Abstract
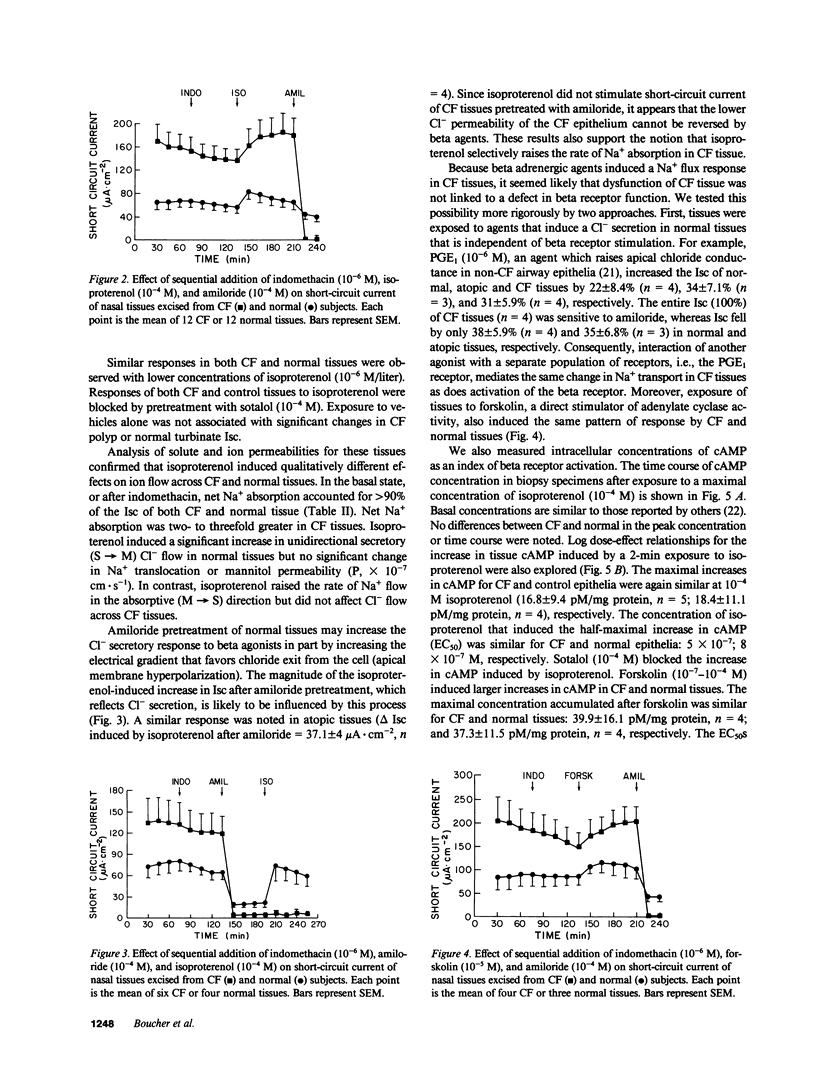
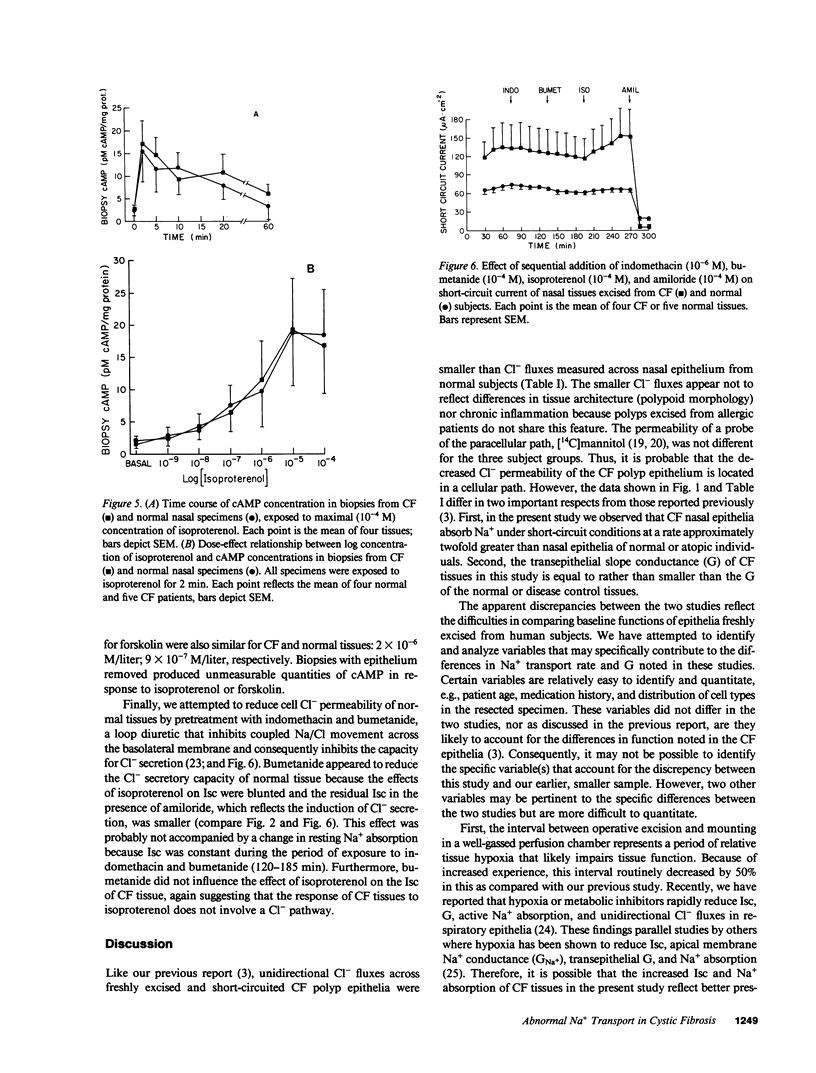
The transepithelial potential difference (PD) of cystic fibrosis (CF) airway epithelium is abnormally raised and the Cl- permeability is low. We studied the contribution of active Na+ absorption to the PD and attempted to increase the Cl- permeability of CF epithelia. Nasal epithelia from CF and control subjects were mounted in Ussing chambers and were short-circuited. The basal rate of Na+ absorption was raised in CF polyps compared with control tissues. Whereas beta agonists induced Cl- secretion in normal and atopic epithelia, beta agonists further increased the rate of Na+ absorption in CF epithelia without inducing Cl- secretion. This unusual effect is not due to an abnormal CF beta receptor because similar effects were induced by forskolin, and because cAMP production was similar in normal and CF epithelia. We conclude that CF airway epithelia absorb Na+ at an accelerated rate. The abnormal response to beta agonists may reflect a primary abnormality in a cAMP-modulated path, or a normal cAMP-modulated process in a Cl- impermeable epithelial cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bazzaz F. J., Cheng E. Effect of catecholamines on ion transport in dog tracheal epithelium. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):397–403. doi: 10.1152/jappl.1979.47.2.397. [DOI] [PubMed] [Google Scholar]
- Al-Bazzaz F., Yadava V. P., Westenfelder C. Modification of Na and Cl transport in canine tracheal mucosa by prostaglandins. Am J Physiol. 1981 Feb;240(2):F101–F105. doi: 10.1152/ajprenal.1981.240.2.F101. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CHERNICK W. S., BARBERO G. J. Composition of tracheobronchial secretions in cystic fibrosis of the pancreas and bronchiectasis. Pediatrics. 1959 Nov;24:739–745. [PubMed] [Google Scholar]
- Davis P. B., Braunstein M., Jay C. Decreased adenosine 3':5'-monophosphate response to isoproterenol in cystic fibrosis leukocytes. Pediatr Res. 1978 Jun;12(6):703–707. doi: 10.1203/00006450-197806000-00005. [DOI] [PubMed] [Google Scholar]
