Abstract
Background
Observational studies of increasingly better quality and in different settings suggest that planned home birth in many places can be as safe as planned hospital birth and with less intervention and fewer complications. This is an update of a Cochrane review first published in 1998.
Objectives
To assess the effects of planned hospital birth compared with planned home birth in selected low-risk women, assisted by an experienced midwife with collaborative medical back up in case transfer should be necessary.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 March 2012) and contacted editors and authors involved with possible trials.
Selection criteria
Randomised controlled trials comparing planned hospital birth with planned home birth in low-risk women as described in the objectives.
Data collection and analysis
The two review authors as independently as possible assessed trial quality and extracted data. We contacted study authors for additional information.
Main results
Two trials met the inclusion criteria but only one trial involving 11 women provided some outcome data and was included. The evidence from this trial was of moderate quality and too small to allow conclusions to be drawn.
Authors’ conclusions
There is no strong evidence from randomised trials to favour either planned hospital birth or planned home birth for low-risk pregnant women. However, the trials show that women living in areas where they are not well informed about home birth may welcome ethically well-designed trials that would ensure an informed choice. As the quality of evidence in favour of home birth from observational studies seems to be steadily increasing, it might be as important to prepare a regularly updated systematic review including observational studies as described in the Cochrane Handbook for Systematic Reviews of Interventions as to attempt to set up new randomised controlled trials.
Medical Subject Headings (MeSH): *Hospitalization; Delivery Rooms; Delivery, Obstetric [*standards]; Home Childbirth [*standards]; Patient Care Planning [*standards]; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Medicalisation of childbirth is a central feature in Western societies (Johanson 2002).The majority of women living in high- and middle-income countries have given birth in hospitals since the middle of the 20th century. However, there are regions where home birth is considered part of normal practice. The most cited case is The Netherlands where planned home birth is supported by the official healthcare system. Here, planned home birth is considered an appropriate choice for a woman of low risk and approximately 30% of all births take place at home (Hendrix 2009). It is of historical interest to note that the transfer of low-risk births from home to hospital in the 1960s, despite lack of high-quality evidence, was one of the pivotal issues when Archie Cochrane laid out the ideological ground for The Cochrane Collaboration. Cochrane awarded ‘the wooden spoon’ to obstetrics (Cochrane 1989), because “The specialty missed its first opportunity in the sixties, when it failed to randomise the confinement of low-risk pregnant women at home or hospital. Then, having filled the emptying beds by getting nearly all pregnant women into hospital, the obstetricians started to introduce a whole series of expensive innovations into the routines of pre- and postnatal care and delivery, without any rigorous evaluation. The list is long, but the most important were induction, ultrasound, foetal monitoring, and placental function tests” (Cochrane 1979). The relationship between hospitalisation, childbirth, and intervention is still an important issue as “Concern about the iatrogenic effects of obstetric intervention in women who do not have a clinical need for it has put “normal” birth firmly on the agenda for the 21st century.” (EURO-PERISTAT 2008). A range of interventions continue to be used routinely in relation to births at many hospitals despite the fact that for a long time they have been proven to have harmful effects, or only marginal or no beneficial effect (Enkin 1995; Sakala 2008; Thorp 2007), e.g. fetal monitoring (Blix 2005; Gourounti 2007), as mentioned by Archie Cochrane, episiotomy (Graham 2005) and early cord clamping (Hutchon, 2010). Even though the use of a few specific interventions have been reduced (e.g. placental function tests (Neilson 2003), in general “Routine medical interventions have […] increased steadily over time” (Hodnett 2010) despite the efforts of the Cochrane Pregnancy and Childbirth Group, its predecessors (Enkin 1995; Sakala 2008; Thorp 2007), and other researchers carrying out systematic reviews (Blix 2005; Gourounti 2007).
The question which motivated this review was (Olsen 1997b): to what extent is it possible to select a group of low-risk women who might benefit from planning a home birth that is backed up by a collaborative, medical support system in case a transfer should turn out to be necessary, rather than plan a hospital birth initially.
Description of the condition
This review is about healthy pregnant women at term for whom no serious complications have been identified prior to the spontaneous initiation of birth and for which the birth is expected to be medically uncomplicated. Generally, between 70% and 80% of all pregnant women may be considered as low risk at the start of labour (WHO 1996).
Description of the intervention
It is debatable whether planned home birth or planned hospital birth should be considered the experimental intervention. In line with Archie Cochrane we will consider planned hospital birth as the experimental intervention. Hospital practices vary a lot (EURO-PERISTAT 2008) and in many places there is a high intervention rate (Sakala 2008). Home birth practices also vary, but it is a common feature that only very few interventions can be carried out without transfer to hospital. Home birth is not only a birth that takes place in a specific place, it is also as a way to perform birth, as a range of childbirth practices (Mansfield 2008). “[…] the act of giving birth to a child is never simply a physiological act, but rather a performance defined by and enacted within a cultural context” (Jordan 1997; Romalis 1981). Thus both the planned hospital and the planned home birth options cover a broad range of actual practices.
How the intervention might work
Two lines of theoretical reasoning exist that each in their own way explain how planned hospital births for low-risk women may have impacts on the labouring woman and the fetus/new-born. They have been labelled the medical model and the midwifery model of care (Rooks 1999). However, it should not be taken for granted that the midwifery model is practiced by midwives and the medical model by doctors; these models describe differences in childbirth practices but they do not apply only to specific professions. Rooks describes the two models as a sort of stereotypes at each end of a continuum. The models can be used to identify and discuss differences even though most practices in real life fall somewhere in between. The models have one thing in common: Proper and timely identification of upcoming complications and access to interventions are at the heart of good care. Otherwise their focus is different.
The rationale behind the medical model is that a hospital provides a safe environment for a labouring woman, due to the capacity to intervene, without delay, in case of complications (e.g. cord prolapse, abruptio placenta, shoulder dystocia and fetal hearth plummeting for no obvious reason). The quote “Birth can only be defined as normal in retrospect” pinpoints this rationale (WHO 1996). Even though women can labour without the need for medical backup, it is not possible to identify those women or newborns beforehand. It is therefore safest for all women to deliver in a hospital fully equipped with modern technology and professionals who can identify women at risk and intervene in case of emergency. All births, including normal births, are attended to as complicated deliveries. Clinicians believe with justification that babies may be rescued by rapid delivery. In their everyday work with average and unselected births obstetricians fairly often encounter complications and not too rarely they also face complications that carry a high risk of maternal and fetal death or severe morbidity. In such emergency circumstances clinicians are often satisfied with interventions even when they have not been tested in randomised trials (e.g. cesarean section or instrumental delivery). And midwives, if in charge of such a seemingly normal birth where complications develop during labour, will transfer responsibility to the obstetricians for surveillance and medical intervention and subsequently assist the obstetrician under his or her supervision. As emergency complications may also occur among women with low-risk pregnancies - although at a much lower frequency - the rationale behind planned hospital births for all women irrespective of their risk status is that immediate availability of emergency interventions will decrease the frequency of the, admittedly rarer, seriously bad outcomes without introducing too many adverse effects that might outweigh the theoretical advantage. Examples of emergency complications that concern clinicians the most in relation to low-risk births, especially if these takes place outside the hospital, are abruptio placenta, cord prolapse, shoulder dystocia and fetal hearth plummeting for no obvious reason.
Among hospital births, abruptio placenta is the complication that carries the highest risk (adjOR = 50 for perinatal mortality (Sheiner 2003)). However, it often develops slowly over time, can take time to diagnose, and among those planning a home birth and going into labour at term at home, abruptio placenta probably occurs for less than 1 in 10,000 (Janssen 2009) so the outcome of pregnancies complicated by abruptio placenta may not be influenced very much by the planned place for the birth, whether at hospital or at home. The complication with the second highest risk is cord prolapse (adjOR = 15 for perinatal mortality (Sheiner 2003)). Among low-risk women cord prolapse is also a rare event, around 1 in 10,000 (Chamberlain 1997; Horn 2010). However, it can happen spontaneously (i.e. the water breaks with an unengaged fetus) and “is one of the emergency situations that can be fatal in both home and hospital” (Horn 2010). At hospital it is usually treated with cesarean section. If encountered at home the “midwife might keep a hand inside you, holding the baby’s head up and off the cord, while waiting for the ambulance to arrive. She may well remain in this position while the ambulance crew transported you to hospital. An interesting sight for the neighbours - but potentially life-saving for your baby. […] this is one complication where any delay could prove fatal; there is little doubt that hospital is the best place to have a cord prolapse” (Horn 2010).
Thus, with regard to the first two emergency complications, the theoretical reasoning why hospitalisation may have a positive impact on the outcome of low-risk pregnancies is perfectly logical. However, it is also obvious that the potential benefits are only realised for a very tiny fraction of women with low-risk pregnancies. Statisticians have grappled with the proper interpretation of events with an extremely low probability in decision making contexts since the concept of risk and probability emerged during the Renaissance, was formalised during the Enlightenment and matured during the ensuing centuries (Gigerenzer 1989; Hacking 1984). One of the solutions to the problem with extremely low probabilities has been to say that probabilities and utility (or loss) do not exist in a vacuum, they exist in a context and have to be interpreted in that context. Thus, while the above mentioned complications and risks are important to care about among women with high risk (and unselected) pregnancies and are important for their caretakers to be prepared for, compared to other potentially devastating life events, this might not be as true for women with low-risk pregnancies. From a low-risk woman’s once-in-a-lifetime perspective, the risk of encountering such a complication is less than the risk of an average person being killed in a traffic accident during one year (Epidemiology of motor vehicle collisions 2012). For a set of parents, the risk that one of the parents (or the child) will get killed in a traffic accident before school age will be fairly much higher than any theoretical potential increase in the chances of survival related to a possible hospitalisation of the birth. This apparent paradox (or dependence on context) is sometimes translated by statisticians into a saying like “an event with very small probability does not occur” (Daston 1979; Shafer 2006; Swijtink 1986). This is of course not true in a strictly mathematical sense. But in a decision-making context it does make some sort of sense. In a decision-making context, it is not meaningful to focus on events with such a small probability of happening that any modifications of the probability that an intervention might have may easily be overruled by other types of events with a much higher probability. One might say that for high-risk pregnancies the pregnant woman and her obstetrician agree in their assessment of what are the most important rare events to prepare for irrespective of whether they see the complications in a life time perspective or in a hospital department perspective, whereas for women with low-risk pregnancies the assessment of the importance of the extremely rare events deviates from the assessment of the same events in a hospital department perspective.
The complication with the third highest risk is shoulder dystocia (adjOR = 7 for perinatal mortality). It has been called “the nightmare of obstetricians” as it is a serious condition complicating 0.1-2.1% of all pregnancies (Sheiner 2006). In addition to a high perinatal mortality there can also be “morbidity associated with the condition, even when it is managed appropriately. Maternal morbidity is also increased, particularly postpartum haemorrhage (11%) and fourth-degree perineal tears (3.8%). [… However,] their incidences remain unchanged by the manoeuvres required to effect delivery” (RCOG 2005). Most procedures that can be carried out at hospital can be carried out at home as well. Midwives are trained in coping with such emergency situations (ICM 2010; RCOG 2005). In a study reporting on the use of McRoberts’ manoeuvre, rotational methods and posterior arm delivery in a hospital setting “The cumulative success rates after the second and the third manoeuvres were 79.0 and 94.6%, respectively” (Leung 2011). In a study of the all-four manoeuvre an even higher success rate was reported (Bruner 1998) with no mortality and a comparably low morbidity. The all-four manoeuvre is even often more easily carried out in home birth settings than in many hospital settings as neither epidurals nor fetal monitors are used at home (Meenan 1991). However, it is not easy to obtain large, valid and comparable case series but to the extent that the mortality and morbidity rates related to shoulder dystocia seem lower in home birth settings than in hospital settings and as the most common ensuing morbidities are either initially treated in the same way at home as in hospital (postpartum haemorrhage) or can be transferred without emergency (fourth-degree perineal tears) the theoretical reasoning why hospitalisation of women with low-risk pregnancies may have a positive impact on the outcome is less clear.
