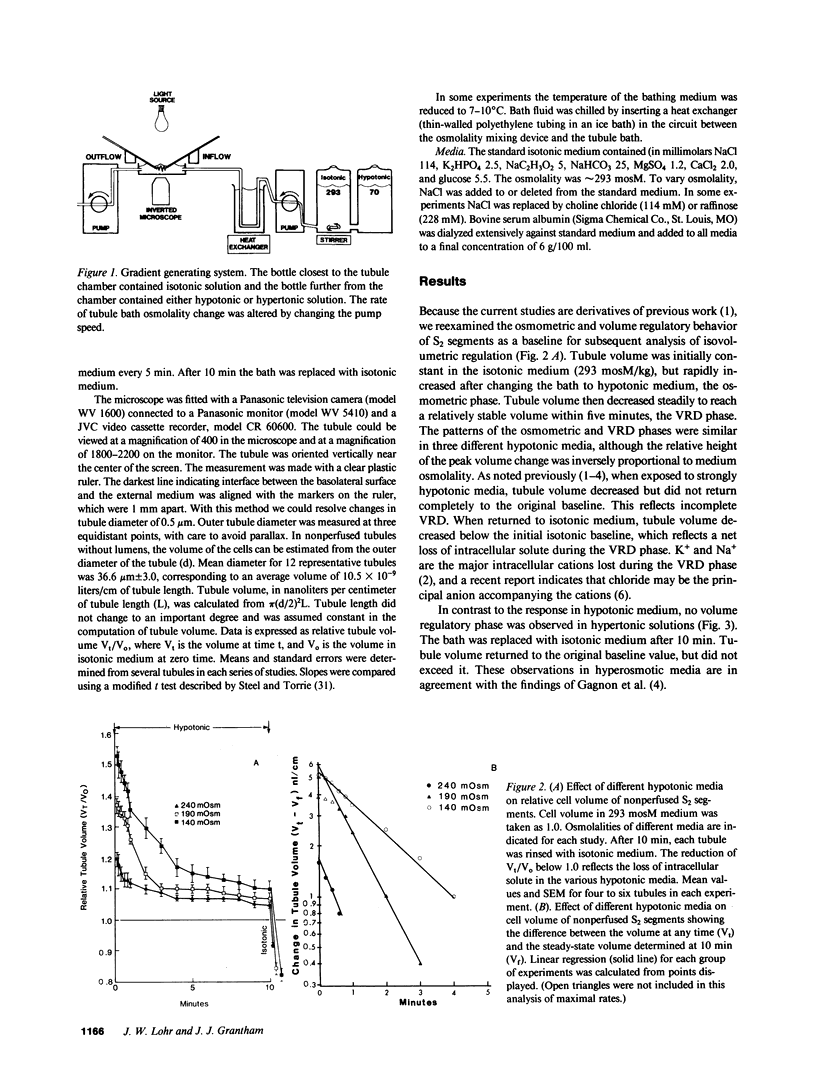
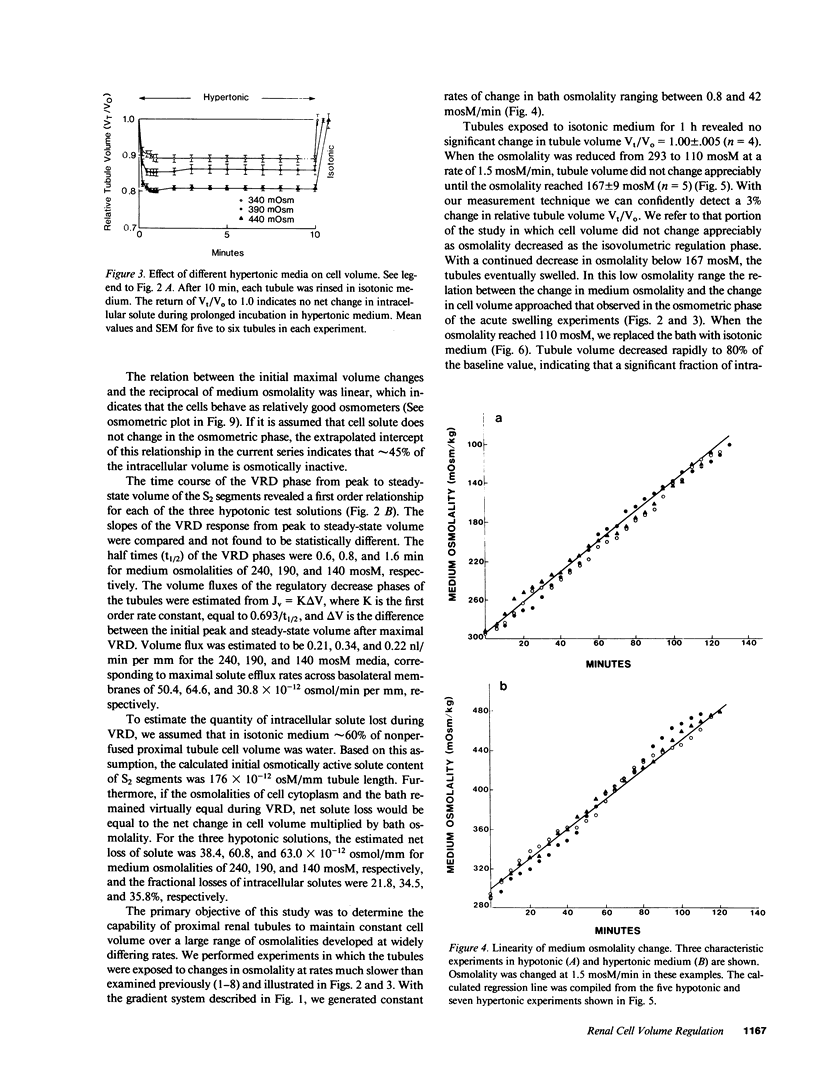
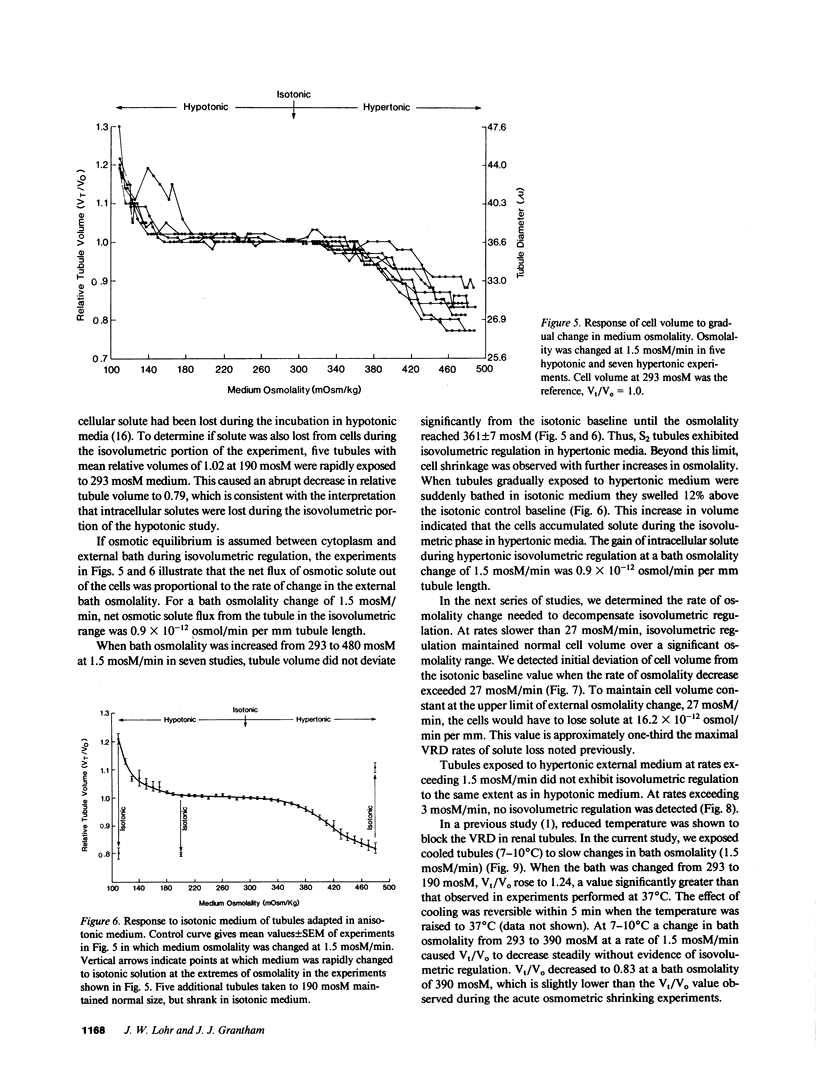
Abstract
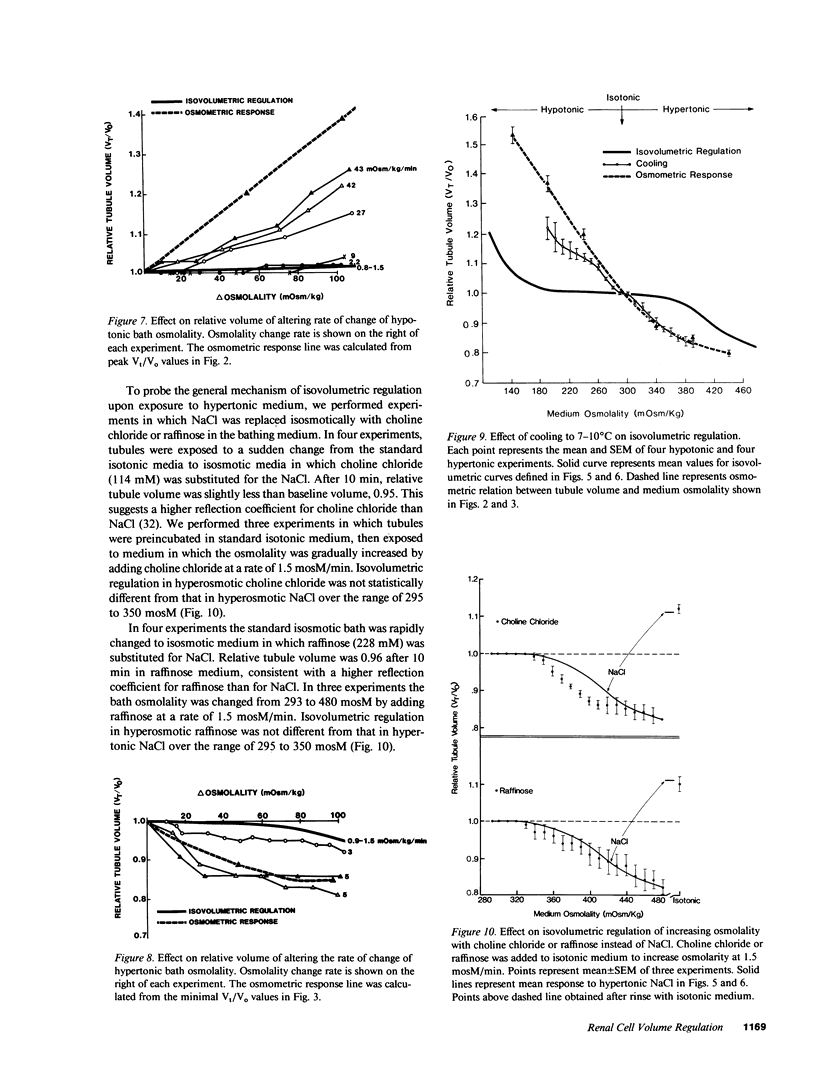
Sudden alteration in medium osmolality causes an osmometric change in proximal tubule cell size followed by restoration of cell volume toward normal in hypotonic but not in hypertonic medium. We determined the capability of isolated nonperfused proximal tubules to prevent a change in cell volume in anisotonic media. The external osmolality was gradually changed over a range from 110 to 480 mosM. At 1.5 mosM/min, cell volume remained constant between 167 +/- 9 and 361 +/- 7 mosM, a phenomenon termed isovolumetric regulation (IVR). Cells lost intracellular solutes in hypotonic and gained intracellular solutes in hypertonic media. Raffinose or choline chloride substitution showed that osmolality, rather than NaCl, signalled cell volume maintenance in hyperosmotic media. Cooling (7-10 degrees C) blocked IVR. IVR was maintained when osmolality was lowered at a rate of 27, but not at 42 mosM/min. IVR was not observed when the rate of osmolality increase exceeded 3 mosM/min. We conclude that proximal tubule cells sensitively regulate intracellular volume in an osmolality range of pathophysiologic interest by mechanisms dependent on the rate of net water movement across basolateral membranes and the absolute intracellular content of critical solutes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arieff A. I., Guisado R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int. 1976 Jul;10(1):104–116. doi: 10.1038/ki.1976.82. [DOI] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by Amphiuma red blood cells. The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol. 1980 Dec;76(6):683–708. doi: 10.1085/jgp.76.