Abstract
Learning and memory abilities tend to decline as people age. The current study examines the question of whether a learning situation that emphasizes collaborative social interaction might help older persons overcome age-related learning and memory changes and thus perform similarly to younger persons. Younger and Older participants (n = 34 in each group) completed the Barrier Task, a game-like social interaction where partners work together to develop labels for a set of abstract tangrams. Participants were also administered standard clinical neuropsychological measures of memory, on which the Older group showed expected inferiority to the Younger group. On the Barrier Task, the Older group performed less well than the Younger group early on, but as the task progressed, the performance of the Older group caught up and became statistically indistinguishable from that of the Younger group. These results can be taken to suggest that a learning milieu characterized by collaborative social interaction can attenuate some of the typical memory disadvantages associated with being older.
Keywords: Aging, Social Interaction, Learning, Memory, Collaborative Discourse
Changes in memory are common among older adults (Zelinski & Stewart, 1998), and up to half of older adults complain about decreased everyday memory function (Jonker, Geerlings, & Schmand, 2000). Even the healthiest older adults display memory decline, particularly after the sixth decade (Schaie, 1996). This decline in memory can be parsimoniously attributed to changes in cellular, morphologic, and volumetric aspects of the hippocampus and related medial temporal lobe (MTL) structures (which support declarative memory) as a function of aging (e.g., Jack et al., 1997; Jernigan et al., 2001; Restom, Bangen, Bondi, Perthen, & Liu, 2007). These age-related changes in hippocampal declarative memory are observed in the context of generally preserved non-declarative memory (LaVoie & Light, 1995).
Interestingly, age-related memory effects can be reduced under certain conditions, such as the manipulation of mood (e.g., Ashby, Isen, & Turken, 1999), emotion (e.g., Denburg, Buchanan, & Tranel, 2003), and novelty (e.g., Nyberg, 2005). For example, material with emotional content can enhance memory performance in older adults (Denburg et al., 2003; Kensinger, 2008), so that differences between younger and older adults are attenuated for such material. Furthermore, when non-declarative memory processes are recruited for successful task completion (e.g., learning to golf putt) age-related differences are reduced (Chauvel, Maquestlaux, Hartley, Joubert, Didierjean, & Masters, 2012).
Of course, alterations to the hippocampus and the medial temporal lobe are not the only repercussion of normal aging. Researchers have also pointed to the frontal lobes as being especially vulnerable to age-related decline, a notion that is termed “The Frontal Aging Hypothesis” (Moscovitch & Winocur, 1995; West, 1996). This is demonstrated by deterioration in neuropsychological performances associated with the prefrontal cortices of the frontal lobes (e.g., West, Murphy, Armilio, Craik, & Stuss, 2002), including frontal memory impairments (Davidson, Troyer, & Moscovitch, 2006). Indeed, along with the hippocampus and other MTL structures, prefrontal cortices are increasingly considered part of the memory network (e.g., Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010).
In the study reported here, we addressed the question of whether a learning and memory situation that emphasized collaborative social interaction might help older persons overcome age-related learning and memory changes and thus perform similarly to younger persons. The rationale for this idea derives from several convergent lines of evidence. For one, persons often perform better when they work in collaborative and social contexts, compared to when they work individually (e.g., Luria 1947/1970; Vygotsky, 1978). Also, cognitive performance in older adults is enhanced by involvement in social activities (e.g., Bassuk, Glass, & Berkman, 1999) and by intellectual engagement in collaborative problem-solving (Stine-Morrow, Parisi, Morrow, & Park, 2008). To illustrate, there is evidence that older adults may be able to compensate for age-related cognitive deficits through collaboration (Dixon & Gould, 1996; Martin & Wight 2008; Strough & Margrett, 2002). The benefits of collaborative recall may be due to re-exposure and cross-cuing phenomenon, which theoretically influence both short- and long-term recall (Blumen, Rajaram, & Henkel, 2013). There is also evidence for decreased memory errors when collaborative interactions include free-flowing conversation (Barber, Rajaram, & Aron, 2010), and when collaborators reach a consensus during collaboration (Harris, Barnier, & Sutton, 2011). Finally, and of particular relevance to the current study, social and collaborative learning has been shown to enhance performance in memory-impaired neurological patients, including patients with amnesia caused by focal hippocampal damage (Duff, Hengst, Tranel, & Cohen, 2006) and patients with Alzheimer’s disease (Duff, Gallegos, Cohen, & Tranel, 2013).
There is a growing body of work on collaborative learning and memory in older adults. Collaborative efforts appear to be influenced by participant age, the nature of the interaction, and the relationship between partners (Blumen, Rajaram, & Henkel, 2013; Rajaram & Pereira-Pasarin, 2010). We note that, on average, collaborative groups remember more information that individuals. Some studies, however, suggest that older adults exhibit a counterintuitive phenomenon (Johansson, Andersson, & Rönnberg, 2000; Ross et al., 2004). That is, when a collaborative group is compared to a nominal group (the same number of individuals remembering alone), it becomes apparent that individuals are not contributing as much information as they are capable of when working alone. This counterintuitive finding is called collaborative inhibition (Basden, Basden, Bryner, & Thomas, 1997). Further evidence supports the notion that most information that is inhibited is recovered over time and collaborative costs do not seem to be long-lasting (Blumen, Rajaram, & Henkel, 2013). In fact, collaborative inhibition can be eliminated when older married couples help each other to remember (Johansson, Andersson, & Rönnberg, 2000a, 2000b), as opposed to individually dividing responsibility between partners during memory tasks (Johansson et al., 2005). Studies have also shown that familiar partners (vs. nominal pairs) are better able to overcome collaborative inhibition effects during problem-solving tasks (Peter-Wight & Martin, 2011; Rajaram & Pereira-Pasarin, 2010). Familiar partners often have a long history of everyday problem solving, which may qualify these partners as “expert” collaborative problem solvers (Peter-Wight & Martin, 2011). These repeated daily experiences with collaborative problem solving allow for optimal distribution of individual strengths and efficient use of cognitive resources during tasks that offer little guidance.
