Abstract
Introduction
Increased expression of IL-33 and its receptor ST2, encoded by the IL1RL1 gene, has been detected in the inflamed arteries of giant cell arteritis (GCA) patients. The aim of the present study was to investigate for the first time the potential influence of the IL33 and IL1RL1 loci on GCA predisposition.
Methods
A total of 1,363 biopsy-proven GCA patients and 3,908 healthy controls from four European cohorts (Spain, Italy, Germany and Norway) were combined in a meta-analysis. Six genetic variants: rs3939286, rs7025417 and rs7044343, within the IL33 gene, and rs2058660, rs2310173 and rs13015714, within the IL1RL1 gene, previously associated with immune-related diseases, were genotyped using predesigned TaqMan assays.
Results
A consistent association between the rs7025417 polymorphism and GCA was evident in the overall meta-analysis, under both allele (PMH = 0.041, OR = 0.88, CI 95% 0.78–0.99) and recessive (PMH = 3.40E-03, OR = 0.53, CI 95% 0.35–0.80) models. No statistically significant differences between allele or genotype frequencies for the other IL33 and IL1RL1 genetic variants were detected in this pooled analysis.
Conclusions
Our results clearly evidenced the implication of the IL33 rs7025417 polymorphism in the genetic network underlying GCA.
Introduction
Giant cell arteritis (GCA) is a chronic granulomatous vasculitis of large and medium size blood vessels which affects predominantly women and people generally older than 50 years [1].
Although the mechanisms triggering this pathology remain unclear, both immune and genetic factors appear to participate in its pathogenesis. The inflammatory process occurring in GCA is mainly driven by Th1 and Th17 lymphocytes and macrophages [2]. Additionally, endothelial cells also play an important role in the induction and perpetuation of the inflammation by promoting neoangiogenesis [3]. Regarding the genetic component of this vasculitis, besides HLA, a few risk factors have been consistently associated with GCA so far [4], [5] and, therefore, most of the genetic contribution to this disorder continues unidentified.
IL-33 has recently been described as a novel IL-1 family member with strong immunomodulatory functions. This cytokine, through binding to its receptor ST2 (suppression of tumorigenicity 2), encoded by the interleukin 1 receptor-like 1 (IL1RL1) gene, activates mast cells and Th2 lymphocytes, leading to the production of chemokines, pro-inflammatory and Th2-associated cytokines, and increased serum immunoglobulin levels [6]. Additionally, it has been shown that IL-33 acts as an activator of endothelial cells promoting angiogenesis and vascular permeability in vitro and in vivo [7].
Different studies have supported a pathogenic role of IL-33 axis in autoimmunity [8], [9], [10], [11]. Regarding genetic studies, several polymorphisms within the IL33 region have been associated with different immune-related conditions. Specifically, rs3939286 was associated with asthma [12], nasal polyposis [13] and inflammatory bowel disease (IBD) [14], rs7025417 was associated with coronary artery disease (CAD) [15], and rs7044343 with rheumatoid arthritis [16]. In addition, three other IL33 polymorphisms, in high linkage disequilibrium (LD) with rs3939286 (rs928413, rs2381416 and rs1342326), have been related to asthma through genome-wide association studies (GWASs) [17], [18], [19]. On the other hand, a number of IL1RL1 polymorphisms have also been implicated in autoimmunity. In this line, the rs2058660, rs2310173 and rs13015714 genetic variants were associated with Crohn's disease [20], ulcerative colitis and ankylosing spondylits [21], [22], and, celiac disease and inflammatory bowel disease [14], [23], respectively. Moreover, an association between three tightly linked polymorphisms (rs10197862, rs13408661 and rs3771180) and asthma has also been described in GWASs [18], [24], [25].
Interestingly, an increased expression of IL-33 and ST2 has been detected in the inflamed arteries of GCA patients, mainly in endothelial cells of newly formed vessels, thus suggesting a possible role of IL-33 in the angiogenesis-dependent inflammation in GCA [26].
The aim of the present study was to assess whether genetic variants at IL33 and IL1RL1, previously associated with immune-mediated diseases, are involved in the genetic predisposition to this vasculitis.