With regard to the fourth mentioned emergency situation, fetal heart plummeting for no obvious reason, we note that many fetal heart rate abnormalities will resolve with simple conservative measures, such as a change in maternal position (to relieve aortocaval compression and pressure on the umbilical cord) […] and short-term maternal oxygen administration” (Enkin 1995). To the extent that “The most common treatment for […] persistent fetal heart rate abnormality […] is prompt delivery” (Enkin 1995), it is obvious that a policy of hospitalisation will have some impact on women with low-risk pregnancies. However, when the fetal heart rate is continuously electronically monitored during labour, the rate of false alarms may be as high as 99.8% (Alfirevic 2008;Nelson 1996), leading to an increase in caesarean sections and instrumental vaginal births without any reduction in cerebral palsy, infant mortality or other standard measures of neonatal well-being (Alfirevic 2008; Nelson 1996). Thus it is not obvious that the impact of a policy of general hospitalisation is necessarily beneficial even though a very small fraction of all persistent fetal heart rate abnormalities for no obvious reason that do not resolve at home may worsen due to the longer transfer time from diagnosis to intervention. It might be noted that many cases of fetal heart rate abnormality observed by obstetricians on duty will be due to interventions that only take place in hospitals, like oxytocin administration or epidural anaesthesia (Enkin 1995) and are in general observed among women with high-risk pregnancies. As many cases of fetal heart plummeting are observed in correlation with the two first mentioned emergency complications or other known high-risk situations (Sheiner 2003), the cases that are both persistent and appears for no obvious reason among women with low-risk pregnancies are probably extremely rare.
In the medical model, the possible iatrogenic effect of labour interventions is given little attention (Davis-Floyd 2009a). If a hospital birth is planned instead of a home birth, it may initiate a cascade of interventions and negative effects. The very first intervention in labour is leaving home which means that the production of oxytocin may be interrupted and labour slow-down. Upon arrival to the hospital the labour may therefore need to be induced or augmented with syntocinon, either because of the slow-down in the natural progress or because of general hospital based routines or time limits (O’Driscoll 1993). Another option is amniotomy that may increase the risk of cord prolapse especially if the fetus has not engaged in the pelvis. The non-familiar environment and the interventions may make it less inviting to remain mobile, actively to change between relaxation, joking, intense labour, managing contraction, visiting the loo, bending over a table, shouting at the partner, kissing, going to the kitchen, in short: to remain in control (Hunter 2004). It is easier to lie down on the hospital bed, give up and ask for pain relief. Busy hospitals often have many routines; in some parts of the world eating and drinking is prohibited if the woman is hospitalised (Rooks 1989; Singata 2010) and many hospitals enrol all women in a standardised regimen such as active management of labour (O’Driscoll 1993) or electronic fetal monitoring. Whether or not hospitals subscribe to active management of labour, augmentation rates may still be high (Clark 2009). In many countries women of low risk are “part of the care in the whole obstetric department, and thus subject to the same rules and arrangements, with little distinction between high-risk and low-risk” (Hodnett 1989). In concert, these aspects may lead to various complications for mother and child - e.g. giving birth in a less physiologically advantageous position lying down on a bed where the body has to work against gravity, where the baby’s exit is impeded, and where the baby may be going into distress (Horn 2010), and where, finally, more dramatic interventions are needed such as instrumental or operative delivery (Anim-Somuah 2005) which in turn may lead to additional problems for mother and baby. Being in hospital also carries risks e.g. from fetal asphyxia from inappropriate over-use of oxytocin. The need for quietness to establish breastfeeding may also be impeded. In addition to such a cascade of events, mother and baby are also inadvertently exposed to unfamiliar pathogens in the hospital (Horn 2010) that may lead to more or less severe consequences. Finally, in some countries there is a trend towards discharging mother and baby still earlier from still larger and more centralised hospitals concentrating the childbirth experience to a condensed episode of objectification, intervention and pain, delimited by long transportation to and from the hospital rather than an empowering, integrated, adventurous personal experience laying out the ground for the joys and challenges of mother- and parenthood.
In addition to the intended and unintended effects on the outcomes traditionally studied in obstetric research (such as Apgar scores, haemorrhage, etc), the midwifery model adds another set of dimensions to the picture. Rooks emphasizes how good obstetric outcomes are not the only goal for the midwife (Rooks 1999). Midwives also value childbirth as an emotionally, socially, culturally, and often spiritually meaningful life experience (ICM 2012). The woman’s transition into motherhood should be a positive experience and breastfeeding and mothercraft are part of the focus of midwifery (Rooks 1999). These dimensions are not seen as external and additional to the obstetric dimensions; they are an integral part of obstetrics as practiced by midwives. Those who practice in accordance with the midwifery model focus on the normalcy of pregnancy, and its potential for health. Birth is viewed as a natural process that has profound meaning to many people and should be treated as normal until there is evidence of a problem. The possibility of complications is not allowed to pre-empt all other values associated with the woman’s experience of bearing and giving birth to a child. Midwives are experts in protecting, supporting, and enhancing the normal physiology of labour, delivery, and breastfeeding, and establish the pregnant woman as an active partner in her own care and recognize her as the primary actor and decisionmaker. The approach is time-intensive and relationship-intensive. Midwives use their own physical and emotional energy to encourage, support, and comfort women during birth. The midwifery model of care is based on respect for the intricacy of the natural physiology of childbirth and belief that women’s bodies are well designed for birth. Midwives try to protect, support, and avoid interfering with the normal processes; thus they try to avoid unnecessary use of obstetric interventions (Rooks 1999). The hospital environment is both a physical and social territory (Jordan 1993) and thus the labour ward shapes the relationship between the woman and the professionals. The woman is prone to meet many caregivers (up to 16 professionals during a 6 hours delivery has been documented, even though the woman may be left alone most of the time with nothing meaningful to do (Hodnett 1989)). Continuous and supportive presence, reassuring, encouragement and praise can often be helpful for labouring women. A setting that allows for continuity of care facilitates a supportive environment for the woman (Kirkham, 2010) and constitutes her as a woman who can cope with labour. Homebirth literature describes the home as an empowering place whereas hospital rooms can be experienced as unfamiliar and intimidating and emphasize the patient role and risk of birth (Mansfield 2008). These non-obstetric dimensions of pregnancy and birth may well be lost if low-risk births as a matter of routine are hospitalised.
To sum up: The first part of the theoretical reasoning described above has its origin among obstetricians who are usually called for and have a focus on the emergency cases at hospitals. In the medical model women’s bodies are viewed as imperfect at giving birth and the model calls for close monitoring and control of the process. For many years “critiques of the biomedical model of childbirth have served to highlight the shortcoming of present-day maternity services” (Walsh 2002) and even though systematic reviews for many years have shown that many obstetric procedures are overused, structural or social interventions to avoid problems are often underused (Sakala 2008) and little attention is given to these aspects. However, if one does not focus on the extremely rare complications, a very different type of theoretical reasoning can emerge, a reasoning that focuses on why hospitalisation may have a negative impact on the vast majority of women who, by selection and prediction, should not develop any serious complications. Here, the hospital environment is understood as a place that may disturb the labour process and lead to iatrogenic effects. The “writing on an alternative social model is less developed” (Walsh 2002) and only just recently proponents seem to have agreed on a common label: “The midwifery model of care” (Davis-Floyd 2009b).
Why it is important to do this review
It is important that pregnant women and public health planners are able to base decisions on the best available evidence. In the previous version of this review (Olsen 1998), the authors concluded that “There is no strong evidence to favour either planned hospital birth or planned home birth for low-risk pregnant women”. The conclusion was solely based on the very limited evidence from randomised studies. The review (Olsen 1998) was originally motivated by a systematic review of observational studies that showed that “the methodologically best, observational, comparative, original studies investigating the mortality related to planned home and planned hospital births revealed no statistical difference in mortality between planned home and planned hospital birth; the confidence interval was not compatible with extreme excess risks in any of the groups (odds ratio (OR) 0.87, 95% confidence interval (CI) 0.54 to 1.41)” and “that fewer medical interventions occurred in the home birth group” followed by a long list (Olsen 1997a). However, the quality of the observational studies was at that time not particularly strong, and a conclusion formally including evidence from these studies would not have been much more informative than the Cochrane review (Olsen 1998).
Since 1997, the amount of observational evidence has grown tremendously (Wax 2010). and the methods for assessing and including evidence from observational studies in systematic reviews have also improved (Schünemann 2011). However, the Pregnancy and Childbirth group has a policy of reviewing only RCTs (Gates 2010) as it is generally acknowledged that it is a great challenge to summarize observational evidence appropriately (Reeves 2011). The intense critique (Davey 2011; Delamothe 2010; Gyte 2011;Hayden 2011; Horton 2010; Johnson 2011; Keirse 2010; Kirby 2011; Michal 2011; Sandall 2011; Zohar 2011) of the reliability of the most recent systematic review of observational studies on the safety of home birth (Wax 2010) demonstrates these challenges. The persistent critiques of this study (Wax 2010) led to publication of supplemental material (Wax 2011), and also led the publishing editors to conclude that “it is clear that we need more rigorous and better designed research on this […] issue” (Anonymous 2011). We thus only include RCTs in this systematic review. However, we briefly present the findings from the largest observational studies included in the most recent systematic review of observational studies on the safety of home birth (Wax 2010) as their literature search seems not to have been criticized and the largest included studies seem not to have been criticized either. As in the previous versions of this review and in accordance with the policy of the Pregnancy and Childbirth group, evidence from observational studies is only mentioned in this subsection and in the discussion section; it is not included in the Authors’ conclusions. The currently largest observational study by far, including more than half a million births, states that “No significant differences were found between planned home and planned hospital birth (adjusted RRs and 95% CIs) intrapartum death and neonatal death up to seven days 1.00 (0.78 to 1.27)” (de Jonge 2009). The study did not report any specific morbidity outcomes, but the second largest study based on 13,000 births did, and they found that “All measures of serious maternal morbidity were lower in the planned home birth group as were rates for all interventions including cesarean section (5.2% versus 8.1%; RR [95% CI]: 0.64 [0.56, 0.73])” (Hutton 2009). The measures of serious maternal morbidity were typically 10% to 30% lower and the rates for all interventions were typically 20% to 60% lower. Thus several findings from the systematic review (Olsen 1997a), quoted in the previous version of this review (Olsen 1998), were supported by the two largest studies included in the most recent review (de Jonge 2009; Hutton 2009). Neither of these two studies reported on birth trauma to the newborn even though it had previously been observed to be consistently different (Olsen 1995; Olsen 1998) but another large observational study (Janssen 2009), confirmed that birth trauma (e.g. cerebral haemorrhage, fracture of clavicle, long bones or skull, fascial nerve injury or nerve injury effecting movement of a child’s shoulder, arm, and hand) were significantly different and three times as frequent in the hospital birth group. Thus the previous conclusions about “no statistical difference” in perinatal mortality (Olsen 1997a; Olsen 1998) seem to have been strengthened, and the results showing significantly lower morbidity rates related to home birth have become more convincing.