In our previous work we asked individuals with hippocampal amnesia to complete a collaborative referencing task with a familiar communication partner (e.g., spouse, friend) (Duff et al., 2006). The task used was similar to the collaborative task used in the current study. The task was presented as a game and encouraged free flowing conversation between familiar partners. Across 24 trials, the individuals with amnesia showed robust learning. Although the patients with amnesia required more time and words to complete the trials, the rate of learning (for both time to complete a trial and number of words used in a trial) across trials was equal to that of healthy comparison participants (Duff et al., 2006).
In short, the aim of the present study was to investigate the question of whether a learning situation that emphasized collaborative social interaction might help older persons overcome age-related learning and memory changes and thus perform similarly to younger persons. The task we used to address this question was a modified version of a “collaborative referencing task” originally devised by Krauss and colleagues (Krauss & Glucksberg, 1969/1977; Krauss & Weinheimer, 1964/1967) to investigate naturalistic communication. This is the same task we used in our previous work with memory-impaired populations described earlier (Duff et al., 2006; 2013). This game-like task emphasizes learning in the setting of a natural social interaction. Specifically, learning occurs in the context of a social situation in which two individuals are working together to solve a particular problem (placing Chinese tangrams into particular locations on a board). The “learning” in this situation involves the partners getting more efficient and thus faster as the task progresses over multiple trials and sessions. The partners develop short-hand labels for the stimuli (labels which are retained over time), and build “common ground” (i.e., a shared perspective in regard to the stimuli and how they are labeled) that facilitates faster, more accurate, and more efficient performance on the task. Critically, observed effects in the collaborative referencing task cannot be attributed to practice effects or repetition alone. Without a communication partner present with whom to interact and collaborate, but with repeated repetition of the same referential expression, there is not a reduction in time to complete the task or a simplification in the references used across trials (Hupet & Chantraine, 1992). Given our previous work showing intact learning in the collaborative referencing task (i.e., a situation that enhances collaborative social interaction) with profoundly memory impaired individuals (e.g., hippocampal amnesia, AD), we predict the typical age-related memory and learning effects would be significantly attenuated or possibly disappear altogether by the end of the task.
Materials and Methods
Participants
Two age groups, Younger and Older, were included. To begin with, we recruited 17 younger participants, aged 25 to 50 years (M = 32.47, SD = 6.87), and 17 older participants, aged 60 to 85 years (M = 70.94, SD = 10.94), into the study, and these 34 participants were administered an extensive battery of neuropsychological tests (see below). Then, each of these 17 younger and 17 older participants invited a “familiar communication partner,” defined as a spouse, sibling, or close friend of a comparable age, to join him or her in the study. Thus, the final sample size came to 34 younger adults (56% female) and 34 older adults (68% female), paired up as 17 younger and 17 older communication partner pairs (or “pairs” for short). A board-certified clinical neuropsychologist conducted a structured health interview (after Tranel et al., 1997) with each of the 68 participants to rule out neurological and psychiatric conditions that could have an unfavorable effect on the brain (e.g., stroke, head injury, Type I diabetes, neurosurgery, seizure disorder, demyelinating disorder, substance abuse, uncontrolled medical condition, vision/hearing loss, psychiatric illness necessitating inpatient treatment, and/or self-reported depression/anxiety exceeding mild severity). All participants were native English speakers. The younger and older communication pairs did not differ in terms of how long the partners had known one another (p = .10, Mann Whitney U test).
The battery of neuropsychological tests administered to the initial 17 younger and 17 older participants comprised standard measures of verbal intellectual ability (Wechsler Adult Intelligence Scale–Third Edition (WAIS-III) Vocabulary and Similarities, Wechsler, 1997); anterograde memory (Rey Auditory-Verbal Learning Test (AVLT), Rey, 1941; Wechsler Memory Scale–Third Edition (WMS-III) Verbal Paired Associates and Visual Reproductions, Wechsler, 1997); language (Token Test, McNeil & Prescott, 1978; Boston Naming Test, Goodglass et al., 2000); executive functioning (Trail Making Tests A and B, Reitan and Wolfson, 1985); and depressive symptoms (Beck Depression Inventory-Second Edition, Beck et al., 1996). We also administered the La Trobe Communication Questionnaire (Douglas et al., 2000), as a measure of perceived communication competence.
Procedures
The procedures for the experimental task in this study, as described below, are the same as those described previously in our work with this task (Duff et al., 2006; Duff, Hengst, Tranel, & Cohen, 2008). The task, which we call the Barrier Task (BT), is performed by two persons (familiar communication partners, as described above), one of whom is the Director and one of whom is the Matcher. The initial 17 younger and 17 older participants (who had their neuropsychological functioning tested) were assigned the role of Director. For each pair, the Director and Matcher sat facing each other across a table, and each of them had a board with 12 numbered spaces, demarcated as 1–6 on the top row and 7–12 on the bottom row. Each participant also had a set of 12 Chinese tangram playing cards. The tangrams were approximately 2 × 3 inches on 3.5 × 5-inch laminated cards. Tangrams are abstract black and white figures that have no pre-established names but that loosely resemble buildings, people, and animals. The Director and Matcher were separated by a low barrier on the table that blocked their boards and cards from view by one another, but allowed them to see each other’s facial expressions and gestures. The Director began with his/her cards on the board (in a unique, predetermined order for each trial), and communicated to the Matcher how to fill the numbered spaces so that at the end of the trial the boards would be identical. Pairs were instructed to treat the BT as a game, to have fun, and to communicate as much as needed to complete the task. The researcher left the room while the pairs played the BT, and returned between trials to give feedback (on overall accuracy) and to set up the next trial. All sessions were videotaped and transcribed following conventions outlined by Duff et al. (2008).
The BT was completed over two consecutive days. There were 24 total trials, with 6 trials conducted in each of 4 sessions, 2 sessions per day. For the Directors, neuropsychological testing consisting of tasks without a memory component was conducted for 30 minutes between sessions, and additional neuropsychological testing was completed at the conclusion of the BT sessions each day. Participants were financially compensated for their time.
