Abstract
To test whether insulin secretion is self-regulatory, canine pancreata were isolated and perfused in vitro and were infused with 0.3, 0.6, or 1.2 mU/ml exogenous insulin. Basal and arginine-stimulated concentrations of C-peptide, glucagon, and somatostatin were measured. There were no significant differences between basal secretion nor the increment of arginine-stimulated secretion for each respective hormone at each exogenous insulin concentration. The second preparation studied was a vascularly isolated, yet innervated, in situ perfused pancreas. Exogenous insulin (1 mU/kg per min) was infused "systemically"; the pancreas received no insulin. Endogenous pancreatic insulin and C-peptide secretion was suppressed, while pancreatic glucagon secretion increased during systemic insulin infusion. No changes in pancreatic hormone secretion occurred after the sympathetic nerves were sectioned. These results suggest that exogenous insulin does not directly suppress the B cell, but can suppress insulin secretion through an indirect neurally mediated, insulin-dependent nerve mechanism.
Full text
PDF

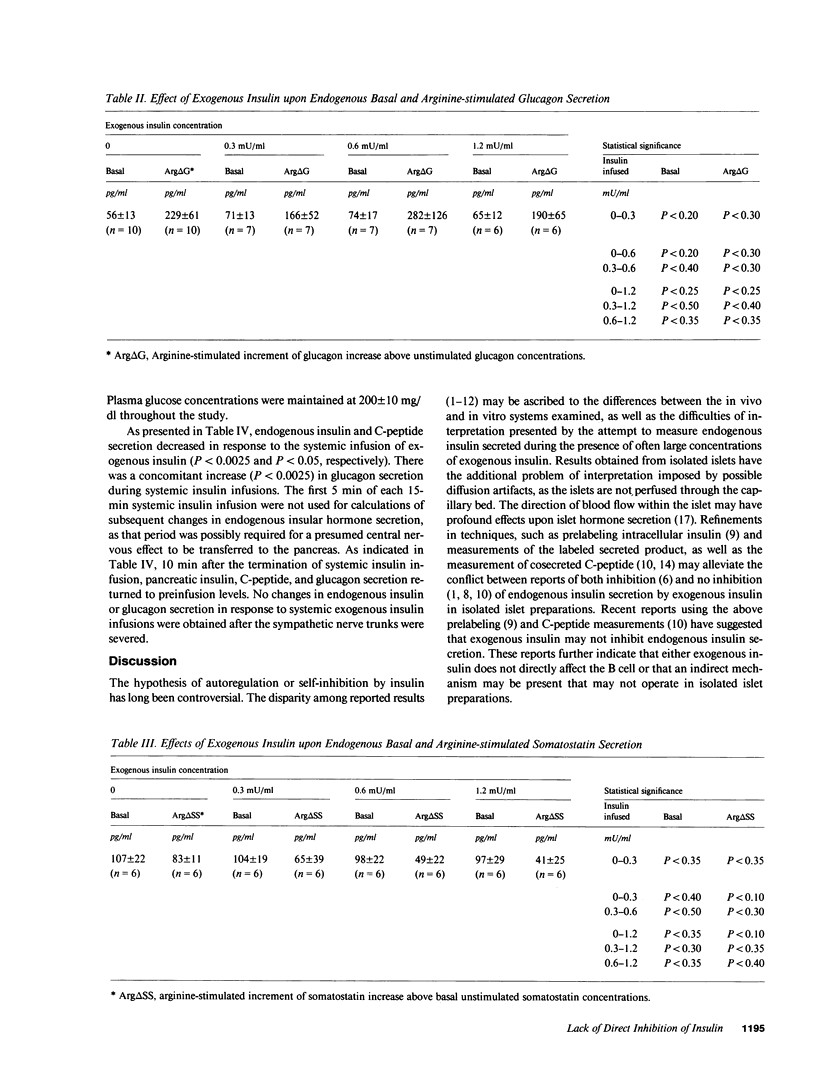
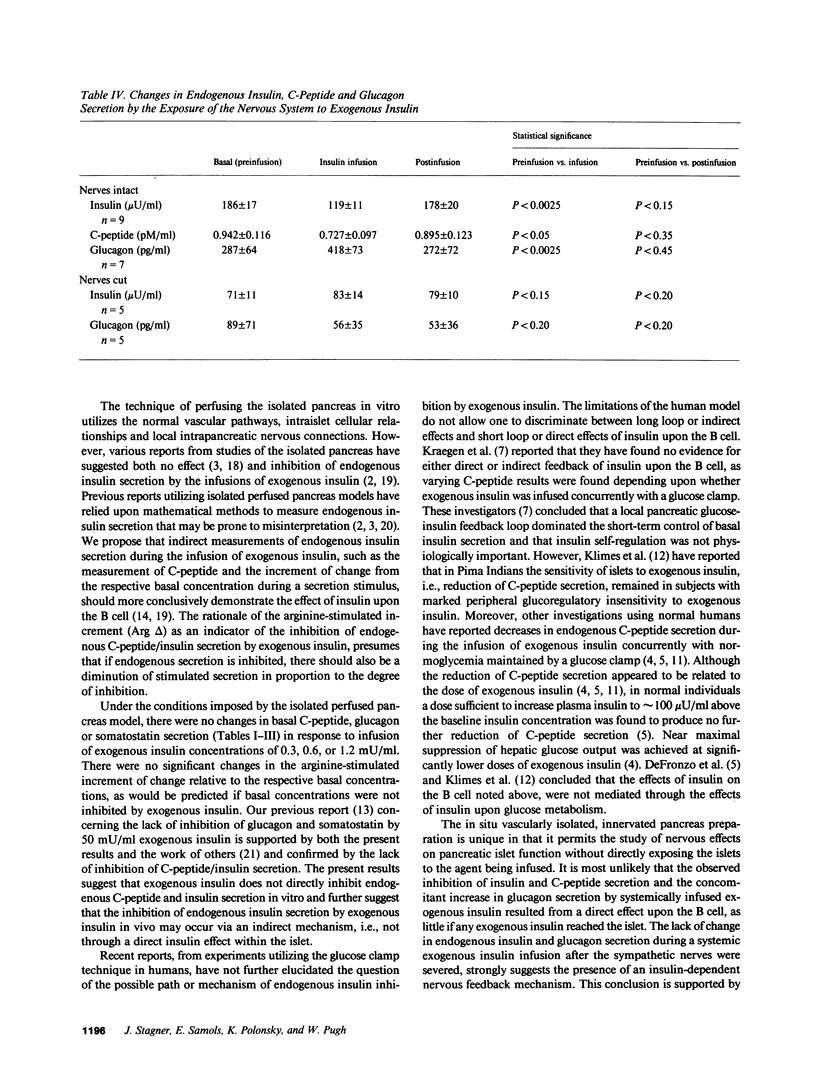


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alteveer R. J., Jaffe M. J., Van Dam J. Hemodynamics and metabolism of the in vivo vascularly isolated canine pancreas. Am J Physiol. 1979 Jun;236(6):E626–E632. doi: 10.1152/ajpendo.1979.236.6.E626. [DOI] [PubMed] [Google Scholar]
- Asplin C. M., Paquette T. L., Palmer J. P. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest. 1981 Jul;68(1):314–318. doi: 10.1172/JCI110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetens D., Vasko M., Unger R. H., Orci L. Ultrastructural detection of granulated cells in the autonomic ganglia of the rat pancreas. Diabetologia. 1985 Nov;28(11):841–846. doi: 10.1007/BF00291075. [DOI] [PubMed] [Google Scholar]
- Baskin D. G., Brewitt B., Davidson D. A., Corp E., Paquette T., Figlewicz D. P., Lewellen T. K., Graham M. K., Woods S. G., Dorsa D. M. Quantitative autoradiographic evidence for insulin receptors in the choroid plexus of the rat brain. Diabetes. 1986 Feb;35(2):246–249. doi: 10.2337/diab.35.2.246. [DOI] [PubMed] [Google Scholar]