Even in well controlled observational studies, observed differences (or lack of differences) may be due to uncontrolled confounding and bias. Thus, some of these findings may be partly or entirely due to bias. Lack of significant difference in various measures of mortality was not refuted by Cochrane reviews of elements of birth care relevant for home birth settings, such as midwifery-led care (Hatem 2008) alternative birth clinics (Hodnett 2010) and continuity of care (Hodnett 2011) and several of the findings showing lower intervention rates at home (Hutton 2009) were supported by significant differences in the Cochrane reviews, although the differences were not as large (Hatem 2008; Hodnett 2010;Hodnett 2011). Most morbidity measures were not significantly different although a few favoured midwifery care or birth clinics, e.g. breastfeeding (Hatem 2008; Hodnett 2010), and a better fiveminute Apgar score favoured continuous support during child-birth (Hodnett 2011). The largest and most marked difference across the three Cochrane reviews (Hatem 2008; Hodnett 2010;Hodnett 2011), is greater satisfaction with the birth experience in the “experimental” setting. The differences and the prevalences in some of the trials (Hodnett 2009; Waldenström 1993) is of such a magnitude that less than 50 participants in a trial are needed to demonstrate a significant difference. Similarly, for a few of the interventions only 100 to 200 participants are needed (Hodnett 2009; Klein 1984). Thus, if the small feasibility trial (Dowswell 1996) identified in the first version of this review (Olsen 1998) had continued until today and had collected the relevant outcomes, we would probably have had sufficient statistical power to make conclusive statements about women’s satisfaction with home birth compared with hospital birth and also about some of the most common and overused interventions (electronic fetal monitoring, augmentation and episiotomy).
In addition to a healthy child many women have additional wishes for the life-changing experience that childbirth is. In order to capture some of these aspects more than 30 “soft” outcomes were included in addition to more than 30 obstetric outcomes in the Cochranre review “Midwife-led versus other models of care for childbearing women” (Hatem 2008). A qualitative review of recent books (Mansfield 2008) shows that proponents of natural childbirth explicitly express a wish for the birth to be ‘wise’, ‘active’, ‘gentle’ and maybe even an ‘adventure’ resulting in ‘personal growth’ with ‘joys, fears, pleasures and pains’. Outcomes attempting to grasp these issues should be included (so far only pain seems to have been of concern). In another Cochrane review, the authors developed one composite outcome to measure all sorts of very positive views of intrapartum care as measured by trial authors, e.g. involvement in the process of birth, freedom to express feelings, support from midwives, and indicators of involvement in decision-making (Hodnett 2011). As the aims and desires related to home birth (and similar minimalistic, humanistic or natural approaches to birth) are so diverse, the preferable primary outcome should be some sort of composite measure that grasps all of these aspects. We believe that satisfaction with the birth experience (Waldenström 2008) as assessed by the women themselves is better than a technically defined composite measure that might tend to average out what is most important to each individual woman.
This review is being updated because a new trial has been identified (Hendrix 2009) and because methodology and references to the evidence in the introduction needed to be updated. According to the protocol the objective was “to determine if the above results [from observational studies] are reproducible in randomised trials”. Maternal and perinatal mortality are so low in low-risk pregnancies that these outcomes cannot be the primary outcome measures. Instead it is of interest to study any excess rates of interventions, complications and morbidity related to planned hospital birth in order to assess the price paid for a general policy based on the belief that planned hospital birth is always the safest (Olsen 1997b). This is still the aim in this updated review. According to the new version of the Handbook “It is normally expected that the […] conclusions of the review will be based in large part on the effects of the interventions on these outcomes [i.e. the review’s primary outcomes] (Higgins 2011)”. If an equivalence trial were to be set up in order to demonstrate that planned hospital birth is at least as safe as planned home birth for low-risk women assisted by an experienced midwife with collaborative medical back up, trialists would need to determine a minimal clinically important difference in maternal mortality (D’Agostino 2003). Despite the fact that regulatory agencies ask for both ‘clinical judgement’ and ‘statistical reasoning’ in the planning of equivalence trials, methodology experts in equivalence trials have in general never come across examples with such clinical input. Thus minimal clinically meaningful differences are often determined as a 10% or 20% difference in the efficacy measure (D’Agostino 2003). Ten to fifty million women would be needed in such a trial depending on the exact circumstance. In order to demonstrate a doubled risk in maternal mortality “only” one to two million women would be needed. More than 100,000 women would be needed in order to falsify the findings on perinatal mortality from the largest observational studies (de Jonge 2009). Nevertheless, we still include maternal and perinatal mortality among the primary outcomes as these play an important role in discussions and decisions concerning place of birth.
In the future it might be worthwhile to prepare a protocol for a separate review focusing on pregnant women who live in areas of the world where hospitals, even though adequate in numbers, are not distributed “within easy reach of all the women and newborns who need them (SOWMY 2011).” Assessments in more than 50 countries have revealed that a deficit of health facilities offering Basic Emergency Obstetric and Newborn Care (BEmONC) is an even larger problem than lack of hospitals. In areas where both levels of care are available it might be worthwhile to consider if resources are better spend maintaining home births in areas within reach of BEmONCs rather than spending all resources on “concentrating staff, equipment, drugs and supplies in a health facility that is open 24 hours a day, 7 days a week (SOWMY 2011).” However, as no observational studies to our knowledge have been published to motivate and inform such a Cochrane review, we doubt that production of a separate Cochrane review with this focus should have a high priority at the moment.
OBJECTIVES
To compare the effects of planned hospital birth attended by a mid-wife or others with midwifery skills (UNFPA 2012) with planned home birth backed up by a modern hospital system in case a transfer should turn out to be necessary. The primary focus is on women with an uncomplicated pregnancy and low risk of medical intervention during birth; any type of hospital birth without restriction will be included, e.g. whether midwifery-led or not, whether with a paediatric department or not, etc. If any trials are identified that include women with a higher risk, secondary analyses will be done for these.
METHODS
Criteria for considering studies for this review
Types of studies
We considered all attempts to conduct randomised controlled trials.
Types of participants
Pregnant women.
Types of interventions
Planned hospital versus planned home birth.
Types of outcome measures
Outcome measures are not part of the criteria for including studies.
Primary outcomes
Maternal mortality
Perinatal mortality (non malformed)
Birth trauma (e.g. cerebral haemorrhage, fracture of clavicle, long bones or skull, fascial nerve injury or nerve injury effecting movement of a child’s shoulder, arm, and hand)
Apgar < 7 at 5 min
Resuscitation
Early cord clamping
Jaundice
Other neonatal morbidity
Transfer to neonatal intensive care unit
Baby not breast fed
Assisted vaginal birth
Caesarean section
Haemorrhage
Perineal trauma
Other maternal morbidity
Epidural
Other (non-epidural) medical pain relief
Non-medical pain relief
Medical augmentation
Episiotomy
Maternal satisfaction
Secondary and non-prespecified outcomes
As low-risk birth is not a disease, as both home and hospital birth can have many diverse physiological and psychosocial effects and side effects (both short- and long-term), and as the aim of the review is to assess the “price paid” for a general policy based on the belief that planned hospital birth is always the safest for all women including low-risk women, it is of interest to include all outcomes recorded in the included trials. Thus we have not listed any specific outcomes as secondary.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (30 March 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For this update we re-assessed the previously included trial that had been awaiting additional data and the newly identified trial, using the following methods. Selection and assessment was carried out as independently as possible but it was a great challenge to be fully independent as most of the necessary information had to be requested from the trialists over several e-mail exchanges.
Selection of studies
The two review authors, as independently as possible, selected the trials to be included in the review. No disagreements occurred but they would have been resolved through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, the two review authors extracted the data using the agreed form. Disagreements were resolved by discussion. When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details. We entered these data into Review Manager software (RevMan 2011) and checked for accuracy.
Assessment of risk of bias in included studies
The two review authors, as independently as possible, assessed risk of bias for each study using the revised Cochrane Pregnancy and Childbbirth Group (CPCG) template data extraction form, supplemented with the CPCG methods standard text (Gates 2010) and the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreement was resolved by discussion or by requesting additional information from the trialists. The assessments in the next update ought to be repeated with a possibly further revised data extraction form.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non-random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence, and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear.
Blinding (checking for possible performance and detection bias)
Women and care providers cannot be blinded to place of birth and assessors can only be blinded in relation to assessment of a small set of outcomes, i.e. outcomes not recorded in direct relation to the birth but followed up for all women (e.g. follow-up for serious perineal trauma). Thus, we did not assess blinding specifically for any outcomes nor for any separate classes of outcomes. Rather, we have substituted the item ‘Blinding’ in the ‘Risk of bias’ table with two non-standard items ‘Standard of performance’ and ‘Integrity of responders’ which we find of greater importance for the overall assessment of bias in trials included in our review.
(3) Standards of performance (performance bias)
As described in the background section, hospital practices vary a lot and actual home birth practices vary, too. One might say that the difference between home and hospital birth lies as much in the performance as in the different geographical locations. Thus to some extent it is meaningless to check for performance bias in the usual way. Instead we described for each included study any performance bias due to poor clinical standards, including skills of the personnel, communication and collaboration between them, when it is needed, in one or both intervention groups. We noted if all cases of perinatal death (and Apgar < seven at five min) were described and that the descriptions did not indicate substandard clinical practice). We also noted any changes in trial design (e.g. in inclusion criteria due to poor recruitment).
We assessed the standards of performance as:
adequate (no indications of substandard clinical practice were apparent)
inadequate (otherwise) or,
unclear.
(4) ‘Integrity of responders’ (checking for possible detection bias)
As one of the primary outcomes in this review is the woman’s overall satisfaction with the birth experience, and as the interaction between woman and birth care provider(s) contribute to this satisfaction, we considered this outcome as adequately collected if it was collected without interaction between birth providers and woman (e.g. interview by a researcher unrelated to caregivers involved in the trial or collected through questionnaires collected and coded by a similarly unrelated researcher or institution with an explicit guarantee to the women that the answers would not be disclosed to care providers and only used for statistical purposes). All outcomes measuring interventions may unavoidably be influenced by performance bias as the decision to apply and subsequently document the interventions rely on the caregivers. We described for each included study the methods used, if any, to minimise the influence of personnel on the women’s own scoring of satisfaction. We assessed the method as:
low risk of bias (if the woman’s overall satisfaction was collected independently, e.g. as described above);
high risk of bias (otherwise) or;
unclear.
(5) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. To be included in the review, data on a given outcome had to be available for at least 80% of those who were originally randomised. For outcomes collected post hospital discharge, we recognise that follow-up can be difficult. Therefore, we included data if the response rate was higher than 75% and there was no obvious imbalance in groups. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re-include missing data in the analyses. We assessed methods as:
low risk of bias (If all patients completed the study and there were no losses to follow-up, no treatment withdrawals, no trial group changes and no major adverse events or in case of only minor deviations from this, e.g. if randomised participants were subsequently found not to have been eligible for the trial, as long as the discovery of ineligibility could not have been affected by the randomised intervention and if the decisions were not made blinded to assignment then the frequency (or risk) of the outcome has to be high compared to the frequency of missing; see Handbook 8.13);
high risk of bias (otherwise);
unclear.
(6) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre-specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(7) Other sources of bias
We planned to describe for each included study any important concerns we had about other possible sources of bias, including, for example, whether the trial was stopped early due to a datadependent process, there was evidence of extreme baseline imbalance, or there had been claims of fraud or misconduct. We assessed whether each study was free of other problems that could put it at risk of bias:
yes, other sources of bias;
no other sources of bias identified;
unclear.
(8) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias along the guidelines in the Handbook (Higgins 2011). With reference to (1) to (7) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings.
Measures of treatment effect
Dichotomous data
One of our primary outcomes is satisfaction, a variable that in some studies is measured as ‘satisfaction’ (Waldenstrom 2000), in other studies as ‘dissatisfaction’ (Hodnett 2010). A similar ‘reversal of scale’ may be seen in other outcomes with a high incidence (e.g. perineal trauma versus intact perineum). As the odds ratio is invariant and equally valid in such cases irrespective of direction, and because we had many sparse data, we presented results as summary Peto odds ratios with 95% confidence intervals.
Continuous data
None among the pre-specified outcomes and none encountered.
Unit of analysis issues
Cluster-randomised trials
Had we found cluster-randomised trials, we would have included them in the analyses along with individually-randomised trials. Our plan was as follows: we would have adjusted their sample sizes using the methods described in the Handbook (Section 16.3.4 or 16.3.6) using an estimate of the intra cluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. In future updates, if we identify both cluster-randomised trials and individually-randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta-analysis.