Methods
Study population
The case-control study included a total of 1,363 biopsy-proven GCA patients and 3,908 unrelated healthy controls, both of European ancestry. First, a discovery cohort, consisting of 894 Spanish GCA cases and 2,047 controls was analyzed. Subsequently, a replication phase was conducted in three independent cohorts from Germany (103 cases and 444 controls), Italy (255 cases and 1,141 controls) and Norway (111 cases and 276 controls). Table S1 summarizes the main characteristics of the analyzed cohorts. Case/control sets were matched by geographical origin and ethnicity. Informed written consent from all participants and approval from the local ethical committees (Comité de Bioética del Consejo Superior de Investigaciones Científicas, Comitato di Etica e Sperimentazione Farmaci Fondazione IRCCS Ca' Granda-Ospedale Maggiore Policlinico di Milano, Ethic Committee Provinciale of Reggio Emilia, Local Ethics Committees of the Hospital Vall d'Hebron, Hospital Clinic, Hospital de la Princesa, Hospital Clínico San Carlos, Hospital Universitario de Bellvitge, Hospital Universitario Marqués de Valdecilla, Oslo University Hospital, Hospital of Southern Norway Trust, Robert-Bosch-Hospital, University of Luebeck, Hannover Medical School, Università degli Studi di Verona, University of Genova and University Hospital of Parma) were obtained in accordance with the tenets of the Declaration of Helsinki. All patients had a positive temporal artery biopsy (disruption of the internal elastic laminae with infiltration of mononuclear cells into the arterial wall with or without multinucleated giant cells) and fulfilled the 1990 American College of Rheumatology classification criteria [27]. Patients were stratified according to the presence or absence of polymyalgia rheumatica (PMR), visual ischaemic manifestations (VIM; if they experienced transient visual loss including amaurosis fugax, permanent visual loss, or diplopia) and irreversible occlusive disease (IOD; if they had at least one of the following features: permanent visual loss, stroke or occlusive disease in the upper extremities or lower extremities).
Genotyping methods
The selection of single-nucleotide polymorphisms (SNPs) was based on their position and previous association with several inflammatory conditions. Polymorphisms with minor allele frequencies lower than 10% were not included as power calculations indicated a lack of statistical power to analyze them. Following these criteria, six polymorphisms, three in the IL33 region (rs3939286, rs7025417 and rs7044343), located in different haplotype blocks of this locus, and three in the IL1RL1 region (rs2058660, rs2310173 and rs13015714), were genotyped using the TaqMan allelic discrimination assay technology on a 7900HT Fast Real-Time PCR System, both from Applied Biosystems (Foster City, California, USA).
Statistical analysis
Table S2 shows the overall statistical power of the analysis (http://www.sph.umich.edu/csg/abecasis/CaTS/). Plink (v1.07) (http://pngu.mgh.harvard.edu/purcell/plink/) and StatsDirect v.2.6.6 (StatsDirect Ltd, Cheshire, UK) were used to perform 2×2 contingency tables and χ2 test. Odds ratios (OR) and 95% confidence intervals (CI) were obtained according to Woolf's method. P-values of the discovery cohort were corrected by The Benjamini & Hochberg (1995) step-up false discovery rate (FDR) control correction for multiple testing [28]. P-values <0.05 were considered statistically significant. The analysis of the combined data from all populations was performed using Plink and StatsDirect. Breslow–Day (BD) test and Cochran's Q and I2 statistics were used to estimate the homogeneity among populations. Pooled analyses were performed by Mantel-Haenszel (MH) test under fixed effects.
Results
Genotypic frequencies did not deviate from those predicted by Hardy-Weinberg (P>0.01) and the genotype success rate were >95%.
Table 1 shows the genotype and allelic frequencies of the IL33 and IL1RL1 polymorphisms analyzed in the Spanish discovery cohort. In the allele test, none of the studied polymorphisms showed a significant association with GCA, although a trend of association was evident for the IL33 rs7025417 SNP (P = 0.082, OR = 0.87 [0.75–1.02]). When the recessive model was considered, the frequency of the minor genotype of IL33 rs7025417 was significantly reduced in patients compared to healthy controls (P = 7.04E-03, OR = 0.46 [0.26–0.81]), even after applying FDR correction (PFDR = 0.042). No significant differences were found between patients with and without specific clinical features, i.e. PMR, VIM and IOD, (data not shown). Regarding IL1RL1, none of the analyzed genetic variants showed association with GCA in the case/control ( Table 1 ) or subphenotype analysis (data not shown).