- Cane P., Artal R., Bergman R. N. Putative hypothalamic glucoreceptors play no essential role in the response to moderate hypoglycemia. Diabetes. 1986 Mar;35(3):268–277. doi: 10.2337/diab.35.3.268. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Binder C., Wahren J., Felig P., Ferrannini E., Faber O. K. Sensitivity of insulin secretion to feedback inhibition by hyperinsulinaemia. Acta Endocrinol (Copenh) 1981 Sep;98(1):81–86. doi: 10.1530/acta.0.0980081. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Epstein G. H., Fanska R., Karam J. H. Pancreatic action of the sulfonylureas. Fed Proc. 1977 Dec;36(13):2714–2719. [PubMed] [Google Scholar]
- Grodsky G. M., Fanska R., Schmid F. G. Evaluation of the role of exogenous insulin on phasic insulin secretion. Diabetes. 1973 Apr;22(4):256–263. doi: 10.2337/diab.22.4.256. [DOI] [PubMed] [Google Scholar]
- Iversen J., Miles D. W. Evidence for a feedback inhibition of insulin on insulin secretion in the isolated, perfused canine pancreas. Diabetes. 1971 Jan;20(1):1–9. doi: 10.2337/diab.20.1.1. [DOI] [PubMed] [Google Scholar]
- Kawai K., Unger R. H. Inhibition of glucagon secretion by exogenous glucagon in the isolated, perfused dog pancreas. Diabetes. 1982 Jun;31(6 Pt 1):512–515. doi: 10.2337/diab.31.6.512. [DOI] [PubMed] [Google Scholar]
- Klimes I., Nagulesparan M., Vasquez B., Hidaka H., Unger R. H. Normal insulin sensitivity of the islets of Langerhans in obese subjects with resistance to its glucoregulatory actions. Diabetes. 1984 Apr;33(4):305–310. doi: 10.2337/diab.33.4.305. [DOI] [PubMed] [Google Scholar]
- Kraegen E. W., Lazarus L., Campbell L. V. Failure of insulin infusion during euglycemia to influence endogenous basal insulin secretion. Metabolism. 1983 Jun;32(6):622–627. doi: 10.1016/0026-0495(83)90034-3. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Lacy P. E., Wright P. H. Insulin secretion by isolated islets in presence of glucose, insulin and anti-insulin serum. Proc Soc Exp Biol Med. 1967 Feb;124(2):497–500. doi: 10.3181/00379727-124-31773. [DOI] [PubMed] [Google Scholar]
- Marincola F., Frank W., Clark W., Douglas M., Merrell R. The independence of insulin release and ambient insulin in vitro. Diabetes. 1983 Dec;32(12):1162–1167. doi: 10.2337/diab.32.12.1162. [DOI] [PubMed] [Google Scholar]