Dealing with missing data
For the included study, we noted levels of attrition. We included data for a given outcome which occurred prior to hospital discharge only if the data were available for at least 80% of those originally randomised. For outcomes collected post-hospital discharge, we included data if the response rate was higher than 75% and there was no obvious imbalance in groups.
For all outcomes we have carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity and reporting biases, data synthesis, subgroup analysis and investigation of heterogeneity, and sensitivity analysis
When more than two trials contribute informative data to this review, we plan to follow standard advice relevant for these issues as published in the then current Cochrane Handbook for Systematic Reviews of Interventions.
RESULTS
Description of studies
Results of the search
Six references were retrieved by the electronic searches for the first version of this review (Olsen 1998). In 2009 an additional reference (Hendrix 2009b) was identified and characterised as relevant to be mentioned in the introduction or discussion section; on closer investigation it was clarified that it referred to a not previously identified trial (Olsen 2010). Thus, two studies met the eligibility criteria for the review. One (Hendrix 2009) is awaiting classification pending further information (See Characteristics of studies awaiting classification), and the other trial (Dowswell 1996) has been fully assessed and included (See Characteristics of included studies).
Included studies
The included study (Dowswell 1996) was conducted in the United Kingdom and recruited multiparous women judged to be at low obstetric risk by a consultant obstetrician (n = 71) and likely to have suitable home support and home circumstances (n = 11). Recruitment was carried out by one consultant obstetrician in an area where planned home birth was otherwise uncommon (0.5% to 1%). The midwives assisting the home births were community midwives who spent a few days each month in hospital; all UK midwives are trained to do home births, but the ones in the trial were probably not experienced with home birth. The hospital births were standard hospital care with intermittent auscultation at a university hospital with consultant obstetrician on call (but not called routinely) and full neonatal facilities. One midwife served one to two women in single rooms, she used intermittent ausculation and was not continuously present.
Studies awaiting classification
Hendrix 2009 was conducted in the Netherlands and recruited nulliparous women of low obstetric risk (n = 1). In this trial, 35 midwives in 14 primary care midwifery practices were involved in recruiting pregnant women in different parts of the Netherlands where 30% of all births are home births (Hendrix 2009).
Excluded studies
Five papers were identified via the search of CENTRAL/CCTR with the MeSH term Home Childbirth. Four papers were not randomised controlled trials or clinical controlled trials (Bateman 1994; Berghs 1995; O’Connor 1986; Truffert 1998), and the fifth paper (MacVicar 1993) compared ‘Simulated home delivery in hospital’ with hospital birth and thus did not fulfil the criteria to be labelled with the MeSH term Home Childbirth nor did it fulfil the criteria to be included in this review.
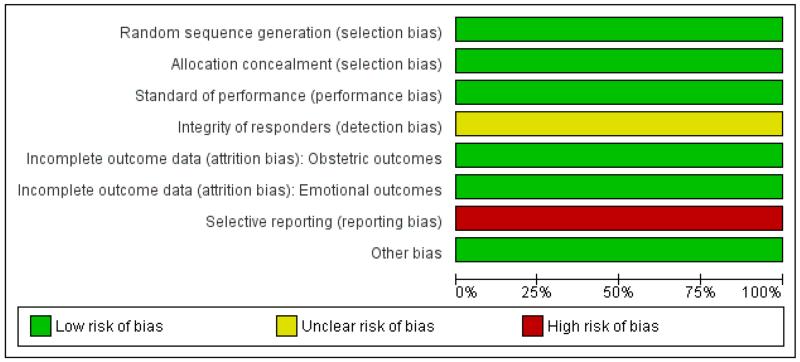
Risk of bias in included studies
The assessed trial was generally of high quality (Figure 1; Figure 2), the only potential problem is that not all outcomes were reported and data seem to have been lost. We consider the outcomes selected for reporting a reasonable choice as it was a feasibility trial that was not published as a full report. We do not consider this to have any appreciable impact on the findings.
Figure 1. ‘Risk of bias’ graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 2. ‘Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
Effects of interventions
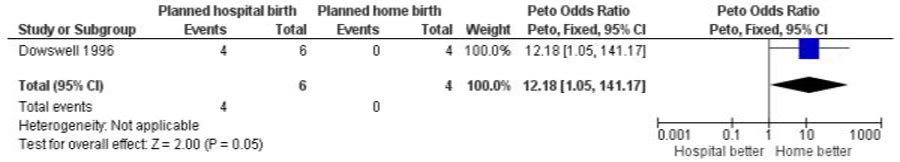
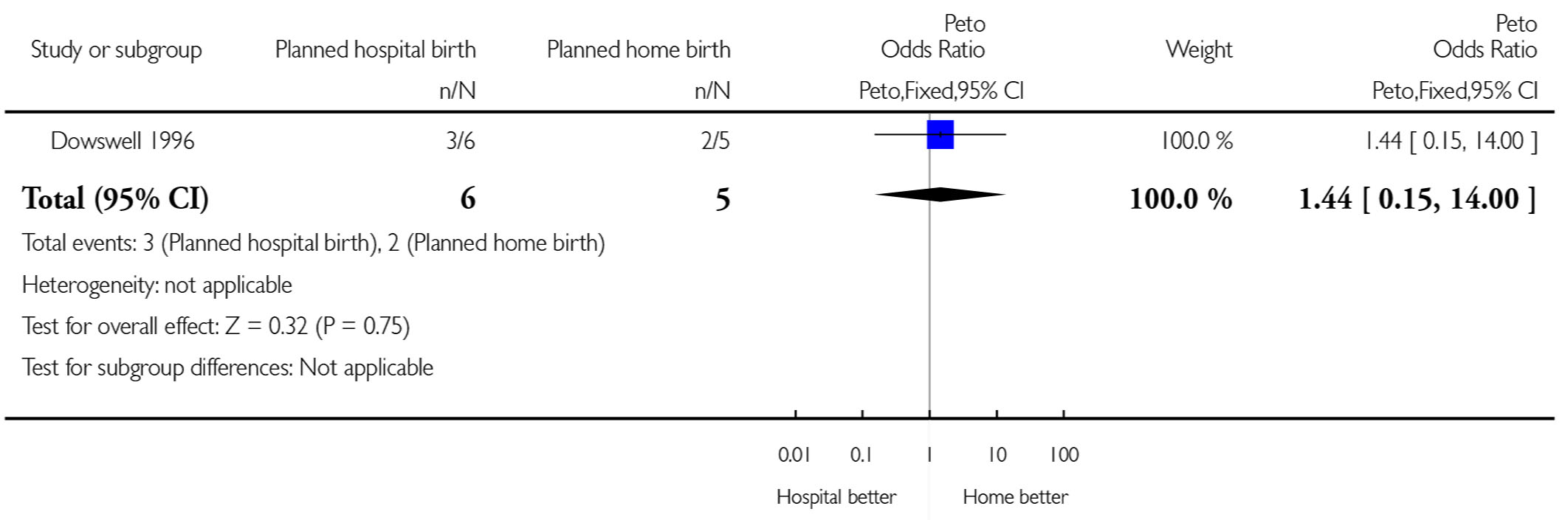
The fully assessed trial with reported outcomes was too small to draw reliable conclusions. Four of the primary outcomes in this review were available for inclusion: baby not breast fed (Analysis 1.1), assisted vaginal birth (Analysis 1.2), caesarean section (Analysis 1.3), and other (non-epidural) medical pain relief (Analysis 1.4). In addition, three other outcomes were reported and these are also included here: perineal sutures (Analysis 1.5), mother disappointed about allocation (Analysis 1.6), and father did not state that he was relieved (Analysis 1.7). One difference seems statistically significant (Figure 3): the majority of mothers in the hospital group were disappointed about the allocation while none of the mothers in the home birth group were disappointed (Peto odds ratio 12.18, 95% confidence interval 1.05 to 141.17); however, the difference is non-significant using a Fisher’s exact test P value = 0.07).
Figure 3. Planned hospital birth versus planned home birth, outcome: 1.6 Mother disappointed about allocation.
DISCUSSION
Summary of main results
The included study was of high methodological quality, except for the small size. It is impossible to balance important benefits against important harms as described in the Background as long as only evidence from randomised studies are acceptable (Gates 2010). Another study is awaiting assessment (Hendrix 2009).
Overall completeness and applicability of evidence
The two identified studies cannot sufficiently address the objectives of this review. The most important finding is that it is possible to randomise women to either home or hospital birth in areas where home birth is available as part of the general maternity care but at the same time is not a commonly known option. On the other hand, it seems impossible to randomise women in areas where women already are well informed about the possibility of home birth (Olsen 2011). Considering that the observational evidence in favour of planned home birth has strengthened since the feasibility trial (Dowswell 1996), it might be worthwhile setting up trials with relevant and fully reported outcomes in areas where home birth is available, but not a commonly known option.
Quality of the evidence
Two trials with a total 12 participants were identified but only one was included and was generally of high quality (n = 11) and provided some useful outcome data. The key methodological limitation of the study was the small size. Thus, the overall quality of evidence from the randomised trial is weak and it should formally be downgraded from high to moderate due to the wide confidence intervals (Schünemann 2011). It may be noted that one of the outcomes in observational studies (birth trauma to the newborn) seems consistently to show a ‘large magnitude of effect’ which might formally increase the quality of this part of the observational evidence from low to moderate (Schünemann 2011). However, as the policy of the Cochrane Pregnancy and Childbirth Group is to include evidence only from randomised trials, this observation cannot be part of the conclusions of this review.
Potential biases in the review process
Inclusion of only randomised trials of a fair quality prevents bias towards strong but misleading conclusions. However, the restriction excludes a large body of evidence from observational studies of a fairly high quality, potentially introducing the misleading impression that almost nothing is known about the potential effects of planned hospital versus home birth (Berghella 2008). In general, over-reliance on only randomised controlled trials should be avoided (Jadad 2007) and this review may provide a good example of a field where evidence from good observational studies are a sine qua non.
Unfortunately, the additional information about the UK trial that was requested back in 1997 now seems to have been lost. Additional details about the Dutch trial have been received and further details have been requested. We did not judge the loss of data to have introduced bias.
Agreements and disagreements with other studies or reviews
The weak evidence from two, not fully reported, randomised trials, one an included study (Dowswell 1996), the other awaiting classification (Hendrix 2009), does not contradict the evidence from the largest observational studies (de Jonge 2009; Hutton 2009; Janssen 2009) identified in the most recent systematic review (Wax 2010), nor does it contradict the results of Cochrane reviews of elements of care typical to home birth (Hatem 2008;Hodnett 2010; Hodnett 2011).
AUTHORS’ CONCLUSIONS
Implications for practice
This review shows that there is no strong evidence to favour either planned hospital or planned home birth for selected, low-risk pregnant women. From an autonomy-based ethical perspective the only justification for practices that restrict a woman’s autonomy and her freedom of choice, would be clear evidence that these restrictive practices do more good than harm (Enkin 1995), as we stated in the previous version of this review (Olsen 1998). A decade later, the European Court of Human Rights in Strasbourg handed down a judgment stating that “the right to respect for private life includes the right to choose the circumstances of birth”. Thus, no matter what the level of evidence is, European governments are not allowed to impose, e.g. “fines on midwives assisting at home births” as it “constitutes an interference in the exercise of the rights … of pregnant mothers” (Registrar 2010). On the other hand, the ethical concept of the fetus as a patient (Chervenak 1992) may lead some to state that “Obstetricians have an ethical obligation to disclose the increased risks of perinatal and neonatal mortality and morbidity from planned home birth in the context of American healthcare and should recommend against it” (Chervenak 2011) and that “In clinical practice it involves recommending … aggressive management (interventions such as fetal surveillance, tocolysis, Caesarean delivery)” (Chervenak 1992). In this ethical perspective recommendations about interventions are acceptable even when they are not supported by randomised controlled trial (RCT) data. The lack of strong evidence from RCTs and an autonomy-based ethical perspective lead to the conclusion that all countries should consider establishing home birth services with collaborative medical back up and offer low-risk pregnant women information about the available evidence and the possible choices.