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by flounder red blood cells in anisotonic media. J Gen Physiol. 1977 May;69(5):537–552. doi: 10.1085/jgp.69.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi-Medina P., Lindemann B., González E., Whittembury G. The continuous measurement of tubular volume changes in response to step changes in contraluminal osmolality. Pflugers Arch. 1984 Apr;400(4):343–348. doi: 10.1007/BF00587530. [DOI] [PubMed] [Google Scholar]
- Dellasega M., Grantham J. J. Regulation of renal tubule cell volume in hypotonic media. Am J Physiol. 1973 Jun;224(6):1288–1294. doi: 10.1152/ajplegacy.1973.224.6.1288. [DOI] [PubMed] [Google Scholar]
- Ericson A. C., Spring K. R. Coupled NaCl entry into Necturus gallbladder epithelial cells. Am J Physiol. 1982 Sep;243(3):C140–C145. doi: 10.1152/ajpcell.1982.243.3.C140. [DOI] [PubMed] [Google Scholar]
- Ericson A. C., Spring K. R. Volume regulation by Necturus gallbladder: apical Na+-H+ and Cl(-)-HCO-3 exchange. Am J Physiol. 1982 Sep;243(3):C146–C150. doi: 10.1152/ajpcell.1982.243.3.C146. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Persson B. E., Spring K. R. Epithelial cell volume regulation: bicarbonate dependence. Science. 1981 Dec 18;214(4527):1357–1359. doi: 10.1126/science.7313695. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Spring K. R. Intracellular activities during volume regulation by Necturus gallbladder. J Membr Biol. 1984;78(3):187–199. doi: 10.1007/BF01925967. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Spring K. R. Involvement of calcium and cytoskeleton in gallbladder epithelial cell volume regulation. Am J Physiol. 