- Davis P. B., Dieckman L., Boat T. F., Stern R. C., Doershuk C. F. Beta adrenergic receptors in lymphocytes and granulocytes from patients with cystic fibrosis. J Clin Invest. 1983 Jun;71(6):1787–1795. doi: 10.1172/JCI110934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. B., Shelhamer J. R., Kaliner M. Abnormal adrenergic and cholinergic sensitivity in cystic fibrosis. N Engl J Med. 1980 Jun 26;302(26):1453–1456. doi: 10.1056/NEJM198006263022605. [DOI] [PubMed] [Google Scholar]
- Dawson D. C. Na and Cl transport across the isolated turtle colon: parallel pathways for transmural ion movement. J Membr Biol. 1977 Dec 15;37(3-4):213–233. doi: 10.1007/BF01940933. [DOI] [PubMed] [Google Scholar]
- Eskesen K., Ussing H. H. Determination of the electromotive force of active sodium transport in frog skin epithelium (Rana temporaria) from presteady-state flux ratio experiments. J Membr Biol. 1985;86(2):105–111. doi: 10.1007/BF01870777. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Welsh M. J., Smith P. L. Hormonal control of chloride secretion by canine tracheal epithelium: an electrophysiologic analysis. Ann N Y Acad Sci. 1981;372:558–570. doi: 10.1111/j.1749-6632.1981.tb15506.x. [DOI] [PubMed] [Google Scholar]
- Galant S. P., Norton L., Herbst J., Wood C. Impaired beta adrenergic receptor binding and function in cystic fibrosis neutrophils. J Clin Invest. 1981 Jul;68(1):253–258. doi: 10.1172/JCI110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981 Dec 17;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983 May;71(5):1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M., Murray G., Shallal J., Askin F., Ranga V., Gatzy J., Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):868–877. doi: 10.1152/jappl.1984.56.4.868. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Boat T. F., Rudolph S. A. Neurohormonal receptors and cyclic AMP-binding proteins in rabbit tracheal mucosa-submucosa. Biochim Biophys Acta. 1982 Nov 24;719(2):169–177. doi: 10.1016/0304-4165(82)90086-1. [DOI] [PubMed] [Google Scholar]