- Polonsky K., Jaspan J., Pugh W., Cohen D., Schneider M., Schwartz T., Moossa A. R., Tager H., Rubenstein A. H. Metabolism of C-peptide in the dog. In vivo demonstration of the absence of hepatic extraction. J Clin Invest. 1983 Sep;72(3):1114–1123. doi: 10.1172/JCI111036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport A. M., Ohira S., Coddling J. A., Empey G., Kalnins A., Lin B. J., Haist R. E. Effects on insulin output and on pancreatic blood flow of exogenous insulin infusion into an in situ isolated portion of the pancreas. Endocrinology. 1972 Jul;91(1):168–176. doi: 10.1210/endo-91-1-168. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Young J. B., Minaker K. L., Stevens A. L., Pallotta J., Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981 Mar;30(3):219–225. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- Schatz H., Pfeiffer E. F. Release of immunoreactive and radioactively prelabelled endogenous (pro)-insulin from isolated islets of rat pancreas in the presence of exogenous insulin. J Endocrinol. 1977 Aug;74(2):243–249. doi: 10.1677/joe.0.0740243. [DOI] [PubMed] [Google Scholar]
- Smythe G. A., Grunstein H. S., Bradshaw J. E., Nicholson M. V., Compton P. J. Relationships between brain noradrenergic activity and blood glucose. Nature. 1984 Mar 1;308(5954):65–67. doi: 10.1038/308065a0. [DOI] [PubMed] [Google Scholar]
- Sodoyez J. C., Sodoyez-Goffaux F., Foà P. P. Evidence for an insulin-induced inhibition of insulin release by isolated islets of Langerhans. Proc Soc Exp Biol Med. 1969 Feb;130(2):568–571. doi: 10.3181/00379727-130-33608. [DOI] [PubMed] [Google Scholar]
- Stagner J. I., Samols E. Retrograde perfusion as a model for testing the relative effects of glucose versus insulin on the A cell. J Clin Invest. 1986 Mar;77(3):1034–1037. doi: 10.1172/JCI112356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhäusl W. K., Gasić S., Bratusch-Marrain P., Korn A., Nowotny P. Feedback inhibition by biosynthetic human insulin of insulin release in healthy human subjects. Am J Physiol. 1982 Dec;243(6):E476–E482. doi: 10.1152/ajpendo.1982.243.6.E476. [DOI] [PubMed] [Google Scholar]
- Ward W. K., Bolgiano D. C., McKnight B., Halter J. B., Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984 Oct;74(4):1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G. C., Samols E., Loo S., Patel Y. C., Gabbay K. H. Somatostatin and pancreatic polypeptide secretion: effects of glucagon, insulin, and arginine. Diabetes. 1979 Jan;28(1):35–40. [PubMed] [Google Scholar]
- van Houten M., Posner B. I., Kopriwa B. M., Brawer J. R. Insulin binding sites localized to nerve terminals in rat median eminence and arcuate nucleus. Science. 1980 Mar 7;207(4435):1081–1083. doi: 10.1126/science.6986652. [DOI] [PubMed] [Google Scholar]