Table 1. Genotype and minor allele frequency of the IL1RL1 and IL33 analyzed polymorphisms in biopsy-proven GCA patients and controls from Spain.
| Genotype, N (%) | Allele test | Recessive model | |||||||||||
| SNP | Locus | 1/2 | Subgroup (N) | 1/1 | 1/2 | 2/2 | MAF (%) | P-value | P FDR * | OR [CI 95%]** | P-value | P FDR * | OR [CI 95%]** |
| rs2310173 | IL1RL1 | T/G | Controls (n = 1972) | 458 (23.23) | 958 (48.58) | 556 (28.19) | 47.52 | ||||||
| GCA (n = 863) | 205 (23.75) | 395 (45.77) | 263 (30.48) | 46.64 | 0.5434 | 0.9304 | 0.97 [0.86–1.08] | 0.7594 | 0.9458 | 1.03 [0.85–1.24] | |||
| rs13015714 | IL1RL1 | G/T | Controls (n = 1944) | 138 (7.10) | 729 (37.50) | 1077 (55.40) | 25.85 | ||||||
| GCA (n = 868) | 61 (7.03) | 329 (37.90) | 478 (55.07) | 25.98 | 0.9178 | 0.9304 | 1.01 [0.88–1.15] | 0.9458 | 0.9458 | 0.98 [0.72–1.35] | |||
| rs2058660 | IL1RL1 | G/A | Controls (n = 2005) | 141 (7.03) | 767 (38.25) | 1097 (54.71) | 26.16 | ||||||
| GCA (n = 858) | 52 (6.06) | 343 (39.98) | 463 (53.96) | 26.05 | 0.9304 | 0.9304 | 0.99 [0.87–1.13] | 0.3425 | 0.6850 | 0.85 [0.61–1.19] | |||
| rs3939286 | IL33 | T/C | Controls (n = 1983) | 190 (9.58) | 826 (41.65) | 967 (48.76) | 30.41 | ||||||
| GCA (n = 862) | 80 (9.28) | 362 (42.00) | 420 (48.72) | 30.28 | 0.9219 | 0.9304 | 0.99 [0.88–1.12] | 0.8014 | 0.9458 | 0.97 [0.73–1.27] | |||
| rs7025417 | IL33 | C/T | Controls (n = 1969) | 72 (3.66) | 533 (27.07) | 1364 (69.27) | 17.19 | ||||||
| GCA (n = 871) | 15 (1.72) | 237 (27.21) | 619 (71.07) | 15.33 | 0.0818 | 0.4907 | 0.87 [0.75–1.02] | 7.04E-03 | 0.0423 | 0.46 [0.26–0.81] | |||
| rs7044343 | IL33 | C/T | Controls (n = 1957) | 220 (11.24) | 865 (44.20) | 872 (44.56) | 33.34 | ||||||
| GCA (n = 864) | 114 (13.19) | 377 (43.63) | 373 (43.17) | 35.01 | 0.2218 | 0.6655 | 1.08 [0.96–1.21] | 0.1393 | 0.4180 | 1.20 [0.94–1.53] | |||
*Benjamini and Hochberg step-up false discovery rate control. ** Odds ratio for the minor allele or genotype.
MAF, minor allele frequency; GCA, giant cell arteritis.
