Abstract
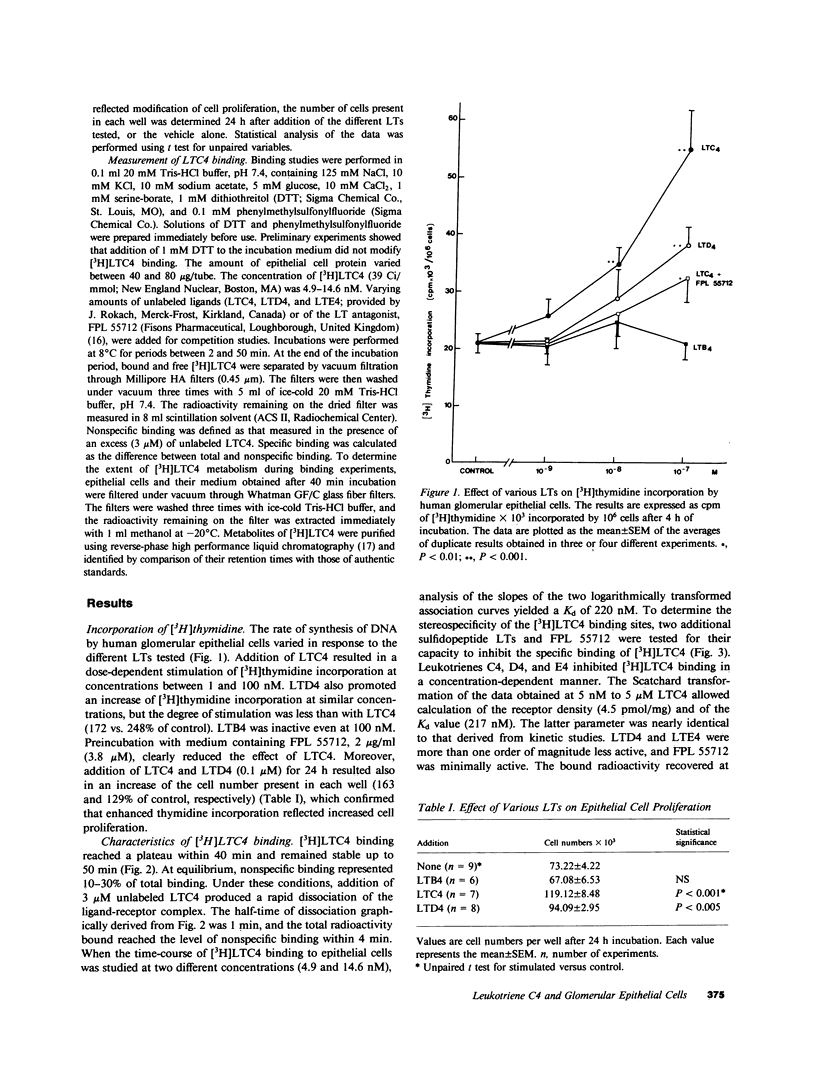
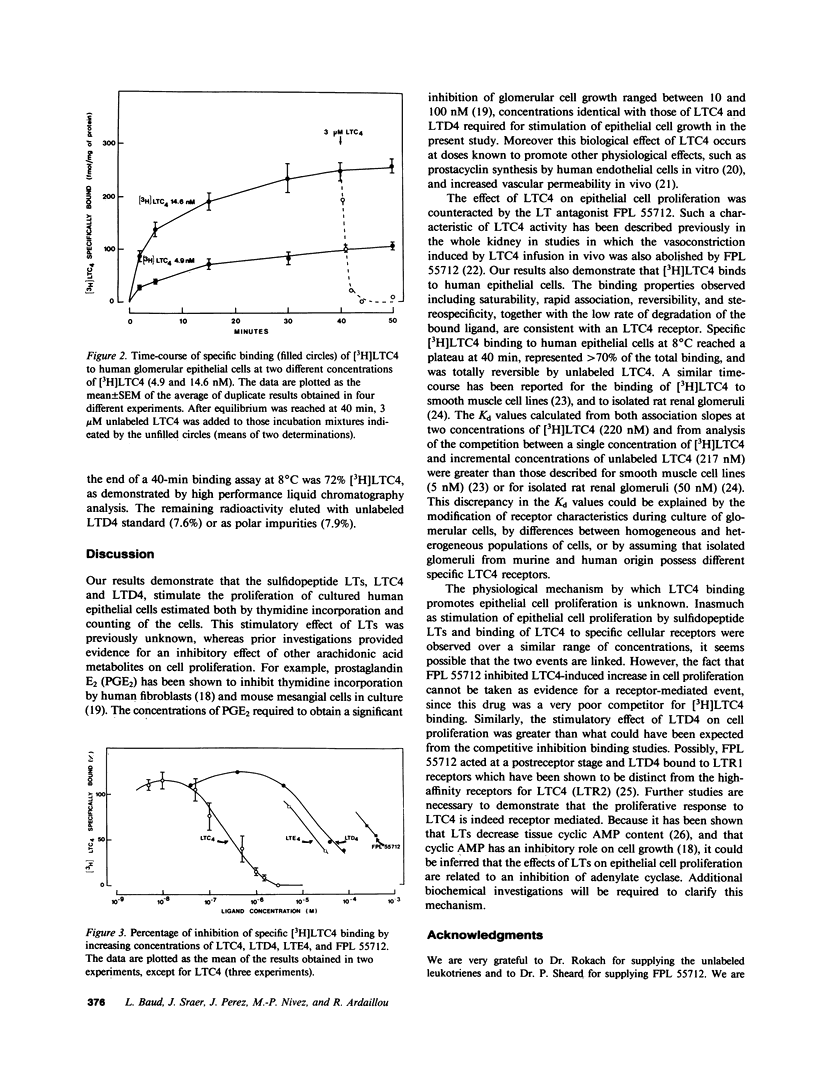
In human and experimental glomerulonephritis, glomerular hypercellularity results both from accumulation of macrophages and proliferation of resident glomerular cells. The recent identification of macrophage-derived factors that stimulate mesangial and epithelial cell proliferation suggests that these factors might contribute to the hypercellularity. To determine the identity of such macrophage-derived growth factors, we studied the effect of leukotrienes (LTs), products that are released from macrophages and leukocytes, on proliferation of human glomerular epithelial cells in culture. Dose-dependent (1-100 nM) stimulation of [3H]thymidine incorporation, an index of cell proliferation, was observed in cells incubated with the sulfidopeptide LTs, LTC4 and LTD4, but not with LTB4. The response was 248 and 172% of control values at 100 nM LTC4 and LTD4, respectively. This effect of LTC4 was abolished by FPL 55712. Subsequent binding studies demonstrated that glomerular epithelial cells possess specific receptors for LTC4. [3H]LTC4 bound rapidly at 8 degrees C to the cells. There was a plateau after 40 min incubation. Maximum specific binding was 70-90% of total binding. Specific binding was totally reversible with addition of an excess of unlabeled LTC4. Analysis of time-course association slopes at two concentrations of [3H]LTC4 and of the competition between a single concentration of [3H]LTC4 and increasing concentrations of unlabelled LTC4 allowed calculation of dissociation constants (Kd) of 220 and 217 nM, respectively. Both LTD4 and LTE4 exhibited ED50 values that were at least one order of magnitude higher than for LTC4. Thus, our findings suggest that LTC4 binds to specific receptors of glomerular epithelial cells, promotes proliferation of these cells, and could contribute to epithelial hypercellularity found in glomerulonephritis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardaillou N., Nivez M. P., Striker G., Ardaillou R. Prostaglandin synthesis by human glomerular cells in culture. Prostaglandins. 1983 Nov;26(5):773–784. doi: 10.1016/0090-6980(83)90061-8. [DOI] [PubMed] [Google Scholar]