1985 Jan;248(1 Pt 1):C27–C36. doi: 10.1152/ajpcell.1985.248.1.C27. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Ouimet D., Nguyen H., Laprade R., Le Grimellec C., Carrière S., Cardinal J. Cell volume regulation in the proximal convoluted tubule. Am J Physiol. 1982 Oct;243(4):F408–F415. doi: 10.1152/ajprenal.1982.243.4.F408. [DOI] [PubMed] [Google Scholar]
- Gilles R., Duchene C., Lambert I. Effect of osmotic shocks on rabbit kidney cortex slices. Am J Physiol. 1983 Jun;244(6):F696–F705. doi: 10.1152/ajprenal.1983.244.6.F696. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Lowe C. M., Dellasega M., Cole B. R. Effect of hypotonic medium on K and Na content of proximal renal tubules. Am J Physiol. 1977 Jan;232(1):F42–F49. doi: 10.1152/ajprenal.1977.232.1.F42. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Dupre A., Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982 May;79(5):849–868. doi: 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györy A. Z., Kweifio-Okai G., Ng J. Hypo- and hyperosmolal saline and raffinose on kidney cortical cell volume at 37 degrees C. Am J Physiol. 1981 Mar;240(3):F180–F184. doi: 10.1152/ajprenal.1981.240.3.F180. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O., Lambert I. H. Volume-induced increase of K+ and Cl- permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+. J Membr Biol. 1984;78(3):211–222. doi: 10.1007/BF01925969. [DOI] [PubMed] [Google Scholar]