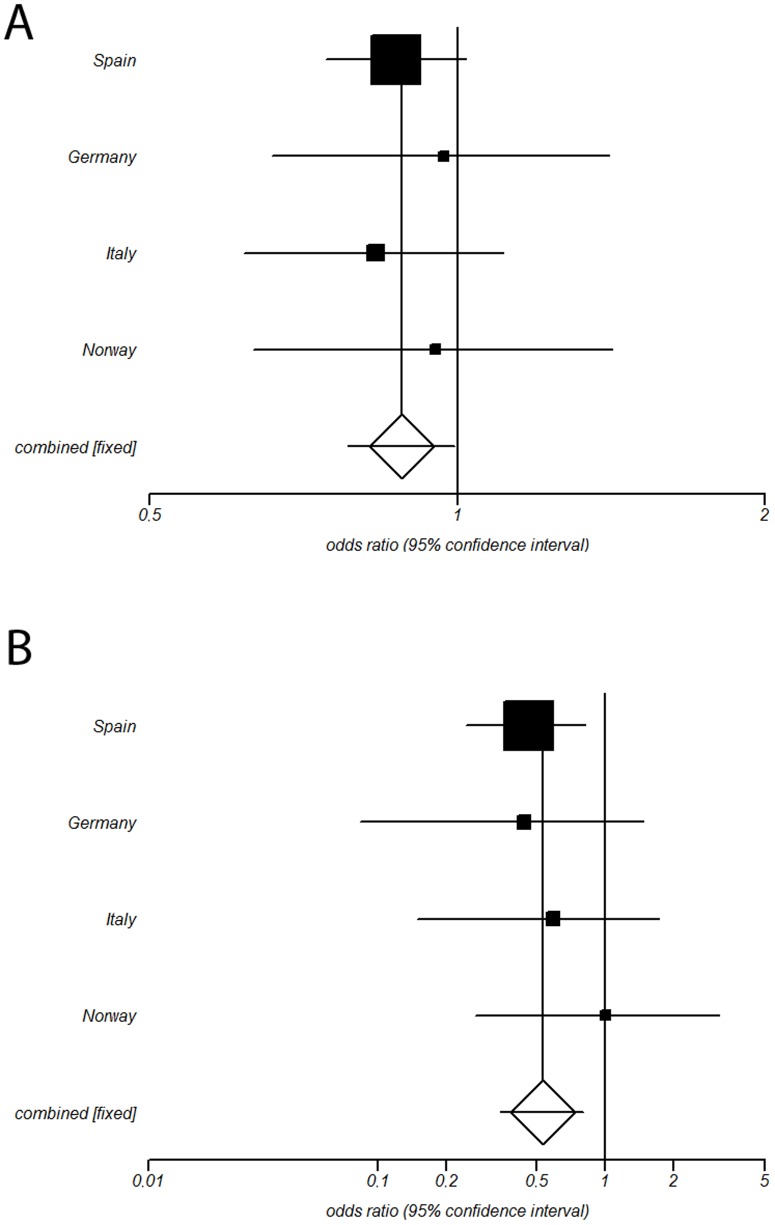
To further examine these findings, the three IL33 genetic variants were analyzed in three independent case/control sets from Germany, Italy and Norway ( Table 2 ). In the Italian set, a clear association between the rs3939286 polymorphism and GCA was found (P = 2.37E-03, OR = 0.70 [0.55–0.88]). No association between IL33 genetic variants and GCA was evident in the German and Norwegian sets. Subsequently, since no heterogeneity between the ORs from the four cohorts was evident (PBD>0.1; Cochran's Q-statistic p>0.1; I2 = 0% for rs7025417 and rs7044343; I2 = 16% for rs3939286), they were combined in a meta-analysis. As shown in Table 2 , only the rs7025417 polymorphism showed a consistent association with GCA in both allele (P = 0.041, OR = 0.88 [0.78–0.99], Figure 1A ) and recessive model (P = 3.40E-03, OR = 0.53 [0.35–0.80], Figure 1B ). The subphenotype analysis according to the main clinical features of GCA yielded negative results in the pooled analysis (data not shown).
Table 2. Replication and pooled analysis of the tested IL33 genetic variants in European biopsy-proven GCA patients and controls.
