Abstract
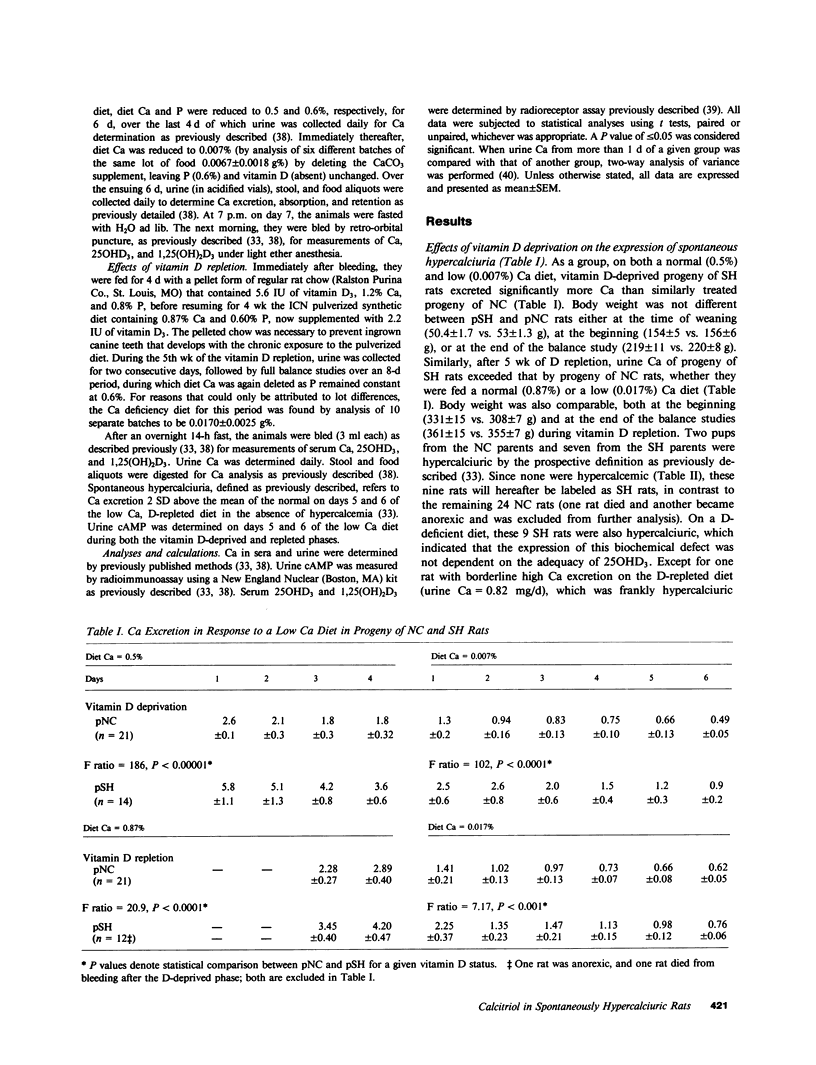
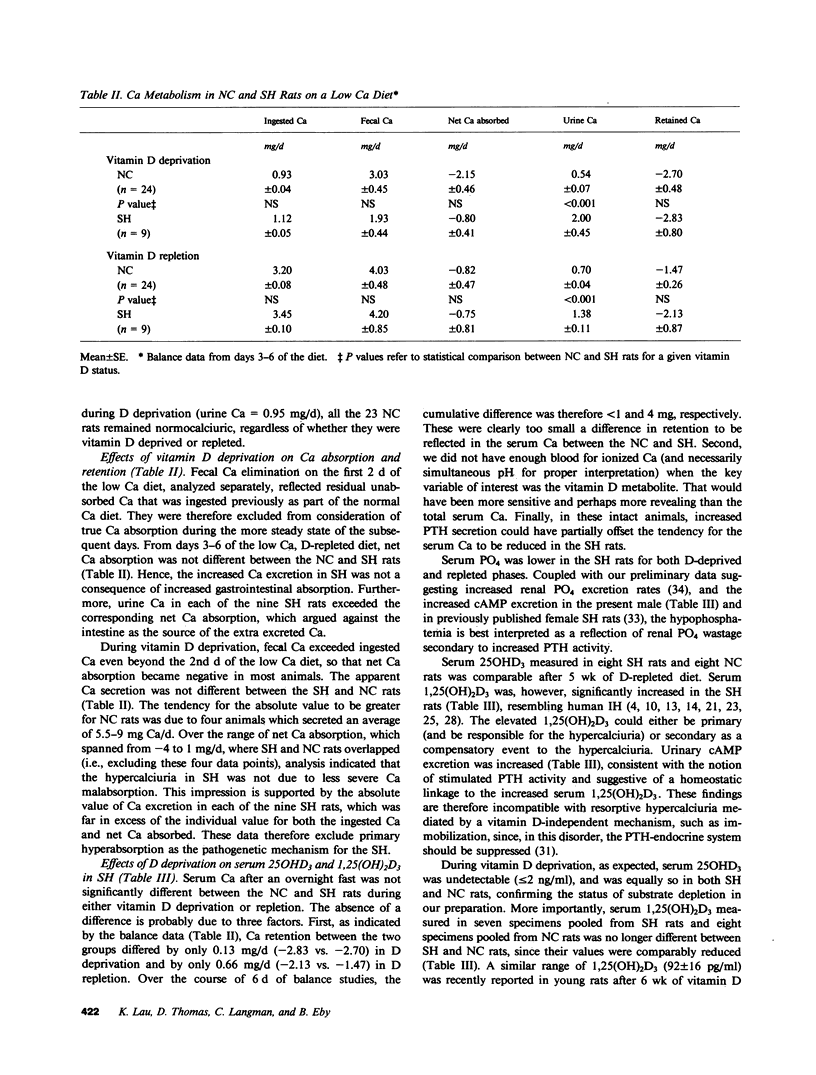
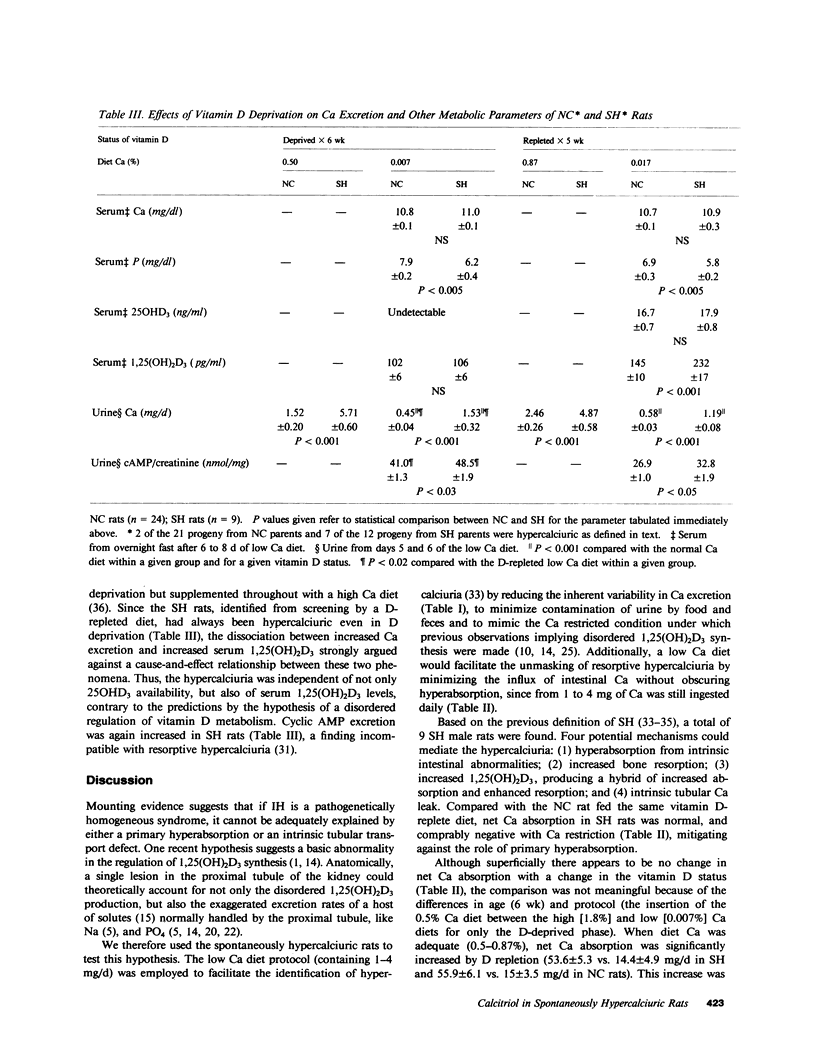
Recent data suggest a causal role of deranged 1,25(OH)2D metabolism in the syndrome of idiopathic hypercalciuria. To test this hypothesis, we evaluated if vitamin D availability and/or increased serum 1,25(OH)2D were critical for the expression of hypercalciuria in laboratory rats. Ca balance, serum 25OHD3, and 1,25(OH)2D3 were studied in D-deprived (-D) and D-repleted (+D) male progeny (p) born to normocalciuric (NC) and spontaneously hypercalciuric (SH) rats. 7 of the 14 pSH and 2 of 21 pNC had SH, which was defined as urinary Ca greater than two standard deviations above the mean of values for control animals on days 5 and 6 of a low Ca +D diet (1.19 vs. 0.58 mg/d, P less than 0.001). Fasting serum Ca and 25OHD3 were similar to control. Serum 1,25(OH)2D3 was elevated in these nine SH rats (232 vs. 145 pg/ml, P less than 0.005). However, during vitamin D deprivation, their Ca excretion was also increased (1.53 vs. 0.45 mg/d, P less than 0.001), despite comparably reduced serum 1,25(OH)2D3 (102 vs. 106 pg/ml) and undetectable serum 25OHD3. Net intestinal Ca absorption on a low Ca diet was comparable during D repletion (-0.75 vs. -0.82 mg/d) or D deprivation (-0.80 vs. -2.15 mg/d), excluding primary hyperabsorption as the mediator of the hypercalciuria. Mild hypophosphatemia was present in SH on +D (5.8 vs. 6.9 mg/dl, P less than 0.005) and -D diets (6.2 vs. 7.9 mg/dl, P less than 0.005), and was associated with higher rates of cyclic adenosine monophosphate excretion (32.8 vs. 26.9 and 48.5 vs. 41.0 nmol/mg of creatinine, respectively). Spontaneous hypercalciuria is therefore dissociable from increased Ca absorption, serum levels of 25OHD3, or 1,25(OH)2D3. The data are most compatible with the hypothesis of a renal Ca leak which stimulates parathyroid hormone activity and