- Hughes P. M., Macknight D. C. The regulation of cellular volume in renal cortical slices incubated in hyposmotic medium. J Physiol. 1976 May;257(1):137–154. doi: 10.1113/jphysiol.1976.sp011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman J. G., Brown W. W., Ware R. A., Schwartz J. H. Cell pH and acid transport in renal cortical tissue. Am J Physiol. 1980 Nov;239(5):F440–F444. doi: 10.1152/ajprenal.1980.239.5.F440. [DOI] [PubMed] [Google Scholar]
- Kleinzeller A., Nedvídková J., Knotková A. Effect of saline osmolarity on the steady-state level of water and electrolytes in kidney cortex cells. Biochim Biophys Acta. 1967 May 2;135(2):286–299. doi: 10.1016/0005-2736(67)90122-8. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M., Caryk T., Siebens A. W. Further studies of the volume-regulatory response of Amphiuma red cells in hypertonic media. Evidence for amiloride-sensitive Na/H exchange. J Gen Physiol. 1985 Oct;86(4):565–584. doi: 10.1085/jgp.86.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):372–395. doi: 10.1085/jgp.58.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Volume adjustment by renal medullary cells in hypo- and hyperosmolal solutions containing permeant and impermeant solutes. J Physiol. 1975 May;247(1):55–70. doi: 10.1113/jphysiol.1975.sp010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linshaw M. A., Grantham J. J. Effect of collagenase and ouabain on renal cell volume in hypotonic media. Am J Physiol. 1980 Jun;238(6):F491–F498. doi: 10.1152/ajprenal.1980.238.6.F491. [DOI] [PubMed] [Google Scholar]
- Paillard M., Leviel F., Gardin J. P. Regulation of cell volume in separated renal tubules incubated in hypotonic medium. Am J Physiol. 1979 Mar;236(3):F226–F231. doi: 10.1152/ajprenal.1979.236.3.F226. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Shigai T., Takeuchi J. Intracellular pH in the isolated perfused rabbit proximal straight tubule. Am J Physiol. 1985 Sep;249(3 Pt 2):F417–F423. doi: 10.1152/ajprenal.1985.249.3.F417. [DOI] [PubMed] [Google Scholar]
