Abstract
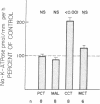
The purpose of this study was to determine the nephron site, time course, and mechanism of mineralocorticoid action on renal tubular Na-K-ATPase in rats and rabbits, without dietary manipulation and by using the natural mineralocorticoid aldosterone. Sustained, high physiologic levels of circulating aldosterone mimicking those produced endogenously during potassium loading or sodium deprivation were provided by constant delivery of the hormone in doses of 5 or 50 micrograms/100 g body wt per 24 h, respectively, from osmotic minipumps implanted subcutaneously. In adrenal-intact rats receiving the 5-microgram dose, aldosterone levels were similar to those seen in animals fed a high K diet and produced a time-dependent increase in Na-K-ATPase activity in the cortical-collecting tubule (CCT) to a level 103% higher than in controls after 7 d (2,007 +/- 178 vs. 989 +/- 72 pmol/mm per h, P less than 0.001); the enzyme activity in the proximal convoluted tubule, medullary thick ascending limb, and the inner stripe of the medullary-collecting tubule did not change significantly. The increment in CCT Na-K-ATPase was larger (142%) in animals receiving for the same period of time the 50-micrograms dose, which produced circulating aldosterone levels similar to those of sodium-deprived rats. A significant stimulation of Na-K-ATPase activity was seen in the CCT of adrenalectomized rats after 24 h of treatment with either dose of the hormone, and at 12 h only in animals receiving the 50 micrograms/100 g per 24 h regimen. To determine whether the enhanced Na-K-ATPase activity produced by aldosterone is due to synthesis of new enzyme units or to alteration in its kinetics, we examined the ouabain-binding capacity and the affinity for Na and K of the enzyme from CCT of rabbits treated with 5 micrograms/100 g body wt per 24 h aldosterone for 3 d. These experiments revealed a parallel increment on Na-K-ATPase activity and specific [3H]ouabain binding in aldosterone-treated rabbits, while the affinity of the enzyme for either sodium or potassium was unaltered. The results of this study indicate that high physiologic levels of aldosterone simulating those measured during K loading or Na deprivation lead to a segment-specific increase in Na-K-ATPase activity in the CCT. This effect was time-and dose-dependent and was due to an increase in the number of active enzyme units. The segmental specificity and time course of the increase in enzyme activity suggest that modulation of Na-K-ATPase by aldosterone plays a role in the chronic adaptation of the CCT to altered availability of sodium and potassium, and therefore in the homeostasis of these cations by the kidney.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doucet A., Katz A. I. Mineralcorticoid receptors along the nephron: [3H]aldosterone binding in rabbit tubules. Am J Physiol. 1981 Dec;241(6):F605–F611. doi: 10.1152/ajprenal.1981.241.6.F605. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I., Morel F. Determination of Na-K-ATPase activity in single segments of the mammalian nephron. Am J Physiol. 1979 Aug;237(2):F105–F113. doi: 10.1152/ajprenal.1979.237.2.F105. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I. Renal potassium adaptation: Na-K-ATPase activity along the nephron after chronic potassium loading. Am J Physiol. 1980 May;238(5):F380–F386. doi: 10.1152/ajprenal.1980.238.5.F380. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I. Short-term effect of aldosterone on Na-K-ATPase in single nephron segments. Am J Physiol. 1981 Sep;241(3):F273–F278. doi: 10.1152/ajprenal.1981.241.3.F273. [DOI] [PubMed] [Google Scholar]
- El Mernissi G., Chabardès D., Doucet A., Hus-Citharel A., Imbert-Teboul M., Le Bouffant F., Montégut M., Siaume S., Morel F. Changes in tubular basolateral membrane markers after chronic DOCA treatment. Am J Physiol. 1983 Jul;245(1):F100–F109. doi: 10.1152/ajprenal.1983.245.1.F100. [DOI] [PubMed] [Google Scholar]
- El Mernissi G., Doucet A. Quantitation of [3H]ouabain binding and turnover of Na-K-ATPase along the rabbit nephron. Am J Physiol. 1984 Jul;247(1 Pt 2):F158–F167. doi: 10.1152/ajprenal.1984.247.1.F158. [DOI] [PubMed] [Google Scholar]
- El Mernissi G., Doucet A. Short-term effect of aldosterone on renal sodium transport and tubular Na-K-ATPase in the rat. Pflugers Arch. 1983 Oct;399(2):139–146. doi: 10.1007/BF00663910. [DOI] [PubMed] [Google Scholar]
- El Mernissi G., Doucet A. Short-term effects of aldosterone and dexamethasone on Na-K-ATPase along the rabbit nephron. Pflugers Arch. 1983 Oct;399(2):147–151. doi: 10.1007/BF00663911. [DOI] [PubMed] [Google Scholar]
- El Mernissi G., Doucet A. Specific activity of Na-K-ATPase after adrenalectomy and hormone replacement along the rabbit nephron. Pflugers Arch. 1984 Nov;402(3):258–263. doi: 10.1007/BF00585508. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Fromm M., Oelkers W., Hegel U. Time course of aldosterone and corticosterone plasma levels in rats during general anaesthesia and abdominal surgery. Pflugers Arch. 1983 Dec;399(4):249–254. doi: 10.1007/BF00652747. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Knepper M. A., Burg M. B. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol. 1981 Jun;240(6):F536–F544. doi: 10.1152/ajprenal.1981.240.6.F536. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Mackie S., Tisher C. C. Effect of low potassium-diet on Na-K-ATPase in rat nephron segments. Pflugers Arch. 1982 Aug;394(2):113–117. doi: 10.1007/BF00582911. [DOI] [PubMed] [Google Scholar]
