi: 10.1016/0092-8674(91)90495-k. [DOI] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- RUUD J. T. Vertebrates without erythrocytes and blood pigment. Nature. 1954 May 8;173(4410):848–850. doi: 10.1038/173848a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Kay R. M., Patient R. K. The nucleotide sequence of the major beta-globin mRNA from Xenopus laevis. Nucleic Acids Res. 1980 Sep 25;8(18):4247–4258. doi: 10.1093/nar/8.18.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]