- Atkins R. C., Glasgow E. F., Holdsworth S. R., Thomson N. M., Hancock W. W. Tissue culture of isolated glomeruli from patients with glomerulonephritis. Kidney Int. 1980 Apr;17(4):515–527. doi: 10.1038/ki.1980.60. [DOI] [PubMed] [Google Scholar]
- Atkins R. C., Holdsworth S. R., Hancock W. W., Thomson N. M., Glasgow E. F. Cellular immune mechanisms in human glomerulonephritis: the role of mononuclear leucocytes. Springer Semin Immunopathol. 1982;5(3):269–296. doi: 10.1007/BF01892089. [DOI] [PubMed] [Google Scholar]
- Augstein J., Farmer J. B., Lee T. B., Sheard P., Tattersall M. L. Selective inhibitor of slow reacting substance of anaphylaxis. Nat New Biol. 1973 Oct 17;245(146):215–217. doi: 10.1038/newbio245215a0. [DOI] [PubMed] [Google Scholar]
- Badr K. F., Baylis C., Pfeffer J. M., Pfeffer M. A., Soberman R. J., Lewis R. A., Austen K. F., Corey E. J., Brenner B. M. Renal and systemic hemodynamic responses to intravenous infusion of leukotriene C4 in the rat. Circ Res. 1984 May;54(5):492–499. doi: 10.1161/01.res.54.5.492. [DOI] [PubMed] [Google Scholar]
- Cattell V., Jamieson S. W. The origin of glomerular crescents in experimental nephrotoxic serum nephritis in the rabbit. Lab Invest. 1978 Dec;39(6):584–590. [PubMed] [Google Scholar]
- Cotran R. S. Monocytes, proliferation, and glomerulonephritis. J Lab Clin Med. 1978 Dec;92(6):837–840. [PubMed] [Google Scholar]
- Cramer E. B., Pologe L., Pawlowski N. A., Cohn Z. A., Scott W. A. Leukotriene C promotes prostacyclin synthesis by human endothelial cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4109–4113. doi: 10.1073/pnas.80.13.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén S. E., Björk J., Hedqvist P., Arfors K. E., Hammarström S., Lindgren J. A., Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois C. H., Foidart J. B., Hautier M. B., Dechenne C. A., Lemaire M. J., Mahieu P. R. Proliferative glomerulonephritis in rats: evidence that mononuclear phagocytes infiltrating the glomeruli stimulate the proliferation of endothelial and mesangial cells. Eur J Clin Invest. 1981 Apr;11(2 Suppl 1):91–104. doi: 10.1111/j.1365-2362.1981.tb02045.x. [DOI] [PubMed] [Google Scholar]