increases serum 1,25(OH)2D3, if provided adequate 25OHD3 as substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams N. D., Gray R. W., Lemann J., Jr, Cheung H. S. Effects of calcitriol administration on calcium metabolism healthy men. Kidney Int. 1982 Jan;21(1):90–97. doi: 10.1038/ki.1982.13. [DOI] [PubMed] [Google Scholar]
- Aladjem M., Modan M., Lusky A., Georgi R., Orda S., Eshkol A., Lotan D., Boichis H. Idiopathic hypercalciuria: a familial generalized renal hyperexcretory state. Kidney Int. 1983 Oct;24(4):549–554. doi: 10.1038/ki.1983.192. [DOI] [PubMed] [Google Scholar]
- Barilla D. E., Tolentino R., Kaplan R. A., Pak C. Y. Selective effects of thiazide on intestinal absorption of calcium and adsorptive and renal hypercalciurias. Metabolism. 1978 Feb;27(2):125–131. doi: 10.1016/0026-0495(78)90158-0. [DOI] [PubMed] [Google Scholar]
- Bordier P., Ryckewart A., Gueris J., Rasmussen H. On the pathogenesis of so-called idiopathic hypercalciuria. Am J Med. 1977 Sep;63(3):398–409. doi: 10.1016/0002-9343(77)90278-9. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Dominguez M., Bartter F. C. Pathophysiological studies in idiopathic hypercalciuria: use of an oral calcium tolerance test to characterize distinctive hypercalciuric subgroups. J Clin Endocrinol Metab. 1978 Oct;47(4):751–760. doi: 10.1210/jcem-47-4-751. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Insogna K. L., Lang R., Ellison A. F., Dreyer B. E. Evidence for disordered control of 1,25-dihydroxyvitamin D production in absorptive hypercalciuria. N Engl J Med. 1984 Jul 12;311(2):73–80. doi: 10.1056/NEJM198407123110201. [DOI] [PubMed] [Google Scholar]
- Coe F. L., Bushinsky D. A. Pathophysiology of hypercalciuria. Am J Physiol. 1984 Jul;247(1 Pt 2):F1–13. doi: 10.1152/ajprenal.1984.247.1.F1. [DOI] [PubMed] [Google Scholar]