- Olver R. E., Davis B., Marin M. G., Nadel J. A. Active transport of Na+ and Cl- across the canine tracheal epithelium in vitro. Am Rev Respir Dis. 1975 Dec;112(6):811–815. doi: 10.1164/arrd.1975.112.6.811. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Edelman I. S., Lindemann B. Current-voltage analysis of apical sodium transport in toad urinary bladder: effects of inhibitors of transport and metabolism. J Membr Biol. 1980 Nov 15;57(1):59–71. doi: 10.1007/BF01868986. [DOI] [PubMed] [Google Scholar]
- Potter J. L., Matthews L. W., Spector S., Lemm J. Studies on pulmonary secretions. II. Osmolality and the ionic environment of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis. 1967 Jul;96(1):83–87. doi: 10.1164/arrd.1967.96.1.83. [DOI] [PubMed] [Google Scholar]
- Quinton P. M., Bijman J. Higher bioelectric potentials due to decreased chloride absorption in the sweat glands of patients with cystic fibrosis. N Engl J Med. 1983 May 19;308(20):1185–1189. doi: 10.1056/NEJM198305193082002. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Chloride impermeability in cystic fibrosis. Nature. 1983 Feb 3;301(5899):421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- Sato K., Sato F. Defective beta adrenergic response of cystic fibrosis sweat glands in vivo and in vitro. J Clin Invest. 1984 Jun;73(6):1763–1771. doi: 10.1172/JCI111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. L., Welsh M. J., Stoff J. S., Frizzell R. A. Chloride secretion by canine tracheal epithelium: I. Role of intracellular c AMP levels. J Membr Biol. 1982;70(3):217–226. doi: 10.1007/BF01870564. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Welsh M. J. Intracellular chloride activities in canine tracheal epithelium. Direct evidence for sodium-coupled intracellular chloride accumulation in a chloride-secreting epithelium. J Clin Invest. 1983 May;71(5):1392–1401. doi: 10.1172/JCI110892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: II. The cellular electrical potential profile. J Membr Biol. 1982;70(3):227–238. doi: 10.1007/BF01870565. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Widdicombe J. H. Pathways of ion movement in the canine tracheal epithelium. Am J Physiol. 1980 Sep;239(3):F215–F221. doi: 10.1152/ajprenal.1980.239.3.F215. [DOI] [PubMed] [Google Scholar]