Implications for research
The UK trial (Dowswell 1996) showed that randomising women to home or hospital delivery is possible (11/71 = 15 % of the invited women accepted to be randomised) whereas the Dutch trial (Hendrix 2009) showed that in places where home birth is part of standard care and where women are already well informed about the two options, women do not wish to be randomised.
Evidence from observational studies suggests that planned home birth is safe and may lead to fewer interventions, fewer complications and fewer neonatal problems. If maternal or perinatal mortality is of prime concern, extremely large trials are required to answer the question with sufficient power; this will hardly ever be achievable. Considering the ongoing and hot debate about the quality of observational studies and systematic reviews of observational studies about home versus hospital birth in relation to perinatal mortality (Delamothe 2010; Hayden 2011; Horton 2010;Keirse 2010; Michal 2011) the best way forward would probably be to conduct a systematic review based on a published protocol in which all steps are prespecified, most importantly inclusion criteria with regard to study design (e.g. prospective studies), population (e.g. various definitions of low risk), type of intervention (e.g. planned home birth backed up by a collaborative medical system) and relevant outcome measures (e.g. perinatal mortality among non-malformed babies).
Clinicians who are uncomfortable with the quality of the observational or indirect evidence and the lack of direct evidence from randomised trials relating to satisfaction, intervention rates, and morbidity may consider setting up or getting involved in trials. With proper choice of outcome measures, and as the number of women randomised (or practitioners in cluster-randomised trials) increases, trials would soon (with a few hundred women) have sufficient power to clarify if the differences seen in observational studies are solely due to selection bias or if planned hospital births really do increase intervention rates and, with larger trials (from a few thousand women), if it also increases morbidity. Conclusive evidence from randomised trials may be reached even quicker (less than one hundred randomised women) in relation to satisfaction (Waldenström 2008) if the difference is as noticeable as seen in randomised trials of elements of care present in home births (Hatem 2008; Hodnett 2010; Hodnett 2011). Women living in areas where they are not well informed about the two options might actually welcome ethically well-designed trials that would ensure all women better information about the available evidence in relation to choice of place of birth (Olsen 2011).
It is well known that it is a great challenge to conclude and act appropriately when “there is inconclusive evidence” (Schünemann 2011), and that the most difficult thing seems to be to say ‘I do not know’ (Chalmers 1983). As long as the available evidence is considered too weak for strong advice and the hot debate continues, it might be worthwhile to use qualitative methods to investigate how clinicians who advice women about place of birth think about home birth in relation to the available evidence (Thorp 2007). Futhermore it could be worthwhile to study the information and advice offered by clinicians to pregnant women with regard to choice of place of birth, and the way such information is offered and received. The role of the media may also be worth studying in a systematic way (Sweet 2010).
It is not obvious that randomised trials are the most needed type of study. It might be worthwhile for the Cochrane Pregnancy and Childbirth Group to consider whether some evidence from observational studies could successfully be included in some reviews under their control (e.g. reviews including only low-risk pregnant women and only for very rare outcomes) on the condition that the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions are followed. As long as low-risk women giving birth at hospital are exposed to overuse of interventions with potentially harmful effects (EURO-PERISTAT 2008; Sakala 2008), all or parts of the above research is needed. As values are so different among and between women, clinicians, scientists, and policy makers, it is difficult to prioritise between the research approaches. They are probably best undertaken in tandem.
PLAIN LANGUAGE SUMMARY.
Benefits and harms of planned hospital birth compared with planned home birth for low-risk pregnant women
Most pregnancies among healthy women are normal, and most births could take place without unnecessary medical intervention. However, it is not possible to predict with certainty that absolutely no complications will occur in the course of a birth. Thus, in many countries it is believed that the safest option for all women is to give birth at hospital. In a few countries it is believed that as long as the woman is followed during pregnancy and assisted by a midwife during birth, transfer between home and hospital, if needed, is uncomplicated. In these countries home birth is an integrated part of maternity care. It seems increasingly clear that impatience and easy access to many medical procedures at hospital may lead to increased levels of intervention which in turn may lead to new interventions and finally to unnecessary complications. In a planned home birth assisted by an experienced midwife with collaborative medical back up in case transfer should be necessary these drawbacks are avoided while the benefit of access to medical intervention when needed is maintained. Increasingly better observational studies suggest that planned hospital birth is not any safer than planned home birth assisted by an experienced midwife with collaborative medical back up, but may lead to more interventions and more complications. However, there is no strong evidence from randomised trials to favour either planned hospital birth or planned home birth for low-risk pregnant women. Only two very small randomised trials have been performed. Only one trial (involving 11 women) contributed data to the review. They did not allow conclusions to be drawn except that women living in areas where they are not well informed about home birth may welcome ethically well-designed trials that would ensure an informed choice.
ACKNOWLEDGEMENTS
We are very grateful to the investigators who provided additional information: J Thornton and M Hendrix. We also thank D Jewell, co-author of the first version of the review; H:S Rigshospitalet, Copenhagen, Denmark for providing internal support for the first version; and The Research Unit for General Practice and Section of General Practice, Department of Public Health, University of Copenhagen, Copenhagen K, Denmark for providing internal support for the current version.
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
H:S Rigshospitalet, Copenhagen, Denmark.
External sources
National Institute for Health Research, UK.
NIHR Programme of centrally-managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation was in the ratio 1:1 in balanced blocks of 8 and performed by opening the next in a series of numbered opaque sealed envelopes containing the trial allocation. No blinding. 1 woman allocated to delivery at home was excluded after randomisation because she was found to have had a previous postpartum haemorrhage. Intention-to-treat analysis of obstetric outcomes; in the analysis of questions regarding satisfaction, the excluded woman was not included | |
| Participants | 11 multiparous women (5 experimental and 6 control) judged to be at low obstetric risk by a consultant obstetrician and likely to have suitable home support and home circumstances | |
| Interventions | Planned delivery at home or in hospital. (More detailed unpublished data were sought in 1997 for first version of the review, in 1998 for the next update and again in 2010 and 2011 for this update. The information has now been supplied by the consultant obstetrician according to his memory.) The midwives assisting the home births were community midwives who spent a few days each month in hospital but were probably not experienced with home birth. All UK midwives are trained to do home births and legally bound to assist a woman who asks for one. However, in practice some feel much more secure than others. So for planned home births midwives tend to self-select enthusiasts. Home birth was generally available in the area to those who asked for it, but not routinely offered. The home birth rate in Leeds at that time was between 0.5% and 1%. The home birth midwives were different from the midwives taking care of the hospital births Hospital birth was normal hospital care with intermittent auscultation, at a university hospital with consultant obstetrician on call (but not called routinely when the childwas born) and full neonatal facilities. 1 midwife who was not continuously present served 12 women in single rooms |
