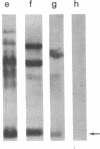
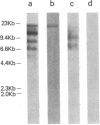
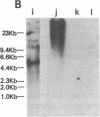
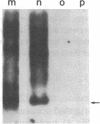
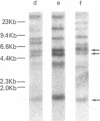
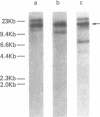
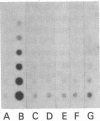
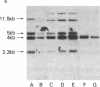
Abstract
Human T lymphotropic virus-I (HTLV-I)-specific T cell lines were established and cloned. K5, an OKT8+ clone bearing multiple proviral integration sites, retained its HTLV-I-specific cytotoxicity and a normal dependence on interleukin 2 (IL-2), indicating that there is a finite number of transforming integration sites. R2, an OKT4+ HTLV-I-infected clone, initially mounted a proliferative response to HTLV-I; but then its IL-2-independent proliferation increased and the antigen specificity was lost. All HTLV-I-infected clones tested including K7, another OKT8+ transformed cytotoxic clone that had lost its reactivity, expressed comparable levels of T cell receptor beta-chain (TCR-beta) messenger (m)RNA. Although clones K5 and K7 had different functional properties, they had the same rearrangement of the TCR-beta gene, suggesting that they had the same clonal origin. These data indicate that HTLV-I-specific T cells retain their immune reactivity for variable periods of time following infection, but then usually lose it; in some cases, however, no alteration in function can be detected. The data also suggest that different consequences can take place in the same clone depending on the pattern of retroviral infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Barker P. E., Ruddle F. H., Royer H. D., Acuto O., Reinherz E. L. Chromosomal location of human T-cell receptor gene Ti beta. Science. 1984 Oct 19;226(4672):348–349. doi: 10.1126/science.6435246. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Blattner W. A., Gallo R. C. Human T-cell leukemia/lymphoma viruses: clinical and epidemiologic features. Curr Top Microbiol Immunol. 1985;115:67–88. doi: 10.1007/978-3-642-70113-9_5. [DOI] [PubMed] [Google Scholar]
- Broder S., Bunn P. A., Jr, Jaffe E. S., Blattner W., Gallo R. C., Wong-Staal F., Waldmann T. A., DeVita V. T., Jr NIH conference. T-cell lymphoproliferative syndrome associated with human T-cell leukemia/lymphoma virus. Ann Intern Med. 1984 Apr;100(4):543–557. doi: 10.7326/0003-4819-100-4-543. [DOI] [PubMed] [Google Scholar]
