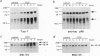Abstract
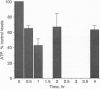
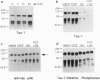
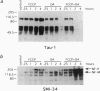
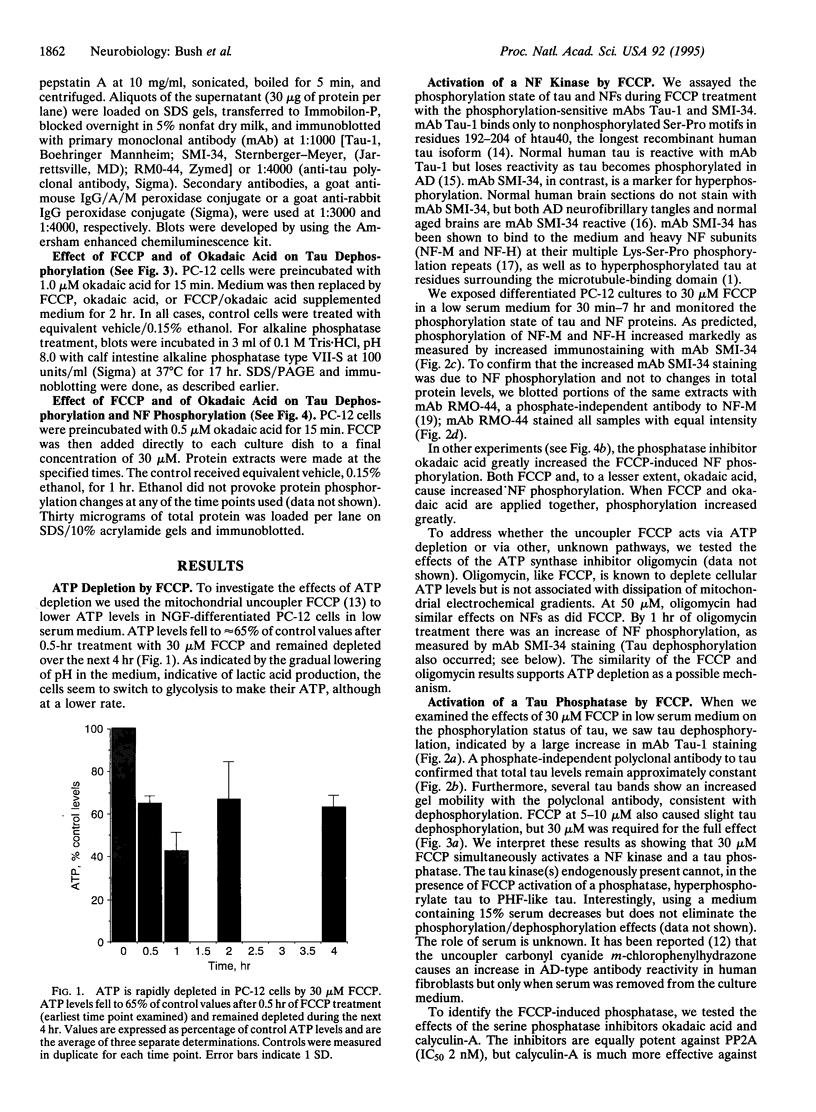
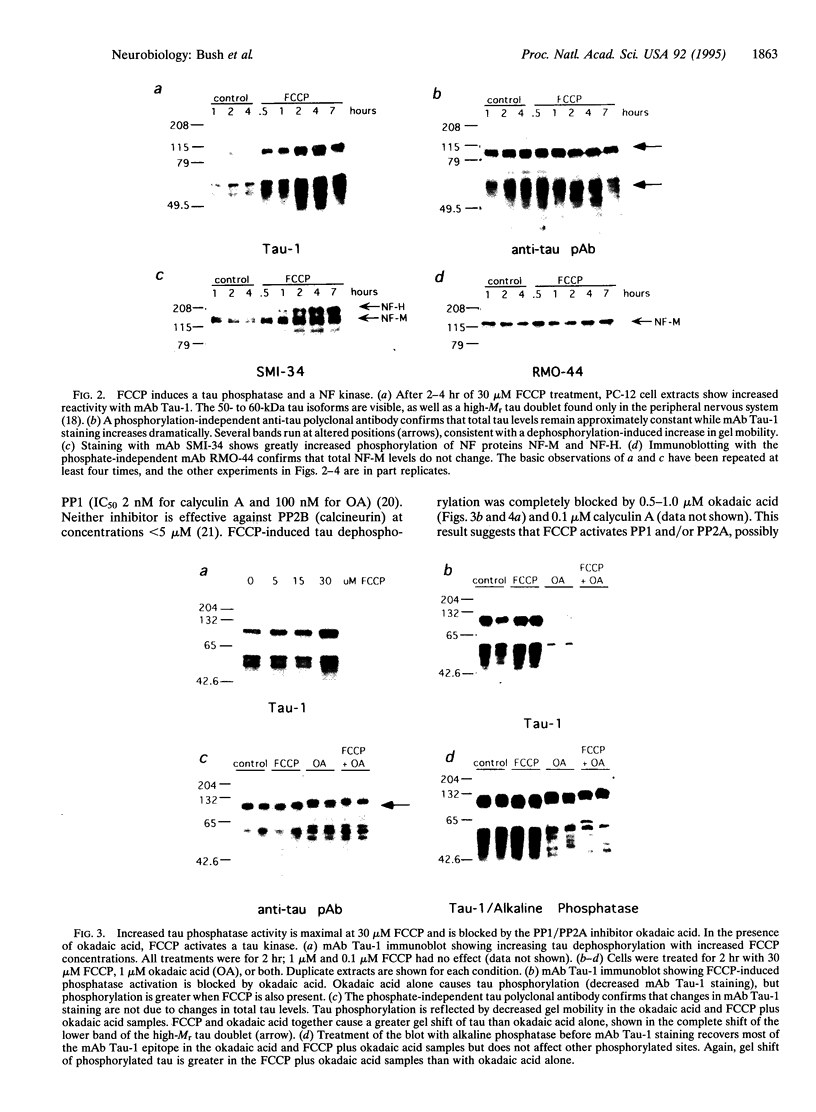
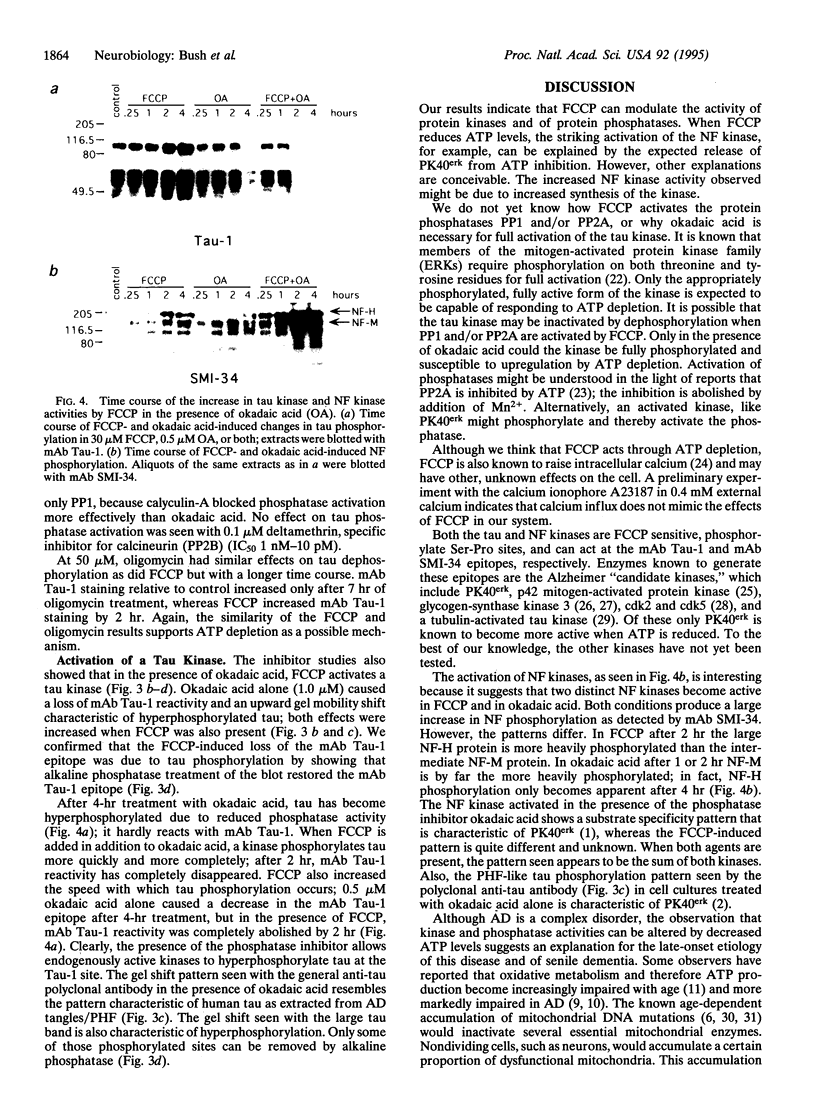
Brain pathology in Alzheimer disease and in aged controls shows hyperphosphorylation of tau and of neurofilament proteins. Roder and Ingram [Roder, H.M. & Ingram, V.M. (1991) J. Neurosci. 11, 3325-3343 and Roder, H.M., Eden, P.A. & Ingram, V.M. (1993) Biochem. Biophys. Res. Commun. 193, 639-647] previously reported that the brain protein kinase PK40erk can hyperphosphorylate both tau and neurofilaments and interestingly, is strongly inhibited by ATP uncomplexed with Mg2+. We now report that the mitochondrial uncoupler carbonyl cyanide p-trifluoro-methoxyphenylhydrazone decreases ATP levels in rat pheochromacytoma (PC-12) cells differentiated with nerve growth factor and activates a neurofilament kinase, a tau kinase, and, unexpectedly, a tau phosphatase--either PP1 or PP2A. Such aberrant modulation of protein phosphorylation patterns could be the common biochemical basis for senile dementia and for Alzheimer disease and could explain the late-onset etiology of both conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann K., Mandelkow E. M., Biernat J., Piwnica-Worms H., Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993 Dec 28;336(3):417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard B. J., Ingram V. M. Age-related neurofilament phosphorylation in normal human brains. Neurobiol Aging. 1989 May-Jun;10(3):253–258. doi: 10.1016/0197-4580(89)90059-6. [DOI] [PubMed] [Google Scholar]
