Abstract
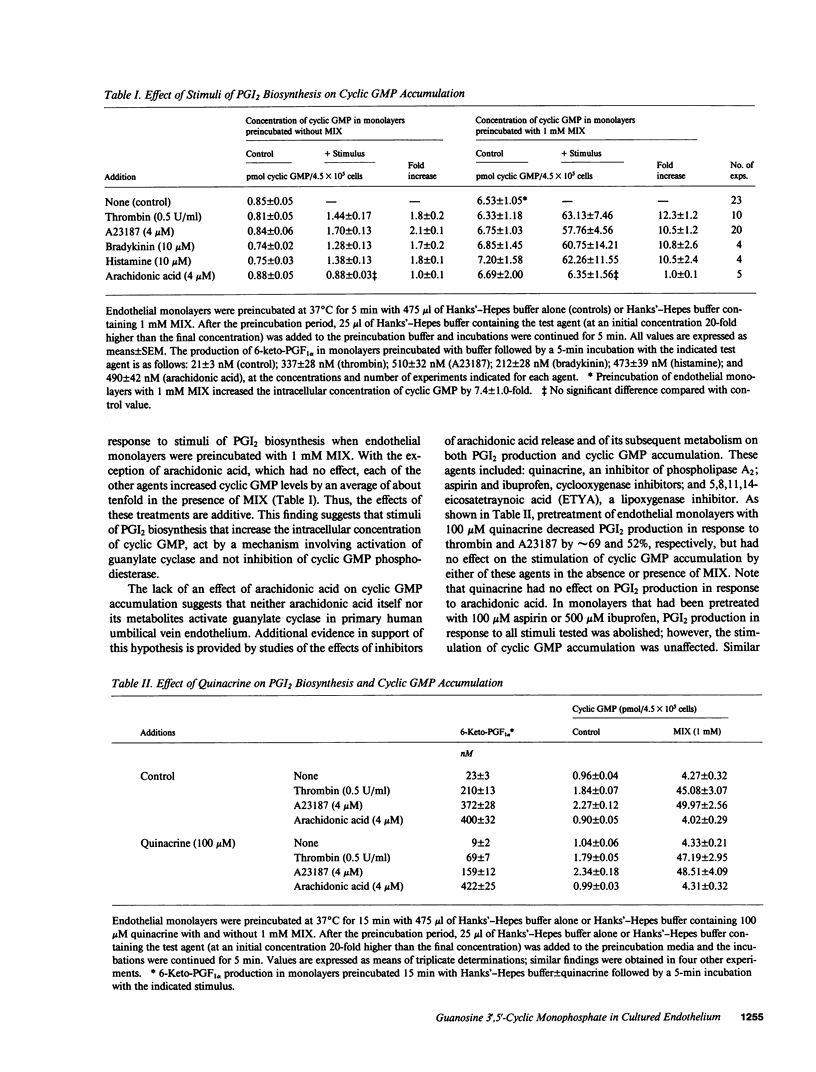
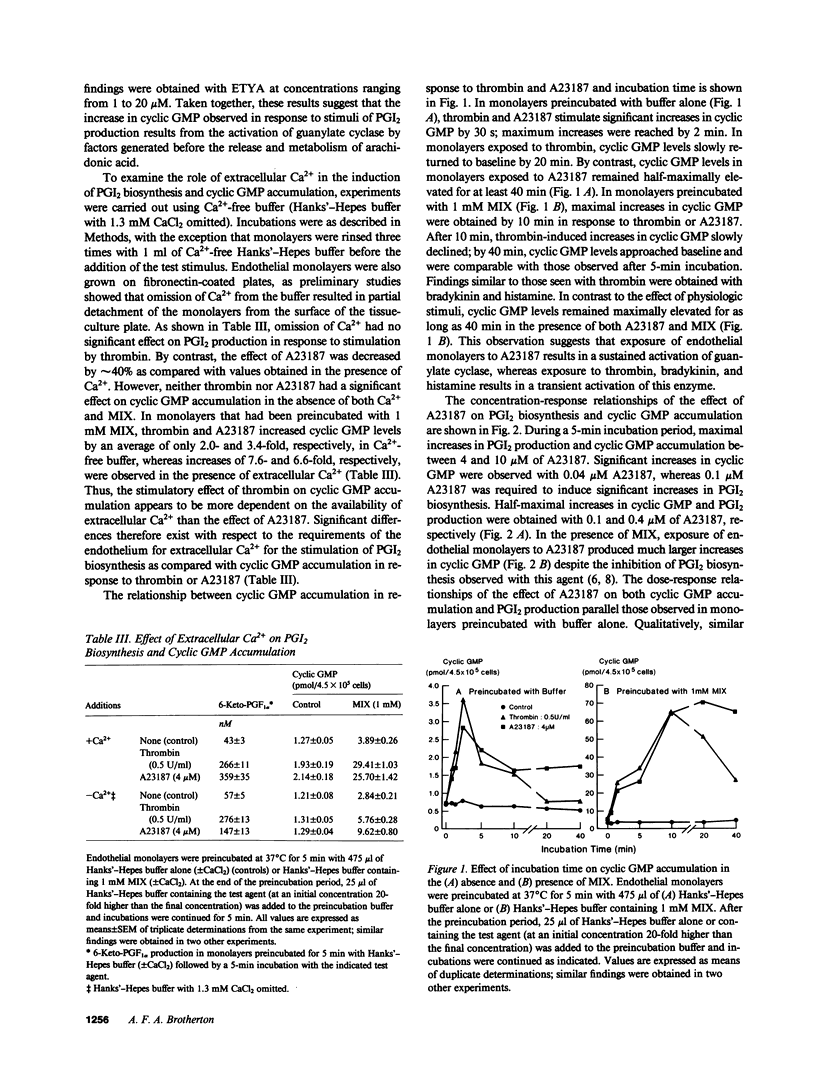
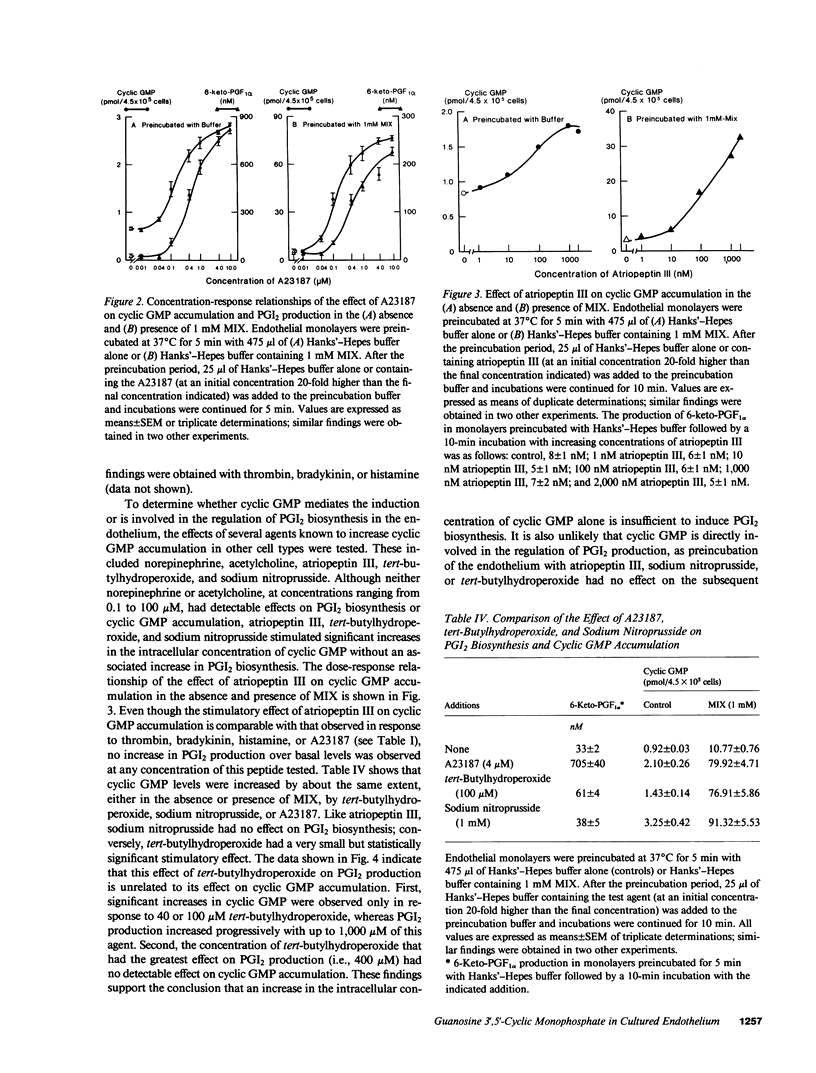
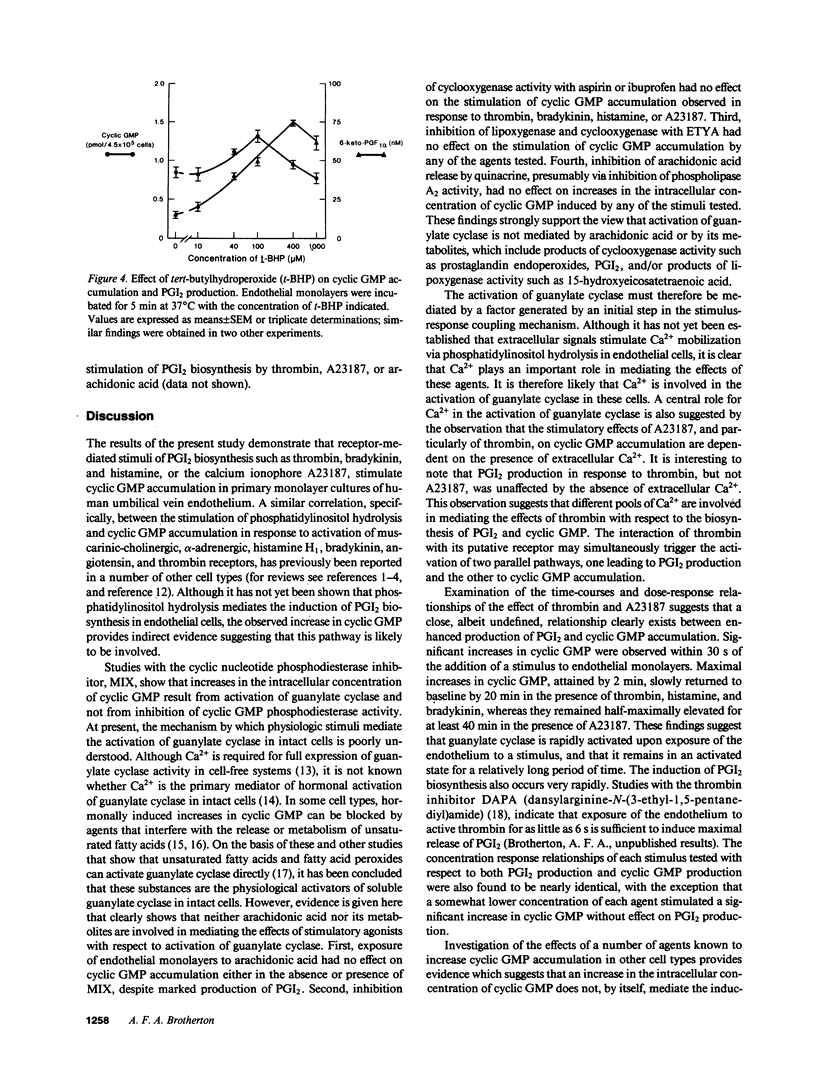
Stimuli of prostacyclin (PGI2) biosynthesis such as thrombin, bradykinin, histamine, and A23187 increase guanosine 3',5'-cyclic monophosphate (cyclic GMP) levels in primary monolayer cultures of human umbilical vein endothelium by about twofold. This effect is dependent on the presence of extracellular Ca2+. Increases of about tenfold are observed when cyclic GMP phosphodiesterase activity is inhibited, which suggests that the observed increases in cyclic GMP involve the activation of guanylate cyclase. Activation of guanylate cyclase appears to involve an early event in the induction of PGI2 biosynthesis, as neither arachidonic acid nor its metabolites stimulate cyclic GMP accumulation. Although activators of guanylate cyclase such as atriopeptin III, sodium nitroprusside, and tert-butylhydroperoxide increase cyclic GMP levels by approximately 2-3-fold, they do not stimulate or modulate PGI2 production. We conclude that cyclic GMP does not play a primary role in mediating the induction or regulation of PGI2 biosynthesis in vascular endothelium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Yamazaki A., Wheeler M. A., George J. S., Rasenick M. M. The mechanism of activation of light-activated phosphodiesterase and evidence