- Bunn P. A., Jr, Schechter G. P., Jaffe E., Blayney D., Young R. C., Matthews M. J., Blattner W., Broder S., Robert-Guroff M., Gallo R. C. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N Engl J Med. 1983 Aug 4;309(5):257–264. doi: 10.1056/NEJM198308043090501. [DOI] [PubMed] [Google Scholar]
- Caccia N., Kronenberg M., Saxe D., Haars R., Bruns G. A., Goverman J., Malissen M., Willard H., Yoshikai Y., Simon M. The T cell receptor beta chain genes are located on chromosome 6 in mice and chromosome 7 in humans. Cell. 1984 Jul;37(3):1091–1099. doi: 10.1016/0092-8674(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Trainor C. D., Mann D. L., Gallo R. C., Reitz M. S. Methylation of human T-cell leukemia virus proviral DNA and viral RNA expression in short- and long-term cultures of infected cells. Virology. 1984 May;135(1):97–104. doi: 10.1016/0042-6822(84)90120-x. [DOI] [PubMed] [Google Scholar]
- Cossman J., Neckers L. M., Arnold A., Korsmeyer S. J. Induction of differentiation in a case of common acute lymphoblastic leukemia. N Engl J Med. 1982 Nov 11;307(20):1251–1254. doi: 10.1056/NEJM198211113072006. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Mann D., Broder S., Ruscetti F. W., Maeda M., Kalyanaraman V. S., Robert-Guroff M., Reitz M. S., Jr Human T-cell leukemia-lymphoma virus (HTLV) is in T but not B lymphocytes from a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5680–5683. doi: 10.1073/pnas.79.18.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Lee J. C. Possible immunological mechanisms in C-type viral leukemogenesis in mice. Curr Top Microbiol Immunol. 1982;98:85–101. doi: 10.1007/978-3-642-68369-5_7. [DOI] [PubMed] [Google Scholar]
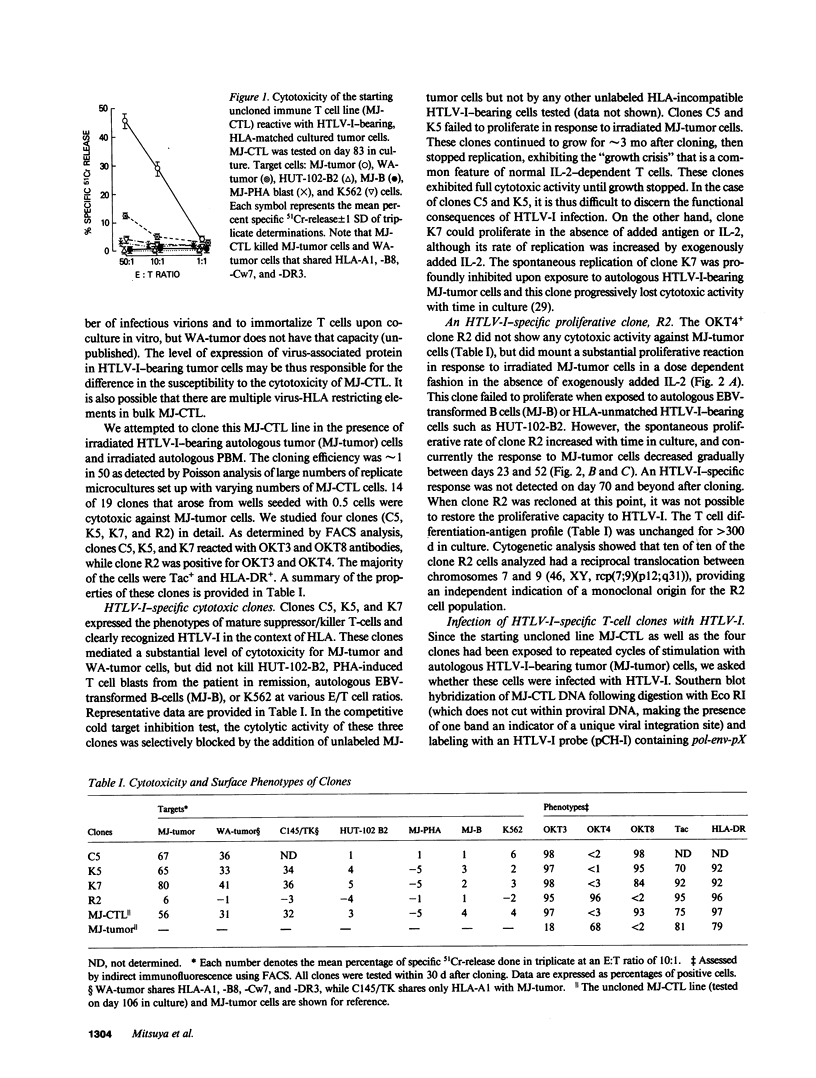
- Jarrett R. F., Mitsuya H., Mann D. L., Cossman J., Broder S., Reitz M. S. Configuration and expression of the T cell receptor beta chain gene in human T-lymphotrophic virus I-infected cells. J Exp Med. 1986 Feb 1;163(2):383–399. doi: 10.1084/jem.163.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Poiesz B., Ruscetti F. W., Gallo R. C. Immunological properties of a type C retrovirus isolated from cultured human T-lymphoma cells and comparison to other mammalian retroviruses. J Virol. 1981 Jun;38(3):906–915. doi: 10.1128/jvi.38.3.906-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi M., Sugamura K., Kinoshita K., Uchino H., Hinuma Y. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J Immunol. 1984 Aug;133(2):1037–1041. [PubMed] [Google Scholar]