|
| Outcomes | Operative delivery, perineal sutures, nitrous oxide and oxygen, pethidine, baby not breast fed, mother disappointed about allocation, father did not state that he was relieved. (Unpublished data were sought in 1997 for first version of the review, in 1998 for the next update and again in 2010 and 2011 for this update; however, the trialists now consider data to be lost.) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
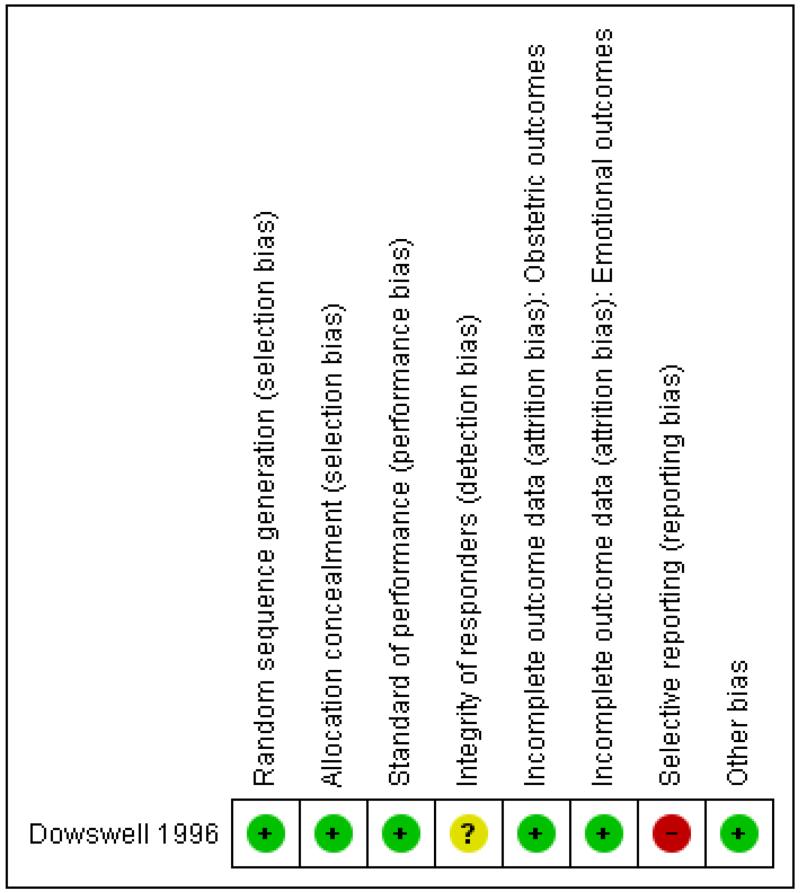
| Random sequence generation (selection bias) | Low risk | “I’m sure it was by computer. Andy Vail generated the sequence for us”. Mail reply from J Thornton, 7 July 2011 |
| Allocation concealment (selection bias) | Low risk | “Randomization was in the ratio 1:1 in balanced blocks of eight and performed by opening the next in a series of numbered opaque sealed envelopes containing the trial allocation/4 |
| Standard of performance (performance bias) | Low risk | No substandard clinical practices suspected. |
| Integrity of responders (detection bias) | Unclear risk | Not applicable (satisfaction with birth experience not reported or lost) |
| Incomplete outcome data (attrition bias) Obstetric outcomes |
Low risk | “intention-to-treat analysis; Table 1” Comment: this is correct. |
| Incomplete outcome data (attrition bias) Emotional outcomes |
Low risk | Data not reported for the excluded participant (9% data loss), and data lost according to trialists Comment: exclusion justifiable (Handbook 8.13.1). |
| Selective reporting (reporting bias) | High risk | Complications recorded according to the text but not reported in table. Data presumably lost according to trialist (mail reply from T Dowswell, Dec 21, 2010) Comment: the reported outcomes are fairly standard and seem to be a fair choice of outcomes considering it is a feasibility trial not published as a full report. So we do not suspect selective reporting though we cannot be sure |
| Other bias | Low risk | No reasons to suspect other types of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Bateman 1994 | This is a retrospective study of unplanned and unattended home births. It is not a trial |
| Berghs 1995 | This is an observational study, not a trial. |
| MacVicar 1993 | The trial is not studying true home birth but ’Simulated home delivery in hospital’ |
| O’Connor 1986 | The trial is not studying home birth but vitamin K1. |
| Truffert 1998 | This is an observational study and not a trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | “one woman had given informed consent for randomisation.” The 1 randomised woman was allocated to the home birth group. Randomised by sealed envelopes The unit of randomisation was not explicitly described, and the time and place for the randomisation was unclear (further information is being sought) |
| Participants | “The trial was conducted in different parts of the Netherlands. Thirty-five midwives in 14 primary care midwifery practices participated in the trial by recruiting pregnant women. The midwives gave information about the trial during the first prenatal visit, usually between 8 and 10 weeks of pregnancy. Only nulliparous women were eligible to participate. Inclusion was possible up till the 18 week of pregnancy.” No exclusion criteria were stated (further information is being sought) |
| Interventions | “Birth at home or at a hospital, in both cases assisted by an(!) registered independent midwife.” |
| Outcomes | Primary outcomes were:
|
| Notes | The quotes are from the paper; the additional information was obtained through mail communication. Further clarification and unpublished data are being sought |
DATA AND ANALYSES
Comparison 1. Planned hospital birth versus planned home birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Baby not breastfed | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.84 [0.15, 23.38] |
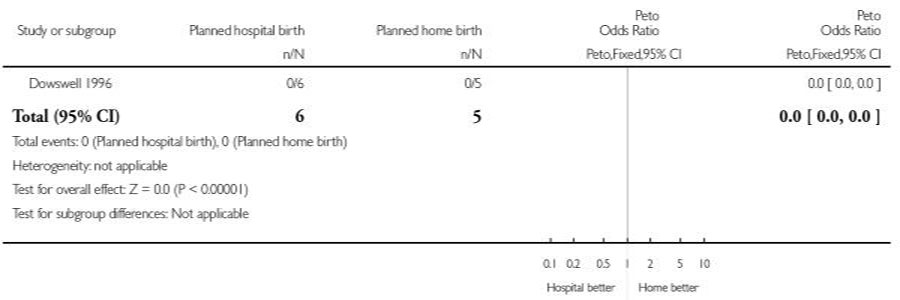
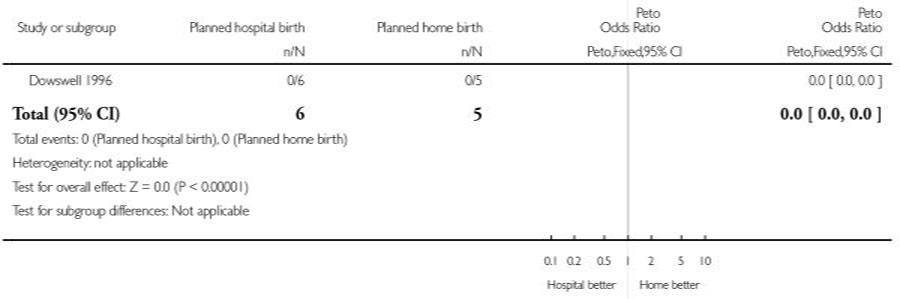
| 2 Assisted vaginal birth | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
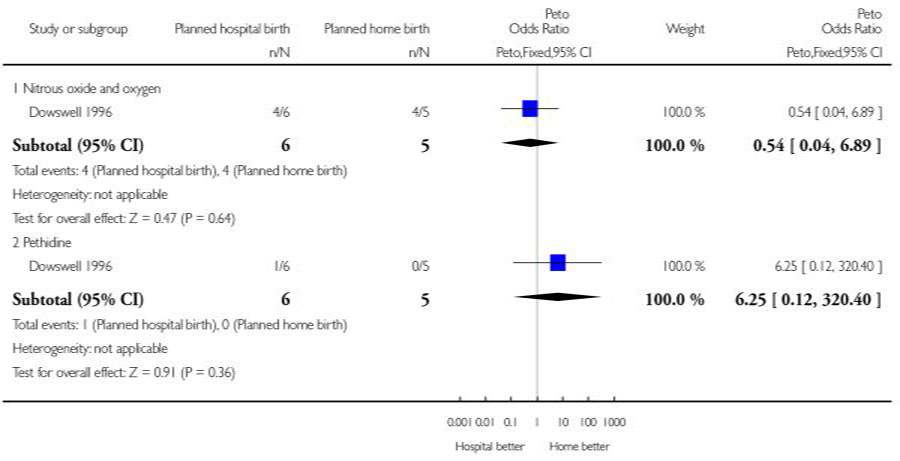
| 4 Other (non-epidural) medical pain relief | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nitrous oxide and oxygen | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.04, 6.89] |
| 4.2 Pethidine | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.25 [0.12, 320.40] |
| 5 Perineal sutures | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.15, 14.00] |
| 6 Mother disappointed about allocation | 1 | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 12.18 [1.05, 141.17] |
| 7 Father did not state that he was relieved | 1 | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.00, 10.32] |
Analysis 1.1. Comparison 1 Planned hospital birth versus planned home birth, Outcome 1 Baby not breastfed.
Review: Planned hospital birth versus planned home birth
Comparison: 1 Planned hospital birth versus planned home birth
Outcome: 1 Baby not breastfed
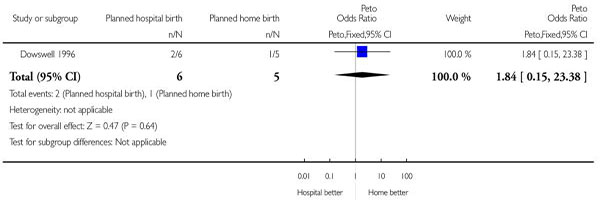 |
Analysis 1.2. Comparison 1 Planned hospital birth versus planned home birth, Outcome 2 Assisted vaginal birth.
Review: Planned hospital birth versus planned home birth
Comparison: 1 Planned hospital birth versus planned home birth
Outcome: 2 Assisted vaginal birth
 |
Analysis 1.3. Comparison 1 Planned hospital birth versus planned home birth, Outcome 3 Caesarean section.
Review: Planned hospital birth versus planned home birth
Comparison: 1 Planned hospital birth versus planned home birth
Outcome: 3 Caesarean section
 |
Analysis 1.4. Comparison 1 Planned hospital birth versus planned home birth, Outcome 4 Other (non-epidural) medical pain relief.
Review: Planned hospital birth versus planned home birth
Comparison: 1 Planned hospital birth versus planned home birth
Outcome: 4 Other (non-epidural) medical pain relief
 |
Analysis 1.5. Comparison 1 Planned hospital birth versus planned home birth, Outcome 5 Perineal sutures.
Review: Planned hospital birth versus planned home birth
Comparison: 1 Planned hospital birth versus planned home birth
Outcome: 5 Perineal sutures
 |
Analysis 1.6. Comparison 1 Planned hospital birth versus planned home birth, Outcome 6 Mother disappointed about allocation.
Review: Planned hospital birth versus planned home birth
Comparison: 1 Planned hospital birth versus planned home birth
Outcome: 6 Mother disappointed about allocation
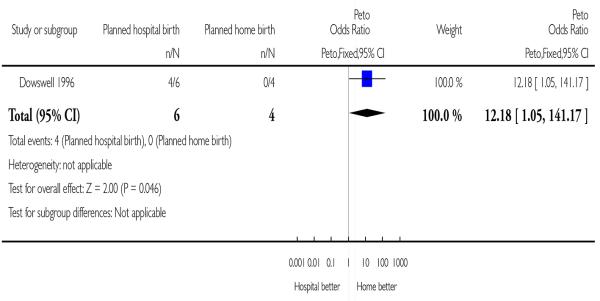 |
Analysis 1.7. Comparison 1 Planned hospital birth versus planned home birth, Outcome 7 Father did not state that he was relieved.
Review: Planned hospital birth versus planned home birth
Comparison: 1 Planned hospital birth versus planned home birth
Outcome: 7 Father did not state that he was relieved
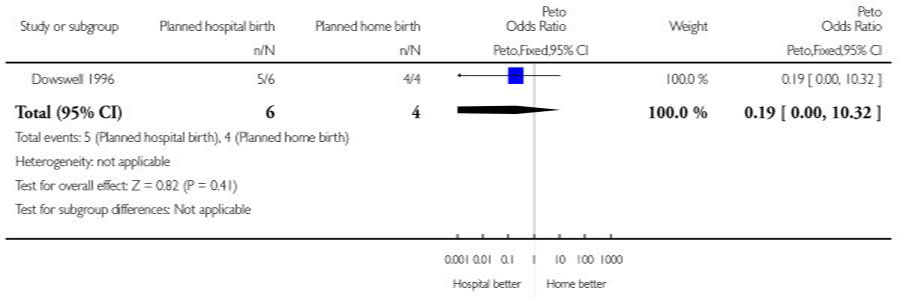 |
FEEDBACK
Feeback from Robin Kidder, 27 August 2013
Summary
The review text states: “Two trials met the inclusion criteria but only one trial involving 11 women provided some outcome data and was included. The evidence from this trial was of moderate quality and too small to allow conclusions to be drawn.”
If this is the case, how can the review authors draw conclusions? As you say, the evidence is of moderate quality and too small to allow conclusions.
Comments submitted by Robin Kidder, August 2013
WHAT’S NEW
Last assessed as up-to-date: 17 April 2012.
| Date | Event | Description |
|---|---|---|
| 27 August 2013 | Feedback has been incorporated | Feedback 1 received from Robin Kidder. |
HISTORY
Protocol first published: Issue 2, 1997
Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 25 January 2013 | Amended | External source of support added. |
| 30 March 2012 | New search has been performed | Search updated. |
| 7 August 2011 | New citation required and conclusions have changed | A new trial has been identified and is awaiting classification (Hendrix 2009). All sections have been almost completely rewritten. A new second author has replaced the previous second author |
| 25 September 2009 | New search has been performed | Search updated. No new trials identified. |
| 3 September 2008 | Amended | Converted to new review format. |
| 30 April 2006 | New search has been performed | Search updated but no new trials identified. |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The Background section has been updated and restructured according to the most recent guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The primary objective is the same as in the protocol for the first version of the review (except that types of people or problem and secondary analysis that were lacking have been added). The Methods section has been amended to be in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and the Cochrane Pregnancy and Childbirth Group: Methodological Guidelines (Gates 2010).