Data Analysis
What typically happens in the BT is that participant pairs get faster and more efficient at communicating with one another and solving the task. This is evidenced by increasingly efficient labels with which to communicate and a reduction in the amount of communicative resources (i.e., turns, words), in addition to a decrease in the time required to complete each trial. As in previous work (Duff et al. 2006, 2008), we analyzed three dependent variables from the BT: Time to Completion, Number of Words, and Number of Turns.1 Time to Completion was the time (in seconds) that it took a pair to finish the game on each trial. The Number of Words and Number of Turns were coded from the recorded transcripts, and totals were calculated. Words were broadly defined with little emphasis on morphological or syntactic form (e.g., fillers such as “um” and “uh” each counted as one word, contractions such as “can’t” counted as one word). For Turns, an interactional turn was defined as an utterance produced by one individual and could include verbal or non-verbal resources (e.g., head nod). Turn boundaries were denoted by a change in speaker. When two individuals spoke simultaneously, each speaker’s utterance was counted as a turn.
For each dependent measure, we took the 24 trials of the BT and binned them into 8 data points (8 “trial bins” representing the average of 3 trials each), in order to reduce trial-by-trial noise in the data. Then, the BT performances of the Younger and Older groups were analyzed with a 2 × 8 linear mixed model analysis for repeated measures using age group (Group: Younger versus Older) as a between-subjects factor and Trial Bin as a repeated measures within-subjects factor. The BT data were analyzed using a repeated measures approach as implemented in SAS Proc Mixed (SAS version 9.1, SAS Institute Inc, Cary, NC). The covariance structure used for the analyses was the first order autoregressive AR(1) process. The analyses were adjusted for inter-correlation of the dependent measures.
Results
Demographic and Neuropsychological Data
We compared Older and Younger Directors on several demographic and cognitive variables, by contrasting the age groups using chi-square or independent samples t-tests (Table 1). Demographically, the groups were indistinguishable with regard to years of education, premorbid intellect as measured by a reading test (WRAT Reading), and sex distribution2. As expected, the groups differed on all anterograde memory (AVLT, and WMS VR and VPA) variables as well as speeded executive functioning (Trail Making Tests A and B). By contrast, the groups performed similarly on tests examining verbal intellect (WAIS Vocabulary and WAIS Similarities), language (Token Test and Boston Naming Test), self-reported depression (BDI-II), and communication (La Trobe).
Table 1.
Demographic and Cognitive Characteristics of Younger and Older Directors
| Characteristic1 | Statistic2 | Directors | p3 | |
|---|---|---|---|---|
| Younger (n = 17) |
Older (n = 17) |
|||
| Age | M | 32.47 | 70.94 | p < .0001 |
| SD | 6.87 | 10.95 | ||
| Education | M | 16.94 | 15.65 | ns |
| SD | 1.25 | 2.45 | ||
| WRAT Reading | M | 110.13 | 106.47 | ns |
| SD | 6.42 | 5.89 | ||
| Sex | % Female | 56% | 68% | ns |
| Handedness | % RH | 88% | 100% | ns |
| WAIS Vocabulary | M | 55.57 | 49.31 | ns |
| SD | 7.68 | 9.44 | ||
| WAIS Similarities | M | 26.64 | 24.31 | ns |
| SD | 2.98 | 4.54 | ||
| WMS VR I | M | 89.18 | 77.29 | p = .003 |
| SD | 9.98 | 11.70 | ||
| WMS VR II | M | 79.88 | 58.29 | p = .006 |
| SD | 13.59 | 25.77 | ||
| AVLT Trials 1–5 | M | 58.88 | 47.75 | p < .001 |
| SD | 7.29 | 6.68 | ||
| AVLT Delay | M | 11.47 | 7.81 | p < .001 |
| SD | 2.70 | 2.51 | ||
| WMS VPA Trials 1–4 | M | 25.82 | 18.31 | p = .001 |
| SD | 5.19 | 6.29 | ||
| WMS VPA Delay | M | 7.59 | 6.38 | p = .015 |
| SD | 0.80 | 1.67 | ||
| Token Test | M | 43.63 | 42.75 | ns |
| SD | 0.81 | 1.77 | ||
| Boston Naming | M | 19.12 | 18.94 | ns |
| SD | 1.11 | 1.44 | ||
| Trail Making A | M | 22.79 | 31.70 | p = .001 |
| SD | 6.73 | 7.52 | ||
| Trail Making B | M | 47.53 | 78.10 | p < .0001 |
| SD | 10.69 | 26.49 | ||
| BDI-II | M | 4.56 | 3.31 | ns |
| SD | 4.55 | 2.98 | ||
| LaTrobe | M | 51.59 | 50.47 | ns |
| SD | 11.91 | 10.26 | ||
Shown are age (in years), education (in years), gender (percent female), and handedness (percent right handed) of the participants; raw scores are provided for Wide Range Achievement Test-3 (WRAT) Reading; Wechsler Adult Intelligence Scale-Third Edition (WAIS) Vocabulary and Similarities; Wechsler Memory Scale-Third Edition (WMS) Verbal Paired Associates (VPA) Trials 1–4 and Delay, and Visual Reproductions (VR) I and II; Rey Auditory Verbal Learning Test (AVLT) Trials 1–5 and Delay; Token Test; Boston Naming Test (20-item); Trail Making Tests A and B (time to completion in seconds); Beck Depression Inventory-II (BDI); and La Trobe Communication Questionnaire.
Means and standard deviations are given for each variable unless otherwise indicated.
Post hoc comparisons were computed using independent-samples t-tests, or chi-square test where appropriate; ns = nonsignificant.
We had demographic and psychometric data (i.e., age, years of education, intellect as estimated from the WRAT Reading test, and sex) for the Matchers, and we contrasted these for the Older versus Younger groups. As with the Directors, the Older and Younger Matchers were statistically indistinguishable in all respects except for age (the Older group being significantly older).