| Genotype, N (%) | Allele test | Recessive model | |||||||||
| SNP | 1/2 | Subgroup (N) | 1/1 | 1/2 | 2/2 | MAF (%) | P-value | OR [CI 95%]* | P-value | OR [CI 95%]* | |
| Germany | rs3939286 | T/C | Controls (n = 427) | 28 (6.56) | 147 (34.43) | 252 (59.02) | 23.77 | ||||
| GCA (n = 102) | 3 (2.94) | 37 (36.27) | 62 (60.78) | 21.08 | 0.4135 | 0.86 [0.59–1.24] | 0.1741 | 0.43 [0.13–1.45] | |||
| rs7025417 | C/T | Controls (n = 430) | 28 (6.51) | 149 (34.65) | 253 (58.84) | 23.84 | |||||
| GCA (n = 101) | 3 (2.97) | 41 (40.59) | 57 (56.44) | 23.27 | 0.8640 | 0.97 [0.67–1.39] | 0.1833 | 0.44 [0.13–1.48] | |||
| rs7044343 | C/T | Controls (n = 436) | 67 (15.37) | 201 (46.10) | 168 (38.53) | 38.42 | |||||
| GCA (n = 101) | 16 (15.84) | 51 (50.50) | 34 (33.66) | 41.09 | 0.4828 | 1.12 [0.82–1.53] | 0.9054 | 1.04 [0.57–1.88] | |||
| Italy | rs3939286 | T/C | Controls (n = 1109) | 94 (8.48) | 437 (39.40) | 578 (52.12) | 28.18 | ||||
| GCA (n = 251) | 13 (5.18) | 82 (32.67) | 156 (62.15) | 21.51 | 2.37E-03 | 0.70 [0.55–0.88] | 0.0830 | 0.59 [0.32–1.07] | |||
| rs7025417 | C/T | Controls (n = 1106) | 29 (2.62) | 282 (25.50) | 795 (71.88) | 15.37 | |||||
| GCA (n = 255) | 4 (1.57) | 59 (23.14) | 192 (75.29) | 13.14 | 0.2023 | 0.83 [0.63–1.10] | 0.3295 | 0.59 [0.21–1.70] | |||
| rs7044343 | C/T | Controls (n = 1116) | 188 (16.85) | 520 (46.59) | 408 (36.56) | 40.14 | |||||
| GCA (n = 252) | 46 (18.25) | 116 (46.03) | 90 (35.71) | 41.27 | 0.6415 | 1.05 [0.86–1.28] | 0.5920 | 1.10 [0.77–1.57] | |||
| Norway | rs3939286 | T/C | Controls (n = 271) | 15 (5.54) | 100 (36.90) | 156 (57.56) | 23.99 | ||||
| GCA (n = 108) | 6 (5.56) | 45 (41.67) | 57 (52.78) | 26.39 | 0.4883 | 1.14 [0.79–1.63] | 0.9937 | 1.00 [0.38–2.66] | |||
| rs7025417 | C/T | Controls (n = 267) | 12 (4.49) | 91 (34.08) | 164 (61.42) | 21.54 | |||||
| GCA (n = 111) | 5 (4.50) | 36 (32.43) | 70 (63.06) | 20.72 | 0.8032 | 0.95 [0.65–1.40] | 0.9965 | 1.00 [0.34–2.92] | |||
| rs7044343 | C/T | Controls (n = 273) | 43 (15.75) | 135 (49.45) | 95 (34.80) | 40.48 | |||||
| GCA (n = 108) | 14 (12.96) | 45 (41.67) | 49 (45.37) | 33.80 | 0.0878 | 0.75 [0.54–1.04] | 0.4924 | 0.80 [0.42–1.53] | |||
| Meta-analysis | rs3939286 | T/C | Controls (n = 3790) | 327 (8.63) | 1510 (39.84) | 1953 (51.53) | 28.55 | ||||
| GCA (n = 1323) | 102 (7.71) | 526 (39.76) | 695 (52.53) | 27.59 | 0.1358 | 0.93 [0.84–1.02] | 0.2036 | 0.85 [0.67–1.08] | |||
| rs7025417 | C/T | Controls (n = 3772) | 141 (3.74) | 1055 (27.97) | 2576 (68.29) | 17.72 | |||||
| GCA (n = 1338) | 27 (2.02) | 373 (27.88) | 938 (70.10) | 15.96 | 0.0408 | 0.88 [0.78–0.99] | 3.40E-03 | 0.53 [0.35–0.80] | |||
| rs7044343 | C/T | Controls (n = 3782) | 518 (13.70) | 1721 (45.51) | 1543 (40.80) | 36.45 | |||||
| GCA (n = 1325) | 190 (14.34) | 589 (44.45) | 546 (41.21) | 36.57 | 0.3747 | 1.04 [0.95–1.14] | 0.2447 | 1.12 [0.93–1.34] | |||
*Odds ratio for the minor allele or genotype.
MAF, minor allele frequency; GCA, giant cell arteritis.
Figure 1. Forest plots showing the odds ratios (OR) and confidence intervals (CI) of the IL33 rs2075417 genetic variant under allelic (A) and recessive (B) genetic models in the discovery and replication cohorts.