for homology with hormone-activated adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:227–237. [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Prostacyclin biosynthesis in cultured vascular endothelium is limited by deactivation of cyclooxygenase. J Clin Invest. 1983 Oct;72(4):1255–1261. doi: 10.1172/JCI111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Macfarlane D. E., Hoak J. C. Prostacyclin biosynthesis in vascular endothelium is not inhibited by cyclic AMP. Studies with 3-isobutyl-1-methylxanthine and forskolin. Thromb Res. 1982 Dec 1;28(5):637–647. doi: 10.1016/0049-3848(82)90155-4. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry G., Parsons T., Hoak J., Sage H., Gingrich R. D., Ercolani L., Nghiem D., Czervionke R. Properties of cultured endothelium from adult human vessels. Arteriosclerosis. 1984 Jan-Feb;4(1):4–13. doi: 10.1161/01.atv.4.1.4. [DOI] [PubMed] [Google Scholar]
- George J. S., Hagins W. A. Control of Ca2+ in rod outer segment disks by light and cyclic GMP. Nature. 1983 May 26;303(5915):344–348. doi: 10.1038/303344a0. [DOI] [PubMed] [Google Scholar]
- Graff G., Stephenson J. H., Glass D. B., Haddox M. K., Goldberg N. D. Activation of soluble splenic cell guanylate cyclase by prostaglandin endoperoxides and fatty acid hydroperoxides. J Biol Chem. 1978 Nov 10;253(21):7662–7676. [PubMed] [Google Scholar]