- Hedman S. E., Andersson R. G. Effects of SRS (slow reacting substance) on cyclic nucleotides in guinea pig tracheal muscle. Acta Pharmacol Toxicol (Copenh) 1982 Jan;50(1):30–34. doi: 10.1111/j.1600-0773.1982.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981 Sep;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Dowling J. P., Atkins R. C. Tissue culture of isolated glomeruli in experimental crescentic glomerulonephritis. J Exp Med. 1978 Jan 1;147(1):98–109. doi: 10.1084/jem.147.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast D., Bartholomaeus W. N. Liquid scintillation technique for the determination of DNA synthesis of lymphocytes cultured in plastic vessels. J Immunol Methods. 1973 Apr;2(3):261–267. doi: 10.1016/0022-1759(73)90052-5. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilis S., Lewis R. A., Corey E. J., Austen K. F. Specific receptors for leukotriene C4 on a smooth muscle cell line. J Clin Invest. 1983 Oct;72(4):1516–1519. doi: 10.1172/JCI111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews W. R., Rokach J., Murphy R. C. Analysis of leukotrienes by high-pressure liquid chromatography. Anal Biochem. 1981 Nov 15;118(1):96–101. doi: 10.1016/0003-2697(81)90162-7. [DOI] [PubMed] [Google Scholar]
- Ooi Y. M., Weiss M. A., Hsu A., Ooi B. S. Mechanisms of suppression of mouse mesangial cell proliferation by macrophage supernatants. J Immunol. 1983 Apr;130(4):1790–1795. [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzel R. B., Pabst R. The temporal relationship between glomerular cell proliferation and monocyte infiltration in experimental glomerulonephritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):337–350. doi: 10.1007/BF02892829. [DOI] [PubMed] [Google Scholar]
- Striker G. E., Killen P. D., Farin F. M. Human glomerular cells in vitro: isolation and characterization. Transplant Proc. 1980 Sep;12(3 Suppl 1):88–99. [PubMed] [Google Scholar]
- Williams J. D., Czop J. K., Austen K. F. Release of leukotrienes by human monocytes on stimulation of their phagocytic receptor for particulate activators. J Immunol. 1984 Jun;132(6):3034–3040. [PubMed] [Google Scholar]