- Blanchard B. J., devi Raghunandan R., Roder H. M., Ingram V. M. Hyperphosphorylation of human TAU by brain kinase PK40erk beyond phosphorylation by cAMP-dependent PKA: relation to Alzheimer's disease. Biochem Biophys Res Commun. 1994 Apr 15;200(1):187–194. doi: 10.1006/bbrc.1994.1432. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Baker A. C., Ko L., Black R. S. Induction of Alzheimer antigens by an uncoupler of oxidative phosphorylation. Arch Neurol. 1990 Aug;47(8):864–869. doi: 10.1001/archneur.1990.00530080046009. [DOI] [PubMed] [Google Scholar]
- Bowling A. C., Mutisya E. M., Walker L. C., Price D. L., Cork L. C., Beal M. F. Age-dependent impairment of mitochondrial function in primate brain. J Neurochem. 1993 May;60(5):1964–1967. doi: 10.1111/j.1471-4159.1993.tb13430.x. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Horton T., Lott M. T., Shoffner J. M., Beal M. F., Wallace D. C. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992 Dec;2(4):324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- Drewes G., Lichtenberg-Kraag B., Döring F., Mandelkow E. M., Biernat J., Goris J., Dorée M., Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992 Jun;11(6):2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff I. S., Liem R. K., Mellado W., Nunez J., Shelanski M. L. High molecular weight tau: preferential localization in the peripheral nervous system. J Cell Sci. 1991 Sep;100(Pt 1):55–60. doi: 10.1242/jcs.100.1.55. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Cairns N. J., Crowther R. A. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992 Jan;8(1):159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- Hanger D. P., Hughes K., Woodgett J. R., Brion J. P., Anderton B. H. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992 Nov 23;147(1):58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Abnormalities of glucose metabolism in Alzheimer's disease. Ann N Y Acad Sci. 1991;640:53–58. doi: 10.1111/j.1749-6632.1991.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Ishiguro K., Takamatsu M., Tomizawa K., Omori A., Takahashi M., Arioka M., Uchida T., Imahori K. Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J Biol Chem. 1992 May 25;267(15):10897–10901. [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Jensen J. R., Rehder V. FCCP releases Ca2+ from a non-mitochondrial store in an identified Helisoma neuron. Brain Res. 1991 Jun 14;551(1-2):311–314. doi: 10.1016/0006-8993(91)90947-t. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Schmidt M. L., Trojanowski J. Q. Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7384–7388. doi: 10.1073/pnas.85.19.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg-Kraag B., Mandelkow E. M., Biernat J., Steiner B., Schröter C., Gustke N., Meyer H. E., Mandelkow E. Phosphorylation-dependent epitopes of neurofilament antibodies on tau protein and relationship with Alzheimer tau. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5384–5388. doi: 10.1073/pnas.89.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E. M., Drewes G., Biernat J., Gustke N., Van Lint J., Vandenheede J. R., Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992 Dec 21;314(3):315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Hougham A. J., Bulpitt K. J. Studies of sequence heterogeneity of mitochondrial DNA from rat and mouse tissues: evidence for an increased frequency of deletions/additions with aging. Mech Ageing Dev. 1988 Jun;43(3):279–293. doi: 10.1016/0047-6374(88)90037-1. [DOI] [PubMed] [Google Scholar]