- Coe F. L., Canterbury J. M., Firpo J. J., Reiss E. Evidence for secondary hyperparathyroidism in idiopathic hypercalciuria. J Clin Invest. 1973 Jan;52(1):134–142. doi: 10.1172/JCI107156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe F. L., Favus M. J., Crockett T., Strauss A. L., Parks J. H., Porat A., Gantt C. L., Sherwood L. M. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med. 1982 Jan;72(1):25–32. doi: 10.1016/0002-9343(82)90567-8. [DOI] [PubMed] [Google Scholar]
- Coe F. L. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med. 1977 Oct;87(4):404–410. doi: 10.7326/0003-4819-87-4-404. [DOI] [PubMed] [Google Scholar]
- Edwards N. A., Hodgkinson A. Metabolic studies in patients with idiopathic hypercalciuria. Clin Sci. 1965 Aug;29(1):143–157. [PubMed] [Google Scholar]
- Favus M. J., Langman C. B. Effects of 1,25-dihydroxyvitamin D3 on colonic calcium transport in vitamin D-deficient and normal rats. Am J Physiol. 1984 Mar;246(3 Pt 1):G268–G273. doi: 10.1152/ajpgi.1984.246.3.G268. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Wilz D. R., Caldas A. E., Lemann J., Jr The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1977 Aug;45(2):299–306. doi: 10.1210/jcem-45-2-299. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Baylink D. J., Hughes M. R., Brumbaugh P. F., Wergedal J. E., Shen F. H., Nielsen R. L., Counts S. J., Bursac K. M., McCain T. A. The assay of 1alpha,25-dihydroxyvitamin D3: physiologic and pathologic modulation of circulating hormone levels. Clin Endocrinol (Oxf) 1976;5 (Suppl):151S–165S. doi: 10.1111/j.1365-2265.1976.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Heller J. E., Konnak J. W., Lau Y. K. Potential pitfalls of the 2-hour calcium-to-creatinine ratio and urinary cyclic adenosine monophosphate excretion in the differential diagnosis of idiopathic hypercalciuria. J Urol. 1984 May;131(5):911–913. doi: 10.1016/s0022-5347(17)50707-7. [DOI] [PubMed] [Google Scholar]