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
- Dowswell 1996 {published data only} .Dowswell T, Thornton JG, Hewison J, Lilford RJL. Should there be a trial of home versus hospital delivery in the United Kingdom? Measuring outcomes other than safety is feasible. BMJ. 1996;312:753. doi: 10.1136/bmj.312.7033.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bateman 1994 {published data only} .Bateman DA, O’Bryan L, Nicholas SW, Heagarty MC. Outcome of unattended out-of-hospital births in Harlem. Archives of Pediatric and Adolescent Medicine. 1994;148:147–52. doi: 10.1001/archpedi.1994.02170020033005. [DOI] [PubMed] [Google Scholar]
- Berghs 1995 {published data only} .Berghs G, Spanjaards E, Driessen L, Doesburg W, Eskes TSO. Neonatal neurological outcome after low-risk pregnancies. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1995;62:167–71. doi: 10.1016/0301-2115(95)02184-9. [DOI] [PubMed] [Google Scholar]
- MacVicar 1993 {published data only} .MacVicar J, Dobbie G, Owen Johnstone L, Jagger C, Hopkins M, Kennedy JSO. Simulated home delivery in hospital: a randomised controlled trial. British Journal of Obstetrics and Gynaecology. 1993;100:316–23. doi: 10.1111/j.1471-0528.1993.tb12972.x. [DOI] [PubMed] [Google Scholar]
- O’Connor 1986 {published data only} .O’Connor ME, Addiego JE., Jr. Use of oral vitamin K1 to prevent hemorrhagic disease of the newborn infant. Journal of Pediatrics. 1986;108(4):616–9. doi: 10.1016/s0022-3476(86)80851-4. [DOI] [PubMed] [Google Scholar]
- Truffert 1998 {published data only} .Truffert P, Goujard J, Dehan M, Vodovar M, Breart G. Outborn status with a medical neonatal transport service and survival without disability at two years. A population-based cohort survey of newborns of less than 33 weeks of gestation. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1998;79(1):13–8. doi: 10.1016/s0301-2115(97)00243-1. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Hendrix 2009 {published and unpublished data} .Hendrix M, Van Horck M, Moreta D, Nieman F, Nieuwenhuijze M, Severens J, et al. Why women do not accept randomisation for place of birth: feasibility of a RCT in The Netherlands. BJOG: An International Journal of Obstetrics and Gynaecology. 2009;116(4):537–42. doi: 10.1111/j.1471-0528.2008.02103.x. Discussion 542-4. [DOI] [PubMed] [Google Scholar]
- Olsen O. Was the Dutch home birth trial properly registered and performed? BJOG: An International Journal of Obstetrics and Gynaecology. 2010;117(9):1162–3. doi: 10.1111/j.1471-0528.2010.02610.x. Author reply 1163. [DOI] [PubMed] [Google Scholar]
Additional references
- Alfirevic 2008 .Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD006066. DOI: 10.1002/14651858.CD006066. [DOI] [PubMed] [Google Scholar]
- Anim-Somuah 2005 .Anim-Somuah M, Smyth R, Howell C. Epidural versus non-epidural or no analgesia in labour. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD000331.pub2. DOI: 10.1002/14651858.CD000331.pub2. [DOI] [PubMed] [Google Scholar]
- Anonymous 2011 .Anonymous Editors’ comment. American Journal of Obstetrics and Gynecology. 2011;204(4):e20. [Google Scholar]
- Berghella 2008 .Berghella V, Baxter JK, Chauhan SP. Evidence-based labor and delivery management. American Journal of Obstetrics and Gynecology. 2008;199(5):445–54. doi: 10.1016/j.ajog.2008.06.093. [DOI] [PubMed] [Google Scholar]
- Blix 2005 .Blix E, Reinar LM, Klovning A, Øian P. Prognostic value of the labour admission test and its effectiveness compared with auscultation only: a systematic review. British Journal of Obstetrics and Gynaecology. 2005;112:1595–604. doi: 10.1111/j.1471-0528.2005.00766.x. [DOI] [PubMed] [Google Scholar]
- Bruner 1998 .Bruner JP, Drummond SB, Meenan AL, Gaskin IM. All-fours maneuver for reducing shoulder dystocia during labor. Journal of Reproductive Medicine. 1998;43:439–43. [PubMed] [Google Scholar]
- Chalmers 1983 .Chalmers I. Scientific inquiry and authoritarianism in perinatal care and education. Birth. 1983;10(3):151–66. doi: 10.1111/j.1523-536x.1983.tb01418.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain 1997 .Chamberlain G, Wraight A, Crowley P, editors. Home Births - The Report of the 1994 Confidential Enquiry by the National Birthday Trust Fund. Parthenon Publishing; 1997. [Google Scholar]
- Chervenak 1992 .Chervenak FA, McCullough LB. What is obstetric ethics? Clinical Obstetrics and Gynecology. 1992;35:709–19. doi: 10.1097/00003081-199212000-00003. [DOI] [PubMed] [Google Scholar]
- Chervenak 2011 .Chervenak FA, McCullough LB, Arabin B. Obstetric ethics: an essential dimension of planned home birth. Obstetrics and Gynecology. 2011;117:1183–7. doi: 10.1097/AOG.0b013e3182172a97. [DOI] [PubMed] [Google Scholar]
- Clark 2009 .Clark SL, Simpson KR, Knox GE, Garite TJ. Oxytocin: new perspectives on an old drug. American Journal of Obstetrics and Gynecology. 2009;200(1):35.e1–6. doi: 10.1016/j.ajog.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Cochrane 1979 .Cochrane AL. 1931-1971: a critical review with particular reference to the medical profession. In: Teeling-Smith G, Wells N, editors. Medicines For The Year 2000. Office of Health Economics; London: 1979. pp. 2–12. [Google Scholar]
- Cochrane 1989 .Cochrane AL. Foreword. In: Chalmers I, Enkin M, Keirse MJN, editors. Effective Care in Pregnancy and Childbirth. Oxford University Press; Oxford: 1989. p. viii. [Google Scholar]
- Daston 1979 .Daston L. d’Alembert’s critique of probability theory. Historia Math. 1979;6:259–79. [Google Scholar]
- Davey 2011 .Davey MA, Flood MM. Perinatal mortality and planned home birth. American Journal of Obstetrics and Gynecology. 2011;204(4):e18. doi: 10.1016/j.ajog.2011.01.037. Author reply e18-20, discussion e20. [DOI] [PubMed] [Google Scholar]
- Davis-Floyd 2009a .Davis-Floyd RE, Barclay L, Daviss BA, Tritten J. Introduction. In: Davis-Floyd RE, Barclay L, Daviss BA, Tritten J, editors. Birth Models That Work. University of California Press; 2009. pp. 1–30. [Google Scholar]
- Davis-Floyd 2009b .Davis-Floyd RE, Barclay L, Daviss BA, Tritten J. Conclusion. In: Davis-Floyd RE, Barclay L, Daviss BA, Tritten J, editors. Birth Models That Work. University of California Press; 2009. pp. 441–62. [Google Scholar]
- de Jonge 2009 .de Jonge A, Van der Goes BY, Ravelli AC, Amelink-Verburg MP, Mol BW, Nijhuis JG, et al. Perinatal mortality and morbidity in a nationwide cohort of 529,688 low-risk planned home and hospital births. BJOG: An International Journal of Obstetrics and Gynaecology. 2009;116(9):1177–84. doi: 10.1111/j.1471-0528.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- Delamothe 2010 .Delamothe T. Throwing the baby back into the bathwater. BMJ. 2010;341:c4292. [Google Scholar]
- D’Agostino 2003 .D’Agostino RB, Sr, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues - the encounters of academic consultants in statistics. Statistics in Medicine. 2003;22:169–86. doi: 10.1002/sim.1425. [DOI] [PubMed] [Google Scholar]
- Enkin 1995 .Enkin M, Keirse MJNC, Renfrew MJ, Neilson JP. A Guide to Effective Care in Pregnancy and Childbirth. 2nd Edition Oxford University Press; Oxford: 1995. [Google Scholar]
- Epidemiology of motor vehicle collisions 2012 . [accessed 15 April 2012];Epidemiology of motor vehicle collisions. 2012 http://en.wikipedia.org/wiki/Epidemiology_of_motor_vehicle_collisions.
- EURO-PERISTAT 2008 . [accessed 2012];EURO-PERISTAT Project, with SCPE, EUROCAT, EURONEOSTAT. European Perinatal Health Report. 2008 http://www.europeristat.com/our-publications/european-perinatal-health-report.html.
- Gates 2010 .Gates S. Cochrane Pregnancy and Childbirth Group: Methodological Guidelines. 2010 Jan; http://pregnancy.cochrane.org/sites/pregnancy.cochrane.org/files/uploads/MethodologicalGuidelines%20%28January%202010%29.doc.
- Gigerenzer 1989 .Gigerenzer G, Swijtink Z, Porter T, Daston L, Beatty J, Kruger L. The Empire of Chance: How Probability Changed Science and Everyday Life. Cambridge University Press; Cambridge: 1989. [Google Scholar]
- Gourounti 2007 .Gourounti K, Sandall J. Admission cardiotocography versus intermittent auscultation of fetal heart rate: effects on neonatal Apgar score, on the rate of caesarean sections and on the rate of instrumental delivery--a systematic review. International Journal of Nursing Studies. 2007;44:1029–35. doi: 10.1016/j.ijnurstu.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Graham 2005 .Graham ID, Carroli G, Davies C, Medves JM. Episiotomy rates around the world: An update. Birth. 2005 Sep;32:219–23. doi: 10.1111/j.0730-7659.2005.00373.x. [DOI] [PubMed] [Google Scholar]
- Gyte 2011 .Gyte GM, Dodwell MJ, Macfarlane AJ. Home birth metaanalysis: does it meet AJOG’s reporting requirements? American Journal of Obstetrics and Gynecology. 2011;204(4):e15. doi: 10.1016/j.ajog.2011.01.035. Author reply e18-20, discussion e20. [DOI] [PubMed] [Google Scholar]
- Hacking 1984 .Hacking I. The Emergence of Probability: A Philosophical Study of Early Ideas About Probability, Induction and Statistical Inference. Cambridge University Press; Cambridge: 1984. [Google Scholar]
- Hatem 2008 .Hatem M, Sandall J, Devane D, Soltani H, Gates S. Midwife-led versus other models of care for childbearing women. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD004667.pub2. DOI: 10.1002/14651858.CD004667.pub2. [DOI] [PubMed] [Google Scholar]
- Hayden 2011 .Hayden EC. [accessed 10 August 2011];Home-birth study investigated. http://www.nature.com/news/2011/110318/full/news.2011.162.html.
- Hendrix 2009b .Hendrix M, Van Horck M, Moreta D, Nieman F, Nieuwenhuijze M, Severens J, et al. Why women do not accept randomisation for place of birth: feasibility of a RCT in The Netherlands. BJOG: An International Journal of Obstetrics and Gynaecology. 2009;116(4):537–42. doi: 10.1111/j.1471-0528.2008.02103.x. Discussion 542-4. [DOI] [PubMed] [Google Scholar]
- Higgins 2011 .The Cochrane Collaboration Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011 Available from www.cochrane-handbook.org.
- Hodnett 1989 .Hodnett ED, Osborn RW. Effects of continuous intrapartum professional support on childbirth outcomes. Research in Nursing and Health. 1989;12(5):289–97. doi: 10.1002/nur.4770120504. [DOI] [PubMed] [Google Scholar]
- Hodnett 2009 .Hodnett ED, Stremler R, Weston JA, Mckeever P. Reconceptualizing the hospital labor room: the PLACE (pregnant and laboring in an ambient clinical environment) pilot trial. Birth. 2009;36(2):159–66. doi: 10.1111/j.1523-536X.2009.00311.x. [DOI] [PubMed] [Google Scholar]
- Hodnett 2010 .Hodnett ED, Downe S, Walsh D, Weston J. Alternative versus conventional institutional settings for birth. Cochrane Database of Systematic Reviews. 2010;(9) doi: 10.1002/14651858.CD000012.pub3. DOI: 10.1002/14651858.CD000012.pub3. [DOI] [PubMed] [Google Scholar]
- Hodnett 2011 .Hodnett ED, Gates S, Hofmeyr GJ, Sakala C, Weston J. Continuous support for women during childbirth. Cochrane Database of Systematic Reviews. 2011;(2) doi: 10.1002/14651858.CD003766.pub3. DOI: 10.1002/14651858.CD003766.pub3. [DOI] [PubMed] [Google Scholar]
- Horn 2010 .Horn A. [accessed 15 April 2012];Home Birth Reference Site. http://www.homebirth.org.uk/ Site was updated 23 March 2010.
- Horton 2010 .Horton R. Offline: urgency and concern about home births. Lancet. 2010;376(9755):1812. [Google Scholar]
- Hunter 2004 .Hunter B. Con icting ideologies as a source of emotion work in midwifery. Midwifery. 2004;20:261–72. doi: 10.1016/j.midw.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Hutchon, 2010 .Hutchon DJ. Why do obstetricians and midwives still rush to clamp the cord? BMJ. 2010;341:c5447. doi: 10.1136/bmj.c5447. DOI: 10.1136/bmj.c5447. [DOI] [PubMed] [Google Scholar]
- Hutton 2009 .Hutton EK, Reitsma AH, Kaufman K. Outcomes associated with planned home and planned hospital births in low-risk women attended by midwives in Ontario, Canada, 2003-2006: a retrospective cohort study. Birth. 2009;36(3):180–9. doi: 10.1111/j.1523-536X.2009.00322.x. [DOI] [PubMed] [Google Scholar]
- ICM 2010 .ICM [accessed 15 April 2012];Essential Competencies for Basic Midwifery Practice 2010. http://www.internationalmidwives.org/assets/uploads/documents/CoreDocuments/ICM%20Essential%20Competencies%20for%20Basic%20Midwifery%20Practice%202010,%20revised%202013.pdf.
- ICM 2012 .ICM [accessed 15 April 2012];The Philosophy and Model of Midwifery Care. http://www.internationalmidwives.org/assets/uploads/documents/CoreDocuments/CD2005_001%20ENG%20Philosophy%20and%20Model%20of%20Midwifery%20Care.pdf.