- Saubermann A. J., Scheid V. L., Dobyan D. C., Bulger R. E. Simultaneous comparison of techniques for x-ray analysis of proximal tubule cells. Kidney Int. 1986 Mar;29(3):682–688. doi: 10.1038/ki.1986.52. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Barfuss D. W. The study of pars recta function by the perfusion of isolated tubule segments. Kidney Int. 1982 Nov;22(5):434–448. doi: 10.1038/ki.1982.196. [DOI] [PubMed] [Google Scholar]
- Siebens A. W., Kregenow F. M. Volume-regulatory responses of Amphiuma red cells in anisotonic media. The effect of amiloride. J Gen Physiol. 1985 Oct;86(4):527–564. doi: 10.1085/jgp.86.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussing H. H. Volume regulation of frog skin epithelium. Acta Physiol Scand. 1982 Mar;114(3):363–369. doi: 10.1111/j.1748-1716.1982.tb06996.x. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J., Ochs T. J. Video measurement of basolateral membrane hydraulic conductivity in the proximal tubule. Am J Physiol. 1983 Jul;245(1):F123–F129. doi: 10.1152/ajprenal.1983.245.1.F123. [DOI] [PubMed] [Google Scholar]
- Welling P. A., Linshaw M. A., Sullivan L. P. Effect of barium on cell volume regulation in rabbit proximal straight tubules. Am J Physiol. 1985 Jul;249(1 Pt 2):F20–F27. doi: 10.1152/ajprenal.1985.249.1.F20. [DOI] [PubMed] [Google Scholar]
- Whittembury G., Grantham J. J. Cellular aspects of renal sodium transport and cell volume regulation. Kidney Int. 1976 Feb;9(2):103–120. doi: 10.1038/ki.1976.15. [DOI] [PubMed] [Google Scholar]