- Henriksen R. A., Owen W. G., Nesheim M. E., Mann K. G. Identification of a congenital dysthrombin, thrombin Quick. J Clin Invest. 1980 Nov;66(5):934–940. doi: 10.1172/JCI109961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. M., Johnson R. M. Possible role of cyclic-GMP-dependent protein kinase in vascular smooth muscle function. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:285–296. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Prendergast F. G., Mann K. G. Interactions of a fluorescent active-site-directed inhibitor of thrombin: dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Biochemistry. 1979 Mar 20;18(6):996–1003. doi: 10.1021/bi00573a010. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Receptor-mediated regulation of calcium mobilization and cyclic GMP synthesis in neuroblastoma cells. Biochem Biophys Res Commun. 1984 Jul 18;122(1):333–339. doi: 10.1016/0006-291x(84)90479-0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Rasmussen H., Kojima I., Kojima K., Zawalich W., Apfeldorf W. Calcium as intracellular messenger: sensitivity modulation, C-kinase pathway, and sustained cellular response. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:159–193. [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Baird C. E., Sutherland E. W. The importance of calcium ions for the regulation of guanosine 3':5'-cyclic monophosphage levels. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3889–3893. doi: 10.1073/pnas.70.12.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider R. M., McKinney M., Fenton J. W., 2nd, Richelson E. Activation of cyclic nucleotide formation in murine neuroblastoma N1E-115 cells by modified human thrombins. J Biol Chem. 1984 Jul 25;259(14):9078–9081. [PubMed] [Google Scholar]
- Snider R. M., McKinney M., Forray C., Richelson E. Neurotransmitter receptors mediate cyclic GMP formation by involvement of arachidonic acid and lipoxygenase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3905–3909. doi: 10.1073/pnas.81.12.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider R. M., McKinney M., Forray C., Richelson E. Neurotransmitter receptors mediate cyclic GMP formation by involvement of arachidonic acid and lipoxygenase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3905–3909. doi: 10.1073/pnas.81.12.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies C., Schultz K. D., Schultz G. Inhibitory effects of mepacrine and eicosatetraynoic acid on cyclic GMP elevations caused by calcium and hormonal factors in rat ductus deferens. Naunyn Schmiedebergs Arch Pharmacol. 1980 Feb;311(1):71–77. doi: 10.1007/BF00500305. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]
- Walter U. Cyclic-GMP-regulated enzymes and their possible physiological functions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:249–258. [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]