- Lee J. C., Ihle J. N. Chronic immune stimulation is required for Moloney leukaemia virus-induced lymphomas. Nature. 1981 Jan 29;289(5796):407–409. doi: 10.1038/289407a0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Manzari V., Wong-Staal F., Franchini G., Colombini S., Gelmann E. P., Oroszlan S., Staal S., Gallo R. C. Human T-cell leukemia-lymphoma virus (HTLV): cloning of an integrated defective provirus and flanking cellular sequences. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1574–1578. doi: 10.1073/pnas.80.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham P. D., Salahuddin S. Z., Macchi B., Robert-Guroff M., Gallo R. C. Transformation of different phenotypic types of human bone marrow T-lymphocytes by HTLV-1. Int J Cancer. 1984 Jan 15;33(1):13–17. doi: 10.1002/ijc.2910330104. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Human T-cell leukemia/lymphoma viruses (HTLV): a unique family of pathogenic retroviruses. Curr Top Microbiol Immunol. 1985;115:33–51. doi: 10.1007/978-3-642-70113-9_3. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Cossman J., Megson M., Reitz M. S., Jr, Broder S. Functional properties of antigen-specific T cells infected by human T-cell leukemia-lymphoma virus (HTLV-I). Science. 1984 Sep 28;225(4669):1484–1486. doi: 10.1126/science.6206569. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Guo H. G., Megson M., Trainor C., Reitz M. S., Jr, Broder S. Transformation and cytopathogenic effect in an immune human T-cell clone infected by HTLV-I. Science. 1984 Mar 23;223(4642):1293–1296. doi: 10.1126/science.6322299. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Matis L. A., Megson M., Bunn P. A., Murray C., Mann D. L., Gallo R. C., Broder S. Generation of an HLA-restricted cytotoxic T cell line reactive against cultured tumor cells from a patient infected with human T cell leukemia/lymphoma virus. J Exp Med. 1983 Sep 1;158(3):994–999. doi: 10.1084/jem.158.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Matis L. A., Megson M., Cohen O. J., Mann D. L., Gallo R. C., Broder S. Immune T cells reactive against human T-cell leukaemia/lymphoma virus. Lancet. 1984 Mar 24;1(8378):649–652. doi: 10.1016/s0140-6736(84)92169-x. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Flomenberg N., Volkman D. J., Mann D., Fauci A. S., Dupont B., Gallo R. C. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science. 1984 Oct 26;226(4673):459–462. doi: 10.1126/science.6093248. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Poiesz B. J., Ruscetti F. W., Gallo R. C. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