- Geering K., Girardet M., Bron C., Kraehenbühl J. P., Rossier B. C. Hormonal regulation of (Na+,K+)-ATPase biosynthesis in the toad bladder. Effect of aldosterone and 3,5,3'-triiodo-L-thyronine. J Biol Chem. 1982 Sep 10;257(17):10338–10343. [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslett J. P., Myketey N., Binder H. J., Aronson P. S. Mechanism of increased potassium secretion in potassium loading and sodium deprivation. Am J Physiol. 1980 Oct;239(4):F378–F382. doi: 10.1152/ajprenal.1980.239.4.F378. [DOI] [PubMed] [Google Scholar]
- Hill J. H., Cortas N., Walser M. Aldosterone action and sodium- and potassium-activated adenosine triphosphatase in toad bladder. J Clin Invest. 1973 Jan;52(1):185–189. doi: 10.1172/JCI107163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horster M., Schmid H., Schmidt U. Aldosterone in vitro restores nephron Na-K-ATPase of distal segments from adrenalectomized rabbits. Pflugers Arch. 1980 Apr;384(3):203–206. doi: 10.1007/BF00584554. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Regulation of the (Na+ + K+)-activated ATP hydrolyzing enzyme system in rat kidney. I. The effect of adrenalectomy and the supply of sodium on the enzyme system. Biochim Biophys Acta. 1968 Jan 8;151(1):212–224. doi: 10.1016/0005-2744(68)90176-9. [DOI] [PubMed] [Google Scholar]
- Kaissling B., Le Hir M. Distal tubular segments of the rabbit kidney after adaptation to altered Na- and K-intake. I. Structural changes. Cell Tissue Res. 1982;224(3):469–492. doi: 10.1007/BF00213746. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Doucet A., Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979 Aug;237(2):F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Kohan D. E., Knox F. G. Localization of the nephron sites responsible for mineralocorticoid escape in rats. Am J Physiol. 1980 Aug;239(2):F149–F153. doi: 10.1152/ajprenal.1980.239.2.F149. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Kaissling B., Dubach U. C. Distal tubular segments of the rabbit kidney after adaptation to altered Na- and K-intake. II. Changes in Na-K-ATPase activity. Cell Tissue Res. 1982;224(3):493–504. doi: 10.1007/BF00213747. [DOI] [PubMed] [Google Scholar]
- Martin R. S., Jones W. J., Hayslett J. P. Animal model to study the effect of adrenal hormones on epithelial function. Kidney Int. 1983 Sep;24(3):386–391. doi: 10.1038/ki.1983.171. [DOI] [PubMed] [Google Scholar]
- Mujais S. K., Chekal M. A., Jones W. J., Hayslett J. P., Katz A. I. Regulation of renal Na-K-ATPase in the rat. Role of the natural mineralo- and glucocorticoid hormones. J Clin Invest. 1984 Jan;73(1):13–19. doi: 10.1172/JCI111183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujais S. K., Chekal M. A., Lee S. M., Katz A. I. Relationship between adrenal steroids and renal Na-K-ATPase. Effect of short-term hormone administration on the rat cortical collecting tubule. Pflugers Arch. 1984 Sep;402(1):48–51. doi: 10.1007/BF00584831. [DOI] [PubMed] [Google Scholar]
- Natke E., Jr, Stoner L. C. Na+ transport properties of the peritubular membrane of cortical collecting tubule. Am J Physiol. 1982 Jun;242(6):F664–F671. doi: 10.1152/ajprenal.1982.242.6.F664. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- Park C. S., Edelman I. S. Dual action of aldosterone on toad bladder: Na+ permeability and Na+ pump modulation. Am J Physiol. 1984 Apr;246(4 Pt 2):F517–F525. doi: 10.1152/ajprenal.1984.246.4.F517. [DOI] [PubMed] [Google Scholar]
- Park C. S., Edelman I. S. Effect of aldosterone on abundance and phosphorylation kinetics of Na-K-ATPase of toad urinary bladder. Am J Physiol. 1984 Apr;246(4 Pt 2):F509–F516. doi: 10.1152/ajprenal.1984.246.4.F509. [DOI] [PubMed] [Google Scholar]
- Petty K. J., Kokko J. P., Marver D. Secondary effect of aldosterone on Na-KATPase activity in the rabbit cortical collecting tubule. J Clin Invest. 1981 Dec;68(6):1514–1521. doi: 10.1172/JCI110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U., Schmid J., Schmid H., Dubach U. C. Sodium- and potassium-activated ATPase. A possible target of aldosterone. J Clin Invest. 1975 Mar;55(3):655–660. doi: 10.1172/JCI107973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Ingram M. J., Williams A. D., Ingram D. Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 1981 Sep;20(3):340–347. doi: 10.1038/ki.1981.144. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest. 1983 Jul;72(1):77–83. doi: 10.1172/JCI110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. B., O'Neil R. G., Pryor J. L., Boulpaep E. L. Modulation of cell membrane area in renal collecting tubules by corticosteroid hormones. J Cell Biol. 1979 May;81(2):439–445. doi: 10.1083/jcb.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenfelder C., Arevalo G. J., Baranowski R. L., Kurtzman N. A., Katz A. I. Relationship between mineralocorticoids and renal Na+-K+-ATPase: sodium reabsorption. Am J Physiol. 1977 Dec;233(6):F593–F599. doi: 10.1152/ajprenal.1977.233.6.F593. [DOI] [PubMed] [Google Scholar]