Barrier Task
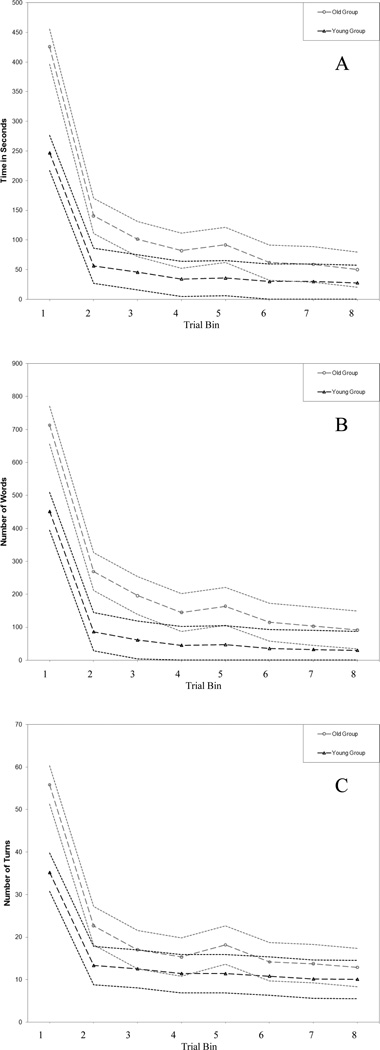
On all three dependent measures from the BT, the results showed the same general trend: the Older group was inferior to the Younger group in the initial phase of the task, but then caught up to the Younger group over trials. The specific outcomes are elaborated below, and depicted graphically in Figure 1.
Figure 1.
Least Squares Means Along with 95% Confidence Band for Younger and Older Groups on: (A) Time to Completion; (B) Number of Words; and (C) Number of Turns
Time to Completion
Figure 1A displays the least squared means at each Trial Bin along with the 95% confidence bands for each Group, for Time to Completion. The analysis yielded a Group main effect (F(1,33) = 19.16, p < .0005), a Trial Bin main effect (F(7,223) = 145.50, p < .0001), and a Group by Trial Bin interaction (F(7,223) = 7.49, p < .0001). We further contrasted the Younger and Older groups at the various Trial Bins under the assumption that the measurements were equally spaced. This follow-up contrast revealed that the Group main effect was a function of differences observed in the first two Trial Bins. Interestingly, the decrease in time from the first to the third Trial Bin was 50% steeper among the Older group relative to the Younger group. From the third Trial Bin on, the Older group was statistically indistinguishable from the Younger group, and as Figure 1A shows, the confidence bands crossed and became especially overlapping in the last three Trial Bins.
Number of Words
Figure 1B displays the least squared means at each Trial Bin along with the 95% confidence bands for each Group, for Number of Words. The analysis yielded a Group main effect (F(1,33) = 16.83, p < .0005), a Trial Bin main effect (F(7,223) = 146.69, p < .0001), and a Group by Trial Bin interaction (F(7,223) = 3.08, p < .005). A follow-up contrast revealed that the Group main effect was a function of differences observed in the first three Trial Bins. For Trial Bins #4 through #8, the Older group was statistically indistinguishable from the Younger group; again, it can be seen that the confidence bands became progressively more overlapping.
Number of Turns
Figure 1C displays the least squared means at each Trial Bin along with the 95% confidence bands for each Group, for Number of Turns. The analysis yielded a Group main effect (F(1,33) = 9.69, p < .005), a Trial Bin main effect (F(7,223) = 85.61, p < .0001), and a Group by Trial Bin interaction (F(7,223) = 4.98, p = .0001). A follow-up contrast revealed that the Group main effect was a function of differences observed in the first two Trial Bins. From the third Trial Bin on, the Older group was statistically indistinguishable from the Younger group, and the confidence bands became more overlapping.
Additional Results
The comparison between the Younger and Older groups on BT performance can be further illustrated by looking at the raw data across Trial Bins. These data are presented in Table 2, as a function of Group and Trial Bin, for the three dependent measures. It is evident that for all measures, both groups continue to get better as the task progresses. Of particular relevance to this study is the fact that the between-group differences shrink across Trial Bins. All three dependent measures show this pattern. For example, for Time to Completion, in Trial Bin #1, the Older group was on average nearly 3 minutes (179.0 seconds) slower than the Younger group, but in Trial Bin #8, the Older group was on average a mere 22.4 seconds slower. For Number of Words, in Trial Bin #1, the Older group used on average 261.4 more words than the Younger group, but in Trial Bin #8, the Older group used on average only 61.6 more words. For Number of Turns, in Trial Bin #1, the Older group required on average almost 20 more turns than the Younger group, but in Trial Bin #8, the Older group required on average less than 3 more turns.
Table 2.
Younger and Older Group Performances on the Barrier Task
| Time to Completion: | ||||||||
| Bin 1 | Bin 2 | Bin 3 | Bin 4 | Bin 5 | Bin 6 | Bin 7 | Bin 8 | |
| Older | 425.8 | 140.6 | 101.4 | 82.2 | 92.0 | 61.9 | 58.8 | 50.2 |
| Younger | 246.8 | 56.4 | 45.5 | 34.5 | 35.8 | 30.0 | 29.9 | 27.8 |
| Difference | 179.0 | 84.2 | 55.9 | 47.7 | 56.2 | 31.9 | 28.9 | 22.4 |
| Effect Size | 1.37 | 2.04 | 2.17 | 2.14 | 2.25 | 1.95 | 1.97 | 1.61 |
| Power | .97 | .99 | .99 | .99 | .99 | .99 | .99 | .98 |
| Number of Words: | ||||||||
| Bin 1 | Bin 2 | Bin 3 | Bin 4 | Bin 5 | Bin 6 | Bin 7 | Bin 8 | |
| Older | 713.3 | 269.1 | 195.8 | 144.9 | 163.8 | 115.2 | 103.1 | 91.6 |
| Younger | 451.9 | 86.5 | 61.2 | 45.4 | 47.3 | 35.4 | 32.7 | 30.0 |
| Difference | 261.4 | 182.6 | 134.6 | 99.5 | 116.5 | 79.8 | 70.4 | 61.6 |
| Effect Size | 1.14 | 2.09 | 2.29 | 1.92 | 1.83 | 1.74 | 1.82 | 1.63 |
| Power | .90 | .99 | 1 | .99 | .99 | .99 | .99 | .99 |
| Number of Turns: | ||||||||
| Bin 1 | Bin 2 | Bin 3 | Bin 4 | Bin 5 | Bin 6 | Bin 7 | Bin 8 | |
| Older | 56.25 | 23.07 | 17.28 | 15.44 | 18.32 | 14.11 | 13.71 | 12.88 |
| Younger | 36.34 | 13.21 | 12.41 | 11.25 | 11.24 | 10.72 | 10.05 | 10.00 |
| Difference | 19.91 | 9.86 | 4.87 | 4.19 | 7.09 | 3.39 | 3.66 | 2.88 |
| Effect Size | 1.04 | 1.52 | 1.5 | 1.1 | 1.55 | 1.27 | 1.33 | 1.39 |
| Power | .84 | .98 | .98 | .86 | .98 | .94 | .96 | .97 |
The data in Table 2 also help address the issue of ceiling effects in performance on the BT. The graphs in Figure 1 suggest that performances tend to reach somewhat of an asymptote by Trial Bins #3 and #4. This is a common pattern of performance on the BT, in a variety of different participant groups (e.g., Duff et al., 2006; Gallegos et al., 2007), attributable in part to the basic logistics of performing the task (e.g., moving the tangram cards around). Nonetheless, it is evident (as shown by the data in Table 2) that both groups in the current study continued to show improvement in their performance across Trial Bins, on all three of the measures, even if the improvement was smaller in magnitude in the latter part of the task. This helps rule out the alternative explanation that the Older group showed less discrepancy from the Younger group (or, in statistical terms, that the Group by Trial Bin interaction can be explained) simply as a function of the Younger group having maxed out their performance on the BT in the latter Trial Bins.