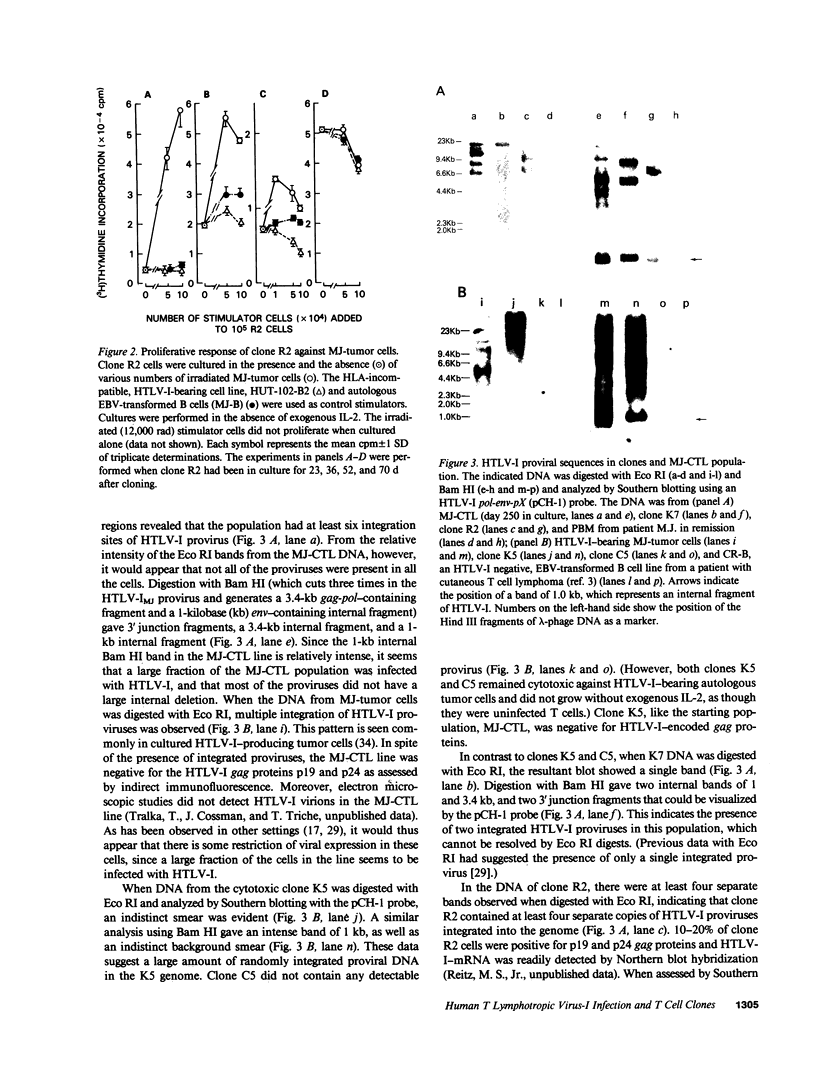
- Romain P. L., Schlossman S. F. Human T lymphocyte subsets. Functional heterogeneity and surface recognition structures. J Clin Invest. 1984 Nov;74(5):1559–1565. doi: 10.1172/JCI111571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., Tunnacliffe A., Smith W. J., Rabbitts T. H. Complexity of human T-cell antigen receptor beta-chain constant- and variable-region genes. Nature. 1984 Dec 6;312(5994):541–545. doi: 10.1038/312541a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor C. D., Wong-Staal F., Reitz M. S., Jr Comparative restriction endonuclease maps of proviral DNA of the primate type C simian sarcoma-associated virus and gibbon ape leukemia virus group. J Virol. 1982 Jan;41(1):298–308. doi: 10.1128/jvi.41.1.298-308.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Uchiyama T., Sagawa K., Takatsuki K., Uchino H. Effect of adult T-cell leukemia cells on pokeweed mitogen-induced normal B-cell differentiation. Clin Immunol Immunopathol. 1978 May;10(1):24–34. doi: 10.1016/0090-1229(78)90005-3. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Weissman I. L., McGrath M. S. Retrovirus lymphomagenesis: relationship of normal immune receptors to malignant cell proliferation. Curr Top Microbiol Immunol. 1982;98:103–112. doi: 10.1007/978-3-642-68369-5_8. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Lee E. C., Sieverts H., Magrath I. T. Burkitt's lymphoma in AIDS: cytogenetic study. Blood. 1984 Apr;63(4):818–822. [PubMed] [Google Scholar]
- Wong-Staal F., Hahn B., Manzari V., Colombini S., Franchini G., Gelmann E. P., Gallo R. C. A survey of human leukaemias for sequences of a human retrovirus. Nature. 1983 Apr 14;302(5909):626–628. doi: 10.1038/302626a0. [DOI] [PubMed] [Google Scholar]
- Yagüe J., White J., Coleclough C., Kappler J., Palmer E., Marrack P. The T cell receptor: the alpha and beta chains define idiotype, and antigen and MHC specificity. Cell. 1985 Aug;42(1):81–87. doi: 10.1016/s0092-8674(85)80103-3. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Takatsuki K., Masuda T. Letter: Two cases of T-cell chronic lymphocytic leukemia in Japan. N Engl J Med. 1974 Mar 7;290(10):572–573. doi: 10.1056/NEJM197403072901018. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Yanagi Y., Suciu-Foca N., Mak T. W. Presence of T-cell receptor mRNA in functionally distinct T cells and elevation during intrathymic differentiation. Nature. 1984 Aug 9;310(5977):506–508. doi: 10.1038/310506a0. [DOI] [PubMed] [Google Scholar]