Discussion
In the present study, we identified for the first time an association between the IL33 gene and GCA through a large meta-analysis of four European cohorts. The minor allele (C) and genotype (CC) of the rs7025417 genetic variant showed a protective effect, consistently with that described in a previous study performed in CAD patients [15]. Interestingly, this polymorphism seems to produce an altered regulation of the IL33 gene expression [15]. In addition, the rs7025417 risk allele (T) correlates with an increase of the IL-33 plasma level in CAD [15]. Our findings would suggest that the rs7025417 genetic variant might influence the development of GCA by regulating the expression of IL-33 [26].
Although the combined analysis of the four cohorts evidenced that only one of the three IL33 tested polymorphisms, rs7025417, was associated with GCA (probably due to the larger sample size of the Spanish set), another different SNP, rs3939286, showed a clear association in the Italian cohort. Both IL33 genetic variants, rs7025417 and rs3939286, have been previously associated with autoimmune diseases [12], [13], [14], [15] and they appear to be regulatory DNA elements according to the public database RegulomeDB [29], but with minimal evidence of being located in a functional region (scores 5 and 6, for rs7025417 and rs3939286, respectively). As for rs7025417, the minor allele of the rs3939286 conferred protection to GCA, which is the opposite effect to that previously reported in asthma, nasal polyposis and IBD [12], [13], [14]. This could be indicating a different molecular effect of this SNP in the pathogenesis of GCA than in other autoimmune diseases. In addition, both SNPs are located in different haplotype blocks and present a low LD (D′ = 0.13 and r2 = 0.01), which suggests that they may represent independent signals. Nevertheless, the fact that different IL33 polymorphisms were associated with GCA in different populations may indicate that none of them is the real causal variant of the association but genetic markers in linkage disequilibrium with it. Small changes in the LD pattern between populations might result in different SNPs being the most associated with the disease in different cohorts.
In spite of an increased expression of the ST2 receptor was detected in the inflamed arteries of GCA patients [26], none of the tested IL1RL1 polymorphisms showed association with this vasculitis. Taking into account that the statistical power of our analysis was high enough to detect a possible weak signal (power >80% to detect an OR >1.20 in the discovery cohort), our data rule out an important role of the analyzed IL1RL1 genetic variants in GCA.
Angiogenesis has been proposed as one of the main mechanisms influencing the progression of GCA [30]. Interplay between inflammation and angiogenesis is mainly mediated by cytokines, chemokines, and growth factors, which also induce increase vascular permeability. In recent years, genetic studies have supported this crucial role of angiogenesis in GCA. In this line, variations within the vascular endothelial growth factor (VEGF) gene, encoding the best known angiogenic factor, have been associated with this vasculitis [4]. Like VEGF, IL-33 also exerts its angiogenic and vasopermeability activities in a nitric oxide (NO)-dependent manner [7]. Interestingly, a role for two genes encoding NO synthases (NOS2A, cytokine-inducible, and NOS3, endothelial) has also been demonstrated in GCA [4].
In conclusion, our results clearly indicate a role of the IL33 rs7025417 polymorphism as a novel genetic risk factor contributing to the GCA susceptibility. The effect of additional and unexplored IL33 and IL1RL1 genetic variants in GCA susceptibility cannot be discarded.
Supporting Information
Main clinical features of the giant cell arteritis patients included in the study.
(DOCX)
Overall statistical power of the study for each analyzed IL1RL1 and IL33 genetic variant at the 5% significance level.
(DOCX)
Other members of Spanish GCA Consortium contributing samples and clinical data to this analysis.
(DOCX)
Acknowledgments
The authors thank Sofía Vargas, Sonia García and Gema Robledo for their excellent technical assistance, and all the patients and healthy controls for kindly accepting their essential collaboration. Banco Nacional de ADN (University of Salamanca, Spain) is thanked for supplying part of the control material. The Norwegian Systemic Vasculitis and Connective Tissue Disease Registry (NOSVAR) at Oslo University Hospital is acknowledged for providing data on the Norwegian patients and the Norwegian Bone Marrow Donor Registry is acknowledged for providing the Norwegian controls.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was partially supported by RETICS Program RD08/0075/0011 (RIER) from ‘Instituto de Salud Carlos III’ (ISCIII) and by Junta de Andalucía, grupo CTS-180. MCC and JH-R were granted by SAF 11/30073. TW was granted by DFG WI 1031/6.1. The sponsors had no role in the design of the study, in the collection, analysis and interpretation of data or in the preparation, review or approval of the manuscript.