A final analysis examined the associations between the three key BT variables (time to completion, number of words, number of turns), each calculated as an average across all 24 trials, and neuropsychological performance, in the Older and Younger Directors, using two-tailed Pearson correlations. Perhaps not surprisingly, several robust and significant associations obtained for the older adults involving the demographic variable of age, as well as the cognitive variables of language and anterograde visual memory, cognitive domains are obviously relevant to the BT. More specifically, time to completion was associated with age (r = .53, p = .03), language (Token Test: r = −.53, p = .035 and Boston Naming Test: r = −.69, p = .003), and anterograde visual memory (Visual Reproductions I: r = −.72, p = .001 and Visual Reproductions II: r = −.73, p = .001). Number of words had very similar findings (Token Test, r = −.51, p < .05; Boston Naming Test, r = −.65, p = .007; Visual Reproductions I, r = −.75, p = .001; and Visual Reproductions II, r = −.81, p < .0001), as did number of turns (Age, r = .55, p = .02; Token Test, r = −.63, p = .008; Boston Naming Test, r = −.68, p = .004; Visual Reproductions I, r = −.62, p = .007; and Visual Reproductions II, r = −.72, p = .001). Among the younger adults, only number of words was associated with neuropsychological performance, namely, premorbid intellect, as measured by WRAT Reading (r = .62, p = .01).
Discussion
Our study sought to address the question of whether a learning situation that emphasizes collaborative social interaction might help older persons overcome age-related learning and memory changes and thus perform similarly to younger persons. The results provide a qualified affirmative answer—yes, but only after some trials of performing the task. Specifically, we found that on the Barrier Task (BT), a collaborative social interactive paradigm that encourages pairs of individuals to learn to communicate efficiently in order to solve a problem, Older participants started with inferior performance compared to Younger participants. As the task progressed, however, the Older group caught up—and by the latter part of the task, the Older group was statistically indistinguishable from the Younger group on all three dependent measures, namely, Time to Completion, Number of Words, and Number of Turns. On standard neuropsychological measures of anterograde memory, the Older group (Directors) was notably inferior to the Younger group (as is typical for such tests). These results can be taken to suggest that a learning milieu characterized by collaborative social interaction can vitiate some of the typical memory disadvantages associated with being older.
We believe that the BT approximates real-world communication (e.g., interaction with familiar partners across multiple days, free-flowing conversation, use of facial expressions, gestures) and encourages participants to engage in meaningful, goal-directed activity and to problem-solve collaboratively, thereby allowing heterogeneity and flexibility to be incorporated into the interactions (Duff et al., 2006; Hengst, Duff, & Dettmer, 2010). This is in contrast to the typical memory tests administered in neuropsychological assessments, which are anything but flexible—e.g., consider the ubiquitous word list learning tasks which call for rote learning and later verbatim reproduction in standard neuropsychological examinations (see Lezak, Howieson, Bigler, & Tranel, 2012). These are some of the factors that we suspect are at play in the results we obtained, and specifically, that differentiate learning in the BT from learning in a typical neuropsychological memory test. This explanation gains additional traction when considered in light of other literature which has demonstrated enhancement of problem-solving and certain aspects of memory in older married couples and other long-standing social dyads when in collaborative settings (see Meegan & Berg, 2002, for a review).
Two previous studies reached conclusions somewhat different from ours. Hupet, Chantraine, and Nef (1993) and Filer and Scukanec (1995) found that older adults were inferior to younger adults on a collaborative referencing task. A closer inspection of the procedures of those studies may help explain why different results were obtained. Specifically, in both of those studies, unacquainted partners were utilized, six learning trials were administered, and a full barrier was placed between the partners. By contrast, in our study, the communication partners (pairs) were very familiar with one another, 24 learning trials were used, and the barrier between partners during the game was only partial. We would submit that the methodological changes in our study, perhaps especially the extension of the task out to 24 learning trials (which allowed the crucial observation that the older participants caught up over time), are what led to a different outcome. Furthermore, given that our older partners were highly familiar with one another, our findings support previous studies showing that older adult spouses outperform older individuals as well as nominal pairs on problem-solving tasks (Peter-Wight & Martin, 2011). This is attributable to partners’ previous experiences in collaborative problem solving during everyday life, which may qualify these couples as “expert” collaborative problem solvers (Peter-Wight & Martin, 2011). These repeated daily experiences with collaborative problem solving allow for optimal distribution of individual strengths and efficient use of cognitive resources during tasks that offer little guidance, such as the Barrier Task.
Returning to the current study, it is intriguing to situate our findings in the context of the social cognition phenomenon referred to as “stereotype threat” (e.g., Steele, 1997). This theory suggests that age-related decline in memory is, in part, a function of older adults’ preconceived beliefs about aging and memory. Specifically, it is widely believed in our culture that memory inexorably declines with age, and hence, tests that explicitly feature memory demands (e.g., “I want you to remember these words”) may evoke performance deficits among older adults in part as a self-fulfilling prophecy. By contrast, when older adults are given a task such as the Barrier Task, which is presented as a social “game” and where learning and memory are incidental and more or less implicit, there is no obvious stereotype threat, and age-related differences tend to be reduced.