- Jadad 2007 .Jadad Alejandro R., Enkin Murray W. Randomised Controlled Trials: Questions, Answers and Musings. 2nd Edition BMJ Books; 2007. [Google Scholar]
- Janssen 2009 .Janssen PA, Saxell L, Page LA, Klein MC, Liston RM, Lee SK. Outcomes of planned home birth with registered midwife versus planned hospital birth with midwife or physician. Canadian Medical Association Journal. 2009;181(6-7):377–83. doi: 10.1503/cmaj.081869. Erratum in: CMAJ. 2009 Oct 27;181(9):617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson 2002 .Johanson R, Newburn M, Macfarlane A. Has the medicalisation of childbirth gone too far? BMJ. 2002;324(7342):892–5. doi: 10.1136/bmj.324.7342.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson 2011 .Johnson KC, Daviss BA. International data demonstrate home birth safety. American Journal of Obstetrics and Gynecology. 2011;204(4):e16–7. doi: 10.1016/j.ajog.2011.01.034. Author reply e18-20, discussion e20. [DOI] [PubMed] [Google Scholar]
- Jordan 1993 .Jordan B. Birth in Four Cultures: a Crosscultural Investigation of Childbirth in Yucatan, Holland, Sweden, and the United States. Waveland Press; 1993. [Google Scholar]
- Jordan 1997 .Jordan B. In: Authoritative knowledge and Its construction. Childbirth and Authoritative Knowledge. Crosscultural Perspectives. Davies-Floyd RE, Sargent CF, editors. University of California Press; 1997. pp. 55–79. [Google Scholar]
- Keirse 2010 .Keirse MJ. Home birth: gone away, gone astray, and here to stay. Birth. 2010;37(4):341–6. doi: 10.1111/j.1523-536X.2010.00431.x. DOI: 10.1111/j.1523-536X.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- Kirby 2011 .Kirby RS, Frost J. Maternal and newborn outcomes in planned home birth vs planned hospital births: a metaanalysis. American Journal of Obstetrics and Gynecology. 2011;204(4):e16. doi: 10.1016/j.ajog.2011.01.031. Author reply e18-20, discussion e20. [DOI] [PubMed] [Google Scholar]
- Kirkham, 2010 .Kirkham M. The Midwife-Mother Relationship. Macmillan; London: 2000. [Google Scholar]
- Klein 1984 .Klein M, Papageorgiou A, Westreich R, Spector-Dunsky L, Elkins V, Kramer M, et al. Care in a birth room versus a conventional setting: a controlled trial. Canadian Medical Association Journal. 1984;131:1461–6. [PMC free article] [PubMed] [Google Scholar]
- Leung 2011 .Leung TY, Stuart O, Suen SS, Sahota DS, Lau TK, Lao TT. Comparison of perinatal outcomes of shoulder dystocia alleviated by different type and sequence of manoeuvres: a retrospective review. BJOG: An International Journal of Obstetrics and Gynaecology. 2011;118:985–90. doi: 10.1111/j.1471-0528.2011.02968.x. DOI: 10.1111/j.1471-0528.2011.02968.x. [DOI] [PubMed] [Google Scholar]
- Mansfield 2008 .Mansfield B. The social nature of natural childbirth. Social Science and Medicine. 2008;66(5):1084–94. doi: 10.1016/j.socscimed.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Meenan 1991 .Meenan AL, Gaskin IM, Hunt P, Ball CA. A new (old) maneuver for the management of shoulder dystocia. Journal of Family Practice. 1991;32:625–9. [PubMed] [Google Scholar]
- Michal 2011 .Michal CA, Janssen PA, Vedam S, Hutton EK, de Jonge A. [accessed 10 August 2011];Planned home vs hospital birth: a meta-analysis gone wrong. http://www.medscape.com/viewarticle/739987.
- Neilson 2003 .Neilson JP. Biochemical tests of placental function for assessment in pregnancy. Cochrane Database of Systematic Reviews. 2003;(2) doi: 10.1002/14651858.CD000108. DOI: 10.1002/14651858.CD000108. [DOI] [PubMed] [Google Scholar]
- Nelson 1996 .Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. New England Journal of Medicine. 1996;334:613–8. doi: 10.1056/NEJM199603073341001. [DOI] [PubMed] [Google Scholar]
- Olsen 1995 .Olsen O. Are home births the future? [Er hjemmefødsler fremtiden?] Tidsskrift for Jordemødre. 1995;105:8–13. [Google Scholar]
- Olsen 1997a .Olsen O. Meta-analysis of the safety of home birth. Birth. 1997;24(1):4–13. doi: 10.1111/j.1523-536x.1997.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Olsen 2010 .Olsen O. Was the Dutch home birth trial properly registered and performed? BJOG: An International Journal of Obstetrics and Gynaecology. 2010;117(9):1162–3. doi: 10.1111/j.1471-0528.2010.02610.x. Author reply 1163. [DOI] [PubMed] [Google Scholar]
- Olsen 2011 .Olsen O. Promoting Normal Birth: Research, Reflections & Guidelines. Fresh Heart Publishing; 2011. Promoting home birth in accordance with the best scientific evidence; pp. 80–7. [Google Scholar]
- O’Driscoll 1993 .O’Driscoll K, Meagher D, Boylan P. Active Management of Labour: the Dublin Experience. 3rd Edition Mosby; 1993. [Google Scholar]
- RCOG 2005 .RCOG [accessed 15 April 2012];Shoulder dystocia - Guideline No. 42. 2005 Dec; http://www.rcog.org.uk/files/rcog-corp/uploaded-files/GT42ShoulderDystocia2005.pdf.
- Reeves 2011 .Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; Mar, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Registrar 2010 .Legal uncertainty prevented mother from giving birth at home. hudoc.echr.coe.int/webservices/content/pdf/003-3372071-3778873.
- RevMan 2011 .The Cochrane Collaboration . The Nordic Cochrane Centre. Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Romalis 1981 .Romalis S. Childbirth, Alternatives to Medical Control. University of Texas Press; Austin: 1981. [Google Scholar]
- Rooks 1989 .Rooks JP, Weatherby NL, Ernst EKM, Stapleton S, Rosen D, Rosenfield A. Outcomes of care in birth centers. New England Journal of Medicine. 1989;321:1804–11. doi: 10.1056/NEJM198912283212606. [DOI] [PubMed] [Google Scholar]
- Rooks 1999 .Rooks JP. The midwifery model of care. Journal of Nurse-Midwifery. 1999;44:370–4. [PubMed] [Google Scholar]
- Sakala 2008 .Sakala C, Corry MP. Evidence-Based Maternity Care: What It Is and What It Can Achieve. 2008 Oct; Available at: http://www.milbank.org/reports/0809MaternityCare/0809MaternityCare.html. Co-published by Childbirth Connection, the Reforming States Group, and the Milbank Memorial Fund.
- Sandall 2011 .Sandall J, Bewley S, Newburn M. Home birth triples the neonatal death rate”: public communication of bad science? American Journal of Obstetrics and Gynecology. 2011;204(4):e17–8. doi: 10.1016/j.ajog.2011.01.036. Author reply e18-20, discussion e20. [DOI] [PubMed] [Google Scholar]
- Schünemann 2011 .Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. The Cochrane Collaboration Higgins JPT, Green S, editors. Chapter 12: Interpreting results and drawing conclusions. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 Mar; Available from www.cochrane-handbook.org.
- Shafer 2006 .Shafer G, Vovk V. The Sources of Kolmogorov’s Grundbegriffe. Statistical Science. 2006;21:70–98. DOI: 10.1214/088342305000000467. [Google Scholar]
- Sheiner 2003 .Sheiner E, Shoham-Vardi I, Hallak M, Hadar A, Gortzak-Uzan L, Katz M, et al. Placental abruption in term pregnancies: clinical significance and obstetric risk factors. Journal of Maternal-Fetal Medicine. 2003;13:45–9. doi: 10.1080/jmf.13.1.45.49. [DOI] [PubMed] [Google Scholar]
- Sheiner 2006 .Sheiner E, Levy A, Hershkovitz R, Hallak M, Hammel RD, Katz M, Mazor M. Determining factors associated with shoulder dystocia: a population-based study. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2006;126:11–5. doi: 10.1016/j.ejogrb.2004.06.010. DOI: http://dx.doi.org/10.1016/j.ejogrb.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Singata 2010 .Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD003930.pub2. DOI: 10.1002/14651858.CD003930.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOWMY 2011 .United Nations Population Fund (UNFPA) [accessed 17 April 2012];The state of the world’s midwifery 2011 delivering health, saving lives. Available at http://www.unfpa.org/sowmy/resources/docs/main_report/en_SOWMR_Full.pdf.
- Sweet 2010 .Sweet M. Science and headlines in the home birth war. BMJ. 2010;340:455. [Google Scholar]
- Swijtink 1986 .Swijtink ZG. D’Alembert and the Maturity of Chances. Studies in History and Philosophy of Science Part A. 1986;17:327–49. [Google Scholar]
- Thorp 2007 .Thorp J. Evidence-based medicine--where is your effectiveness? BJOG: An International Journal of Obstetrics and Gynaecology. 2007;114(1):1–2. doi: 10.1111/j.1471-0528.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- UNFPA 2012 .UNFPA [accessed 16 April 2012];Skilled Attendance at Birth. http://www.unfpa.org/public/mothers/pid/4383#.
- Waldenstrom 2000 .Waldenstrom U, Brown S, McLachlan H, Forster D, Brennecke S. Does team midwife care increase satisfaction with antenatal, intrapartum, and postpartum care? A randomized controlled trial. Birth. 2000;27(3):156–67. doi: 10.1046/j.1523-536x.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- Waldenström 1993 .Waldenstrom U, Nilsson CA. Women’s satisfaction with birth center care: a randomized, controlled study. Birth. 1993;20:3–13. doi: 10.1111/j.1523-536x.1993.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Waldenström 2008 .Waldenström U, Rudman A. Satisfaction with maternity care: how to measure and what to do. Womens Health. 2008;4(3):211–4. doi: 10.2217/17455057.4.3.211. [DOI] [PubMed] [Google Scholar]
- Walsh 2002 .Walsh D, Newburn M. Towards a social model of childbirth: part one. British Journal of Midwifery. 2002;10:476–81. [Google Scholar]
- Wax 2010 .Wax JR, Lucas FL, Lamont M, Pinette MG, Cartin A, Blackstone J. Maternal and newborn outcomes in planned home birth vs planned hospital births: a metaanalysis. American Journal of Obstetrics and Gynecology. 2010;203(3):243.e1–8. doi: 10.1016/j.ajog.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Wax 2011 .Wax J. Supplementary material of interest to our readers. American Journal of Obstetrics and Gynecology. 2011;204(4):e7–13. doi: 10.1016/j.ajog.2011.02.056. [DOI] [PubMed] [Google Scholar]
- WHO 1996 .World Health Organization. Maternal and Newborn Health/Safe Motherhood Unit . Care in Normal Birth: a Practical Guide (WHO/FRH/MSM/96.24) WHO; Geneva: 1996. [Google Scholar]
- Zohar 2011 .Zohar N, De Vries R. Study validity questioned. American Journal of Obstetrics and Gynecology. 2011;204(4):e14. doi: 10.1016/j.ajog.2010.08.052. Author reply e14-5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Olsen 1997b .Olsen O. Home versus hospital birth. Cochrane Database of Systematic Reviews. 1997;(2) doi: 10.1002/14651858.CD000352. DOI: 10.1002/14651858.CD000352. [DOI] [PubMed] [Google Scholar]
- Olsen 1998 .Olsen O, Jewell D. Home versus hospital birth. Cochrane Database of Systematic Reviews. 1998;(3) doi: 10.1002/14651858.CD000352. DOI: 10.1002/14651858.CD000352. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study