- Kaplan R. A., Haussler M. R., Deftos L. J., Bone H., Pak C. Y. The role of 1 alpha, 25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest. 1977 May;59(5):756–760. doi: 10.1172/JCI108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnik B. R., Hruska K. A. Effects of 1,25-dihydroxycholecalciferol on phosphate transport in vitamin D-deprived rats. Am J Physiol. 1984 Jul;247(1 Pt 2):F177–F184. doi: 10.1152/ajprenal.1984.247.1.F177. [DOI] [PubMed] [Google Scholar]
- Lau K., Chen S., Eby B. Evidence for the role of PO4 deficiency in antihypertensive action of a high-Ca diet. Am J Physiol. 1984 Mar;246(3 Pt 2):H324–H331. doi: 10.1152/ajpheart.1984.246.3.H324. [DOI] [PubMed] [Google Scholar]
- Lau K., Eby B. K. Tubular mechanism for the spontaneous hypercalciuria in laboratory rat. J Clin Invest. 1982 Oct;70(4):835–844. doi: 10.1172/JCI110680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. K., Wasserstein A., Westby G. R., Bosanac P., Grabie M., Mitnick P., Slatopolsky E., Goldfarb S., Agus Z. S. Proximal tubular defects in idiopathic hypercalciuria: resistance to phosphate administration. Miner Electrolyte Metab. 1982;7(5):237–249. [PubMed] [Google Scholar]
- Maierhofer W. J., Gray R. W., Cheung H. S., Lemann J., Jr Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 1983 Oct;24(4):555–560. doi: 10.1038/ki.1983.193. [DOI] [PubMed] [Google Scholar]
- NASSIM J. R., HIGGINS B. A. CONTROL OF IDIOPATHIC HYPERCALCIURIA. Br Med J. 1965 Mar 13;1(5436):675–681. doi: 10.1136/bmj.1.5436.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C. Y., Kaplan R., Bone H., Townsend J., Waters O. A simple test for the diagnosis of absorptive, resorptive and renal hypercalciurias. N Engl J Med. 1975 Mar 6;292(10):497–500. doi: 10.1056/NEJM197503062921002. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., McGuire J., Peterson R., Britton F., Harrod M. J. Familial absorptive hypercalciuria in a large kindred. J Urol. 1981 Dec;126(6):717–719. doi: 10.1016/s0022-5347(17)54715-1. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Oata M., Lawrence E. C., Snyder W. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974 Aug;54(2):387–400. doi: 10.1172/JCI107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F. H., Baylink D. J., Nielsen R. L., Sherrard D. J., Ivey J. L., Haussler M. R. Increased serum 1,25-dihydroxyvitamin D in idiopathic hypercalciuria. J Lab Clin Med. 1977 Dec;90(6):955–962. [PubMed] [Google Scholar]
- Stewart A. F., Adler M., Byers C. M., Segre G. V., Broadus A. E. Calcium homeostasis in immobilization: an example of resorptive hypercalciuria. N Engl J Med. 1982 May 13;306(19):1136–1140. doi: 10.1056/NEJM198205133061903. [DOI] [PubMed] [Google Scholar]
- Sutton R. A., Walker V. R. Responses to hydrochlorothiazide and acetazolamide in patients with calcium stones. Evidence suggesting a defect in renal tubular function. N Engl J Med. 1980 Mar 27;302(13):709–713. doi: 10.1056/NEJM198003273021302. [DOI] [PubMed] [Google Scholar]
- Wilz D. R., Gray R. W., Dominguez J. H., Lemann J., Jr Plasma 1,25-(OH)2-vitamin D concentrations and net intestinal calcium, phosphate, and magnesium absorption in humans. Am J Clin Nutr. 1979 Oct;32(10):2052–2060. doi: 10.1093/ajcn/32.10.2052. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kawanobe Y., Takahashi H., Shimazawa E., Kimura S., Ogata E. Vitamin D deficiency and renal calcium transport in the rat. J Clin Invest. 1984 Aug;74(2):507–513. doi: 10.1172/JCI111448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendt E. R., Cohanim M. Prevention of calcium stones with thiazides. Kidney Int. 1978 May;13(5):397–409. doi: 10.1038/ki.1978.58. [DOI] [PubMed] [Google Scholar]
- Zerwekh J. E., Pak C. Y. Selective effects of thiazide therapy on serum 1 alpha,25-dihydroxyvitamin D and intestinal calcium absorption in renal and absorptive hypercalciurias. Metabolism. 1980 Jan;29(1):13–17. doi: 10.1016/0026-0495(80)90091-8. [DOI] [PubMed] [Google Scholar]