Our data also contribute some novel findings to the burgeoning field of social neuroscience. There is a growing literature in the social neuroscience of aging, with data to suggest that social interaction and involvement may provide a protection against cognitive decline. For example, Stine-Morrow et al. (2007) put forth the engagement hypothesis, which proposes that social and intellectual engagement produces its effects via the stimulation and development of new neural pathways. In a 20-week course, entitled The Senior Odyssey Program (Parisi, Greene, Morrow, & Stine-Morrow, 2007), 50 middle-aged and older adult participants underwent a cognitive intervention involving an emphasis on collaboration and creativity, and 24 participants served as controls. Relative to the controls, the Program participants displayed reliable improvements in processing speed, divergent thinking, and mindfulness.
The barrier task is, by nature, a fun and interactive task compared to standardized memory tests, which can be viewed by older participants as emotionally negative. Older adults sometimes show a “positivity effect” in memory (Mather & Carstensen, 2005). With age, the amygdala shows increasing reactivity to positive information and decreased reactivity to negative information (Mather, et al., 2004). Older adults remember relatively less emotionally negative information and relatively more emotionally positive information compared with younger adults (Charles, Mather, & Carstensen, 2003; Fung & Carstensen, 2003). These findings are supported by our current study, which shows that older adults perform as well as the younger adults by the end of the BT. The BT’s use of positive and game-like interactions may be a viewed as emotionally positive information, thus contributing to the improved performance seen in the older adults.
The BT is thought to involve multiple aspects of learning and memory, which may help account for some of the benefits seen. In our previous work we asked individuals with hippocampal amnesia to complete a similar collaborative referencing task with a familiar communication partner (e.g., spouse, friend) (Duff et al., 2006). Across 24 trials, the individuals with amnesia showed robust learning arriving at increasingly concise labels for the abstract shapes (e.g., siesta man). Although the patients with amnesia required more time and words to complete the trials, the rate of learning (for both time to complete a trial and number of words used in a trial) across trials was equal to that of healthy comparison participants (Duff et al., 2006). Critically, the collaborative learning observed here did not require the acquisition of novel semantic information or arbitrary word pairs, both types of learning thought to require an intact hippocampus and declarative memory. Rather, the participants used pre-existing knowledge to self generate the labels for the cards, i.e., producing a label of siesta man for a card that looks like a man slumped over taking a nap). This gradual tuning of conceptual, semantic, and visual information over the course of the task suggests that the learning is most consistent with non-declarative mechanisms (e.g., procedural memory) (Duff et al., 2006; 2013). This account fits well with relatively preserved aspects of memory and learning in healthy aging including remote semantic knowledge (Piolini et al., 2002) and non-declarative memory (LaVoie & Light, 1995). That said, these preserved aspects of memory and learning do not alone account for the performance of the older pairs. Recall that without a partner with whom to collaborate and interact the learning across trials is not observed (Hupet & Chantraine, 1992) and that our results here are in contrast to other versions of the collaborative referencing task in aging that do not preserve many aspects of routine social interaction (e.g., familiar partners, visualization of facial expressions and gesture) (Filer & Scukanec, 1995; Hupet et al., 1993). Thus, the benefits of a social and collaborative environment appear to be above and beyond what would be observed in the presence of intact non-declarative memory alone.
There are also declarative memory contributions to successful task completion. Further analysis of these sessions revealed that the comparison participants developed and used multiple perspectives (e.g., squirrelly Viking ship) and quickly moved to the use of definite references (e.g., the windmill) as the trial progressed whereas the amnesic participants did not (Duff et al., 2008; 2012). While these features are not required to show intact learning (i.e., the amnesic participants were not disadvantaged on the learning variables) they should be seen as aspects of task performance that do rely on intact declarative memory.
Our previous work involving the BT and individuals with amnesia and Alzheimer’s Disease (Duff et al., 2006; 2013) has also shown us that age may affect the nature of the interactions between the older and younger. As mentioned earlier these memory-impaired individuals show a normal rate of learning on the BT. However, in follow-up analyses focusing on the content of their interactions (the use of verbal play; the playful manipulation of language to make puns, tell jokes or funny stories, tease), we found clear differences in the use of verbal play between the older participants in the AD study (those with and without AD; mean age = 77 years; SD = 5.8) and the younger participants in the amnesia study (those with and without amnesia; mean age = 49 years; SD = 3.6) (Duff et al., 2009; Shune & Duff, 2012). Verbal play productions of Younger participants with amnesia and their matched comparison participants were overwhelmingly simple episodes for referencing the cards during the task trials. This differs from the older participants in the AD study and their matched comparison participants, who displayed more extended verbal play episodes, spanning for than 3 contiguous turns. These extended episodes often involved telling funny stories or teasing both during and outside of the task. These differences in simple vs. extended play episodes are likely attributable to age and support successful completion of the task, irrespective of the presence of brain damage. These findings fit well with Carstensen’s (1992; 1995) socioemotional selectivity theory, which predicts more social and emotional investment in the interactions of older participants.
However, while these findings do suggest age-related differences in how older and younger participants approach the task and structure their interactions, these differences did not appear to influence the nature of the learning. Recall that both the amnesic and AD participants exhibited normal learning on the task itself. While the younger participants in the amnesia study used verbal play in a different manner than the older participants in the AD study, the different communicative style of verbal play use did not disadvantage either group on the learning variables of the task itself. That said, given our previous work, we do not believe any observed differences in the content of the interactions present in our current study would account for performance on the learning measures of the task.