References
- 1. Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, et al. (2009) Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum 61: 1454–1461. [DOI] [PubMed] [Google Scholar]
- 2. Weyand CM, Goronzy JJ (2013) Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol 9: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ly KH, Regent A, Tamby MC, Mouthon L (2010) Pathogenesis of giant cell arteritis: More than just an inflammatory condition? Autoimmun Rev 9: 635–645. [DOI] [PubMed] [Google Scholar]
- 4. Carmona FD, Gonzalez-Gay MA, Martin J (2014) Genetic component of giant cell arteritis. Rheumatology (Oxford) 53: 6–18. [DOI] [PubMed] [Google Scholar]
- 5. Serrano A, Marquez A, Mackie SL, Carmona FD, Solans R, et al. (2013) Identification of the PTPN22 functional variant R620W as susceptibility genetic factor for giant cell arteritis. Ann Rheum Dis 72: 1882–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, et al. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23: 479–490. [DOI] [PubMed] [Google Scholar]
- 7. Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, et al. (2009) Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 114: 3117–3126. [DOI] [PubMed] [Google Scholar]
- 8. Demyanets S, Konya V, Kastl SP, Kaun C, Rauscher S, et al. (2011) Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 31: 2080–2089. [DOI] [PubMed] [Google Scholar]
- 9. Li M, Li Y, Liu X, Gao X, Wang Y (2012) IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neuroimmunol 247: 25–31. [DOI] [PubMed] [Google Scholar]
- 10. Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, et al. (2010) IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A 107: 4448–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu D, Jiang HR, Kewin P, Li Y, Mu R, et al. (2008) IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A 105: 10913–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, et al. (2009) Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 41: 342–347. [DOI] [PubMed] [Google Scholar]
- 13. Buysschaert ID, Grulois V, Eloy P, Jorissen M, Rombaux P, et al. (2010) Genetic evidence for a role of IL33 in nasal polyposis. Allergy 65: 616–622. [DOI] [PubMed] [Google Scholar]
- 14. Latiano A, Palmieri O, Pastorelli L, Vecchi M, Pizarro TT, et al. (2013) Associations between genetic polymorphisms in IL-33, IL1R1 and risk for inflammatory bowel disease. PLoS One 8: e62144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tu X, Nie S, Liao Y, Zhang H, Fan Q, et al. (2013) The IL-33-ST2L pathway is associated with coronary artery disease in a Chinese Han population. Am J Hum Genet 93: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li C, Mu R, Guo J, Wu X, Tu X, et al. (2014) Genetic variant in IL33 is associated with susceptibility to rheumatoid arthritis. Arthritis Res Ther 16: R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, et al. (2010) A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 363: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, et al. (2011) Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet 43: 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Moller E, Mercader JM, et al. (2014) A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 46: 51–55. [DOI] [PubMed] [Google Scholar]
- 20. Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, et al. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42: 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, et al. (2010) Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 42: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, et al. (2011) Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, et al. (2008) Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet 40: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramasamy A, Kuokkanen M, Vedantam S, Gajdos ZK, Couto Alves A, et al. (2012) Genome-wide association studies of asthma in population-based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS One 7: e44008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferreira MA, Matheson MC, Tang CS, Granell R, Ang W, et al. (2014) Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol 133: 1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciccia F, Alessandro R, Rizzo A, Raimondo S, Giardina A, et al. (2013) IL-33 is overexpressed in the inflamed arteries of patients with giant cell arteritis. Ann Rheum Dis 72: 258–264. [DOI] [PubMed] [Google Scholar]
- 27. Hunder GG, Arend WP, Bloch DA, Calabrese LH, Fauci AS, et al. (1990) The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum 33: 1065–1067. [DOI] [PubMed] [Google Scholar]
- 28. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300. [Google Scholar]
- 29. Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, et al. (2012) Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22: 1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cid MC, Cebrian M, Font C, Coll-Vinent B, Hernandez-Rodriguez J, et al. (2000) Cell adhesion molecules in the development of inflammatory infiltrates in giant cell arteritis: inflammation-induced angiogenesis as the preferential site of leukocyte-endothelial cell interactions. Arthritis Rheum 43: 184–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main clinical features of the giant cell arteritis patients included in the study.
(DOCX)
Overall statistical power of the study for each analyzed IL1RL1 and IL33 genetic variant at the 5% significance level.
(DOCX)
Other members of Spanish GCA Consortium contributing samples and clinical data to this analysis.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.