- Pleasure S. J., Selzer M. E., Lee V. M. Lamprey neurofilaments combine in one subunit the features of each mammalian NF triplet protein but are highly phosphorylated only in large axons. J Neurosci. 1989 Feb;9(2):698–709. doi: 10.1523/JNEUROSCI.09-02-00698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder H. M., Eden P. A., Ingram V. M. Brain protein kinase PK40erk converts TAU into a PHF-like form as found in Alzheimer's disease. Biochem Biophys Res Commun. 1993 Jun 15;193(2):639–647. doi: 10.1006/bbrc.1993.1672. [DOI] [PubMed] [Google Scholar]
- Roder H. M., Ingram V. M. Two novel kinases phosphorylate tau and the KSP site of heavy neurofilament subunits in high stoichiometric ratios. J Neurosci. 1991 Nov;11(11):3325–3343. doi: 10.1523/JNEUROSCI.11-11-03325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N. R., Finegan J. M., Blass J. P., Bowen D. M., Neary D. Mitochondrial function in brain tissue in primary degenerative dementia. Brain Res. 1987 Dec 8;436(1):30–38. doi: 10.1016/0006-8993(87)91553-8. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendrei G. I., Lee V. M., Otvos L., Jr Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J Neurosci Res. 1993 Feb 1;34(2):243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Saitoh Y., Fukunaga K., Nishimura H., Miyamoto E. Dephosphorylation of microtubule proteins by brain protein phosphatases 1 and 2A, and its effect on microtubule assembly. J Neurochem. 1988 May;50(5):1614–1623. doi: 10.1111/j.1471-4159.1988.tb03051.x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Sternberger N. H., Rubinstein L. J., Herman M. M., Binder L. I., Sternberger L. A. Abnormal processing of multiple proteins in Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8045–8049. doi: 10.1073/pnas.86.20.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]