In sum, we found that in a collaborative social learning procedure, older adults started out at a disadvantage to younger adults, but over time, the older adults were able to make up ground and to achieve a level of performance that became statistically indistinguishable from that of the younger adults. The initial disadvantage seen in the older group is likely attributable to normal age-related processes in learning and memory, such as changes in frontal, MTL, and hippocampal brain regions. The similar learning seen in older adults over the course of the collaborative task can be accredited to the unique aspects of the BT, which are not present in the standardly administered neuropsychological tests, such as: the novelty of the BT, the social and collaborative environment, utilization of familiar communication partners, free-flowing conversation, reaching a consensus (“common-ground”), and eliciting emotion through the game-like atmosphere,
This novel finding may have practical significance (e.g., the teaching of older adults could be conducted in milieus that emphasized collaborative social interactions, rather than rote, verbatim learning), although it will be important to replicate the results with additional participants. Also, it will be important to conduct further work aimed at isolating the putative “active ingredients” of the Barrier Task—especially the social and collaborative nature of the learning milieu—to solidify the interpretation that these ingredients are in fact the cause of better performance in the older participants. Future studies may benefit by focusing on a more exacting analysis of the collaborative interactions, such as investigating differences in utterances and gestures in older vs. younger, differences in how older vs. younger adults approach the task, and further analysis of the variability in the type and length of the relationship between familiar partners. Finally, it would be interesting to conduct standard learning and memory procedures (e.g., list learning, paired associate learning) over extended trials and days, to more closely resemble the BT paradigm and enable a more direct contrast between standard learning and the learning presumed to occur in the context of the BT. Even with these caveats, though, the current study provides new evidence that a collaborative social milieu might be especially conducive to effective learning and memory in older persons.
Acknowledgements
Preparation of this article was supported by a National Institute on Aging Career Development Award (K01 AG022033) and by fellowship funding from the Iowa Scottish Rite Masonic Foundation to NLD; NINDS P01 NS19632, NIDA R01 DA022549, and the Spastic Paralysis Research Foundation to DT.
Footnotes
We also looked at Accuracy, defined as the number of correct placements on the Matcher’s board. However, similar to previous studies involving the BT (e.g., Duff et al., 2006; Hupet, Chantraine, & Neff, 1993), the pairs reached ceiling on accuracy within the first couple of trials, and thus accuracy was not subjected to formal statistical analysis and is not discussed further.
We note that none of our analyses adjusted for sex because of the lack of sex differences within group (i.e., when comparing Younger Director to Younger Matcher or Older Director to Older Matcher), with one exception, Block Design, in which younger males outperformed younger females. Perhaps more importantly, no sex differences within group were observed on the Barrier Task outcome measures (time to completion, number of words, number of turns).
References
- Andrews-Hanna J, Reidler J, Sepulcre J, Poulin R, Buckner R. Functional- anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby F, Isen A, Turken A. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Barber SJ, Rajaram S, Aron A. When two is too many: Collaborative encoding impairs memory. Memory and Cognition. 2010;38:255–264. doi: 10.3758/MC.38.3.255. [DOI] [PubMed] [Google Scholar]
- Basden BH, Basden DR, Bryner S, Thomas RL., III A comparison of group and individual remembering: does collaboration disrupt retrieval strategies? Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:1176–1191. doi: 10.1037//0278-7393.23.5.1176. [DOI] [PubMed] [Google Scholar]
- Bassuk S, Glass T, Berkman L. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory manual. 2nd ed. Texas: Psychological Corporation; 1996. [Google Scholar]
- Blumen HM, Rajaram S, Henkel L. The applied value of collaborative memory research in aging: Behavioral and neural considerations. Journal of Applied Research in Memory and Cognition. 2013;2(2013):107–117. [Google Scholar]
- Carstensen LL. Social and emotional patterns in adulthood: support for socioemotional selectivity theory. Psychology and Aging. 1992;7:331–338. doi: 10.1037//0882-7974.7.3.331. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science. 1995;4:151–156. doi: 10.1177/09637214211011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Chauvel G, Maquestiaux F, Hartley A, Joubert S, Didierjean A, Masters R. Age effects shrink when motor learning is predominantly supported by nondeclarative, automatic memory processes: Evidence from golf putting. Journal of Experimental Psychology. 2012;65:25–38. doi: 10.1080/17470218.2011.588714. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Troyer AK, Moscovitch M. Frontal lobe contributions to recognition and recall: Linking basic research with clinical evaluation and remediation. Journal of the International Neuropsychological Society. 2006;12:210–223. doi: 10.1017/S1355617706060334. [DOI] [PubMed] [Google Scholar]
- Denburg N, Buchanan T, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal elderly persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Gould ON. Interactive minds: Life-span perspectives on the social foundation of cognition. Cambridge University Press; 1996. pp. 221–241. [Google Scholar]
- Douglas J, O'Flaherty C, Snow P. Measuring perception of communicative ability: The development and evaluation of the La Trobe communication questionnaire. Aphasiology. 2000;14:251–268. [Google Scholar]
- Duff M, Gallegos D, Cohen N, Tranel D. Learning in Alzheimer’s disease in facilitated by social interaction. Journal of Comparative Neurology. 2013;521:4356–4369. doi: 10.1002/cne.23433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Gupta R, Hengst J, Tranel D, Cohen NJ. The use of definite references signals declarative memory: Evidence from hippocampal amnesia. Psychological Science. 2011;22:666–673. doi: 10.1177/0956797611404897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff M, Hengst J, Tranel D, Cohen N. Development of shared information in communication despite hippocampal amnesia. Nature Neuroscience. 2006;9:140–146. doi: 10.1038/nn1601. [DOI] [PubMed] [Google Scholar]
- Duff M, Hengst J, Tranel D, Cohen J. Collaborative discourse facilitates efficient communication and new learning in amnesia. Brain and Language. 2008;106:41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer L, Scukanec G. Collaborative referencing in elderly women. Perceptual and Motor Skills. 1995;81:995–1000. doi: 10.2466/pms.1995.81.3.995. [DOI] [PubMed] [Google Scholar]
- Fung HH, Carstensen LL. Sending memorable messages to the old: age differences in preferences and memory for advertisements. Journal of Personality and Social Psychology. 2003;85:163. doi: 10.1037/0022-3514.85.1.163. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. The Assessment of Aphasia and Related Disorders. 3rd ed. Philadelphia: Lea & Febiger; 2000. [Google Scholar]
- Harris CB, Barnier AJ, Sutton J. Consensus collaboration enhances group and individual recall accuracy. The Quarterly Journal of Experimental Psychology. 2011;65:179–194. doi: 10.1080/17470218.2011.608590. [DOI] [PubMed] [Google Scholar]
- Hengst JA, Duff MC, Dettmer A. Rethinking repetition in therapy: Repeated engagement as the social ground of learning. Aphasiology. 2010;24:887–901. [Google Scholar]
- Hupet M, Chantraine Y. Changes in repeated references: Collaboration or repetition effects. Journal of Psycholinguistic Research. 1992;21:485–496. [Google Scholar]
- Hupet M, Chantraine Y, Nef F. References in conversation between young and old normal adults. Psychology and Aging. 1993;8:339–346. doi: 10.1037//0882-7974.8.3.339. [DOI] [PubMed] [Google Scholar]
- Jack C, Petersen R, Xu Y, Waring S, O'Brien P, Tangalos E, Smith G, Ivnik R, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T, Archibald S, Fennema-Notestine C, Gamst A, Stout J, Bonner J, Hesselink J. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Johansson O, Andersson J, Rönnberg J. Do elderly couples have a better prospective memory than other elderly people when they collaborate? Applied Cognitive Psychology. 2000;14:121–133. [Google Scholar]
- Johansson O, Andersson J, Rönnberg J. Compensating strategies in collaborative remembering in very old couples. Scandinavian Journal of Psychology. 2005;46:349–359. doi: 10.1111/j.1467-9450.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- Jonker C, Geerlings M, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. International Journal of Geriatric Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kensinger E. Emotional Memory Across the Adult Lifespan. New York: Psychology Press; 2008. [Google Scholar]
- Krauss R, Glucksberg S. The development of communication: Competence as a function of age. Child Development. 1969;40:255–266. [Google Scholar]
- Krauss R, Glucksberg S. Social and nonsocial speech. Scientific American. 1977;236:100–105. [Google Scholar]
- Krauss R, Weinheimer S. Changes in references phrases as a function of frequency usage in social interactions. Psychonomic Science. 1964;1:113–114. [Google Scholar]
- Krauss R, Weinheimer S. Effect of referent similarity and communication mode on verbal encoding. Journal of Verbal Learning and Verbal Behavior. 1967;6:359–363. [Google Scholar]
- Lezak M, Howieson D, Bigler E, Tranel D. Neuropsychological Assessment. 5th ed. New York: Oxford University Press; 2012. [Google Scholar]
- Luria A. Traumatic Aphasia: ItsSsyndromes, Psychology and Treatment. Mouton: The Hague; (1947/1970). [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JDE, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Science. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Meegan S, Berg C. Contexts, functions, forms, and processes of collaborative everyday problem solving in older adulthood. International Journal of Behavioral Development. 2002;26:6–15. [Google Scholar]
- McNeil M, Prescott T. Revised Token Test. Austin, Texas: PRO-ED, Inc; 1978. [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Annals of the New York Academy of Sciences. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Nyberg L. Any novelty in hippocampal formation and memory? [Neuroimaging] Current Opinion in Neurology. 2005;18:424–428. doi: 10.1097/01.wco.0000168080.99730.1c. [DOI] [PubMed] [Google Scholar]
- Parisi J, Greene J, Morrow D, Stine-Morrow E. The Senior Odyssey: Participant experiences of a program of social and intellectual engagement. Activities, Adaptation, and Aging. 2007;31:31–49. [Google Scholar]
- Peter-Wight M, Martin M. When 2 is better than 1 + 1. European Psychologist. 2011;16:288–294. [Google Scholar]
- Piolino P, Desgranges B, Benali K, Eustache F. Episodic and semantic remote autobiographical memory in aging. Memory. 2002;10:239–257. doi: 10.1080/09658210143000353. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Pereira-Pasarin L. Collaborative Memory: Cognitive research and theory. Perspectives on Psychological Science. 2010;5:649–663. doi: 10.1177/1745691610388763. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tuscon: Neuropsychology Press; 1985. [Google Scholar]
- Restom K, Bangen K, Bondi M, Perthen J, Liu T. Cerebral blood flow and BOLD responses to a memory encoding task: A comparison between healthy young and elderly adults. NeuroImage. 2007;37:430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. Rey Auditory Verbal Learning Test. Psychological Assessment Resources, Inc.; 1941. [Google Scholar]
- Ross M, Spencer SJ, Linardatos L, Lam KCH, Perunovic M. Going shopping and identifying landmarks: Does collaboration improve older people’s memory? Applied Cognitive Psychology. 2004;18:683–696. [Google Scholar]
- Schaie K. Intellectual development in adulthood. In: Birren JE, Schaie KW, editors. Handbook of Psychology and Aging. California: Academic Press; 1996. pp. 266–286. [Google Scholar]
- Shune S, Duff MC. Verbal play as an interactional discourse resource in Alzhemier’s disease. Aphasiology. 2012;26:811–825. doi: 10.1080/02687038.2011.650626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. A threat in the air: How stereotypes shape intellectual identity and performance. American Psychologist. 1997;52:613–629. doi: 10.1037//0003-066x.52.6.613. [DOI] [PubMed] [Google Scholar]
- Stine-Morrow E, Parisi J, Morrow D, Greene J, Park D. An engagement model of cognitive optimization through adulthood. Journal of Gerontology: Psychological Sciences. 2007;62B:62–69. doi: 10.1093/geronb/62.special_issue_1.62. [DOI] [PubMed] [Google Scholar]
- Stine-Morrow E, Parisi J, Morrow D, Park D. The effects of an engaged lifestyle on cognitive vitality: A field experiment. Psychology and Aging. 2008;23:778–786. doi: 10.1037/a0014341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strough J, Margrett J. Overview of the special section on collaborative cognition in later adulthood. International Journal of Behavioral Development. 2002;26:2–5. [Google Scholar]
- Tranel D, Benton A, Olson K. A 10-Year longitudinal study of cognitive changes in elderly persons. Developmental Neuropsychology. 1997;13:87–96. [Google Scholar]
- Vygotsky L. Mind in Society: The Development of Higher Psychological Processes. Massachusetts: Harvard University Press; 1978. [Google Scholar]
- Wechsler D. Wechsler Memory Scale –Third Edition (WMS-III) New York: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Adult Scale of Intelligence. New York: Psychological Corporation; 1997. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armilio ML, Craik FIM, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test – 3. Delaware: Jastak Associates, Inc; 1993. [Google Scholar]
- Zelinski E, Stewart S. Individual differences in 16-year memory changes. Psychology and Aging. 1998;13:622–630. doi: 10.1037//0882-7974.13.4.622. [DOI] [PubMed] [Google Scholar]