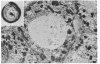
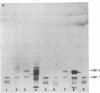
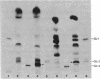
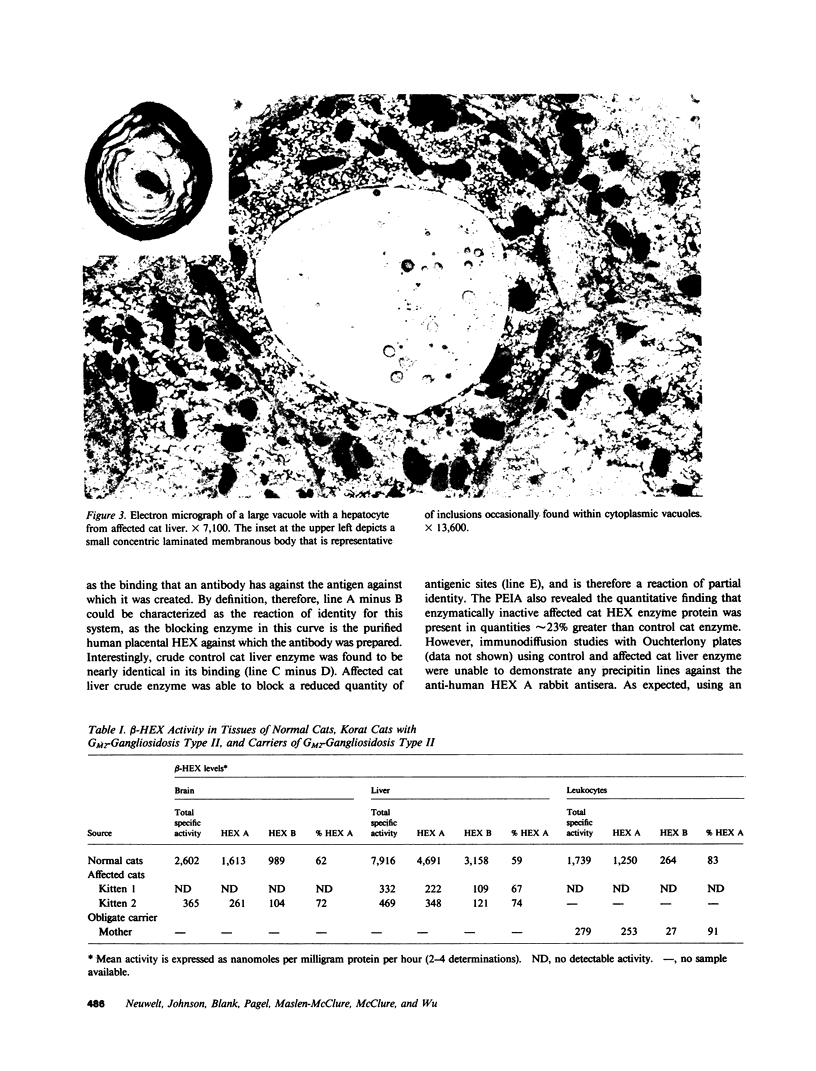
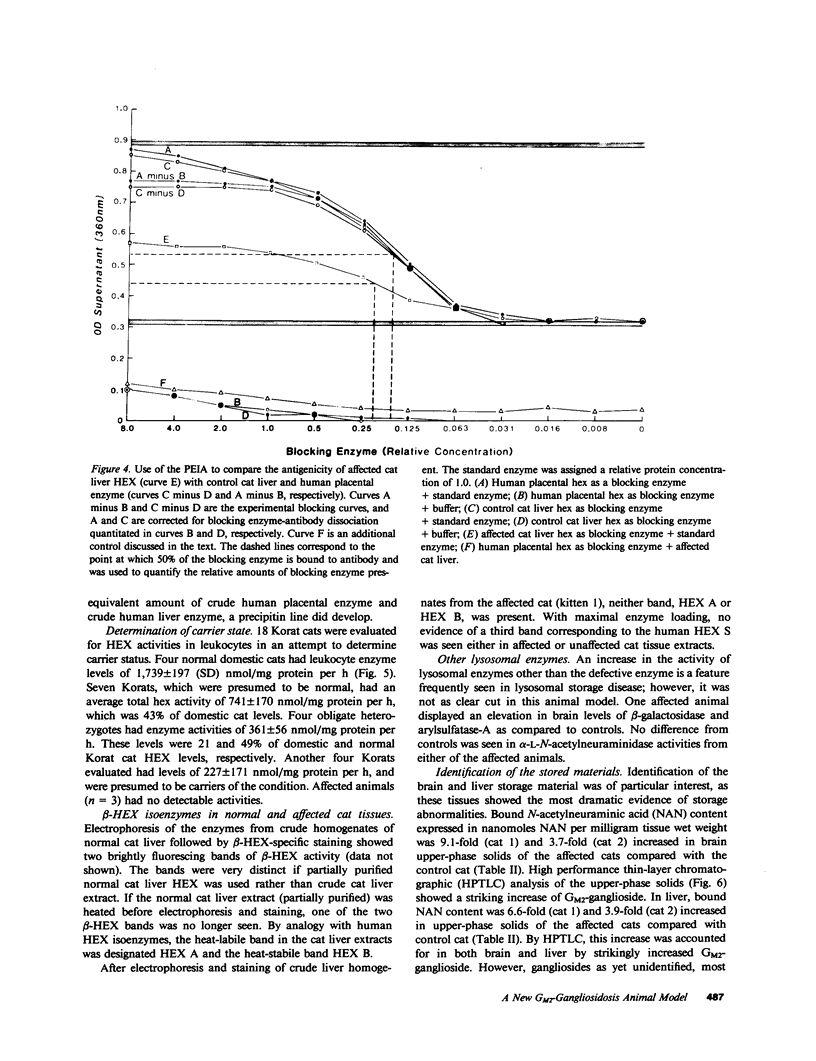
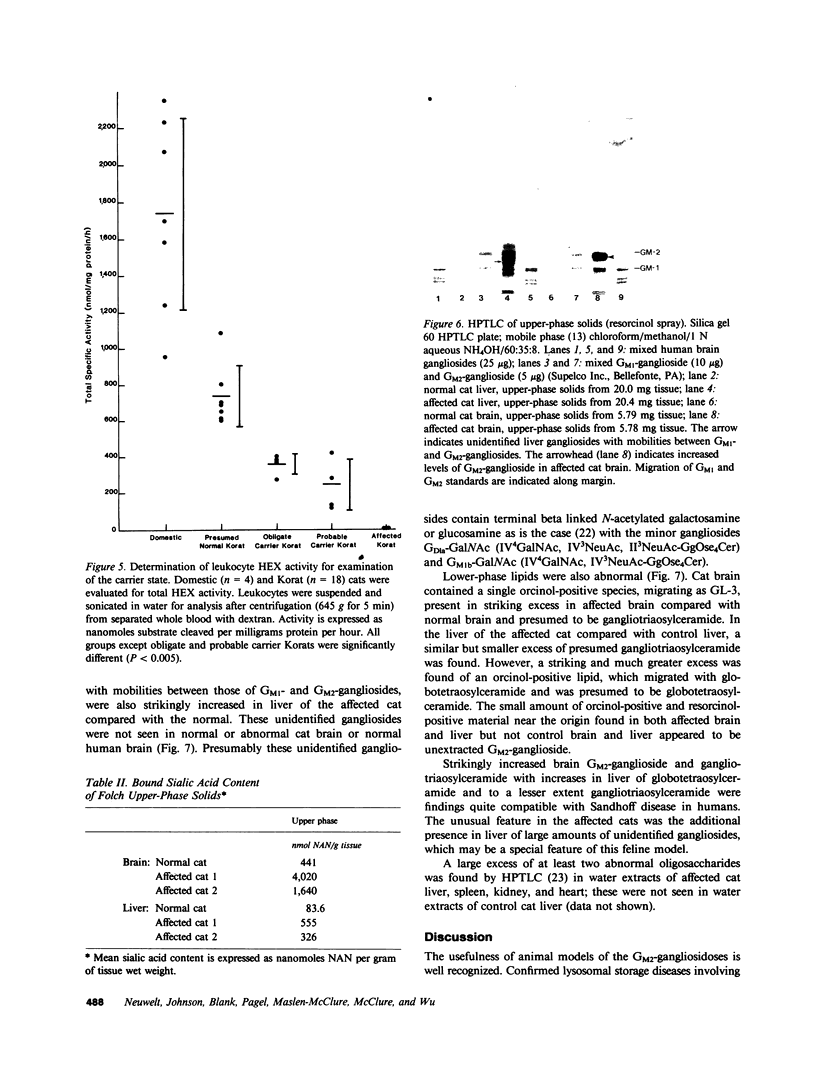
Abstract
We have detected a disorder in Korat cats (initially imported from Thailand) that is analogous to human Sandhoff's disease. Pedigree analysis indicates that this disease in an autosomal recessive disorder in the American Korat. Postmortem studies on one affected cat showed hepatomegaly that was not reported in the only other known feline model of GM2-gangliosidosis type II. Histologic and ultra-structural evaluation revealed typical storage vacuoles. There was a marked deficiency in the activity of hexosaminidase (HEX) A and B in affected brain and liver as compared to controls. Electrophoresis of a liver extract revealed a deficiency of normal HEX A and B in the affected animals. The blocking primary enzyme immunoassay verified the presence of antigenically reactive HEX present in affected cat livers in quantities slightly elevated with respect to the normal HEX concentration in control cats. In leukocytes, obligate heterozygotes had intermediate levels of total HEX activity with a slight increase in the percent activity due to HEX A. Indeed, 4 of 11 phenotypically normal animals in addition to four obligate heterozygotes appear to be carriers using this assay. Affected brain and liver compared with control brain and liver contained a great excess of bound N-acetylneuraminic acid in the Folch upper-phase solids; thin-layer chromatography showed a marked increase in GM2-ganglioside. In summary, we have characterized the pedigree, pathology, and biochemistry of a new feline model of GM2-gangliosidosis which is similar to but different from the only other known feline model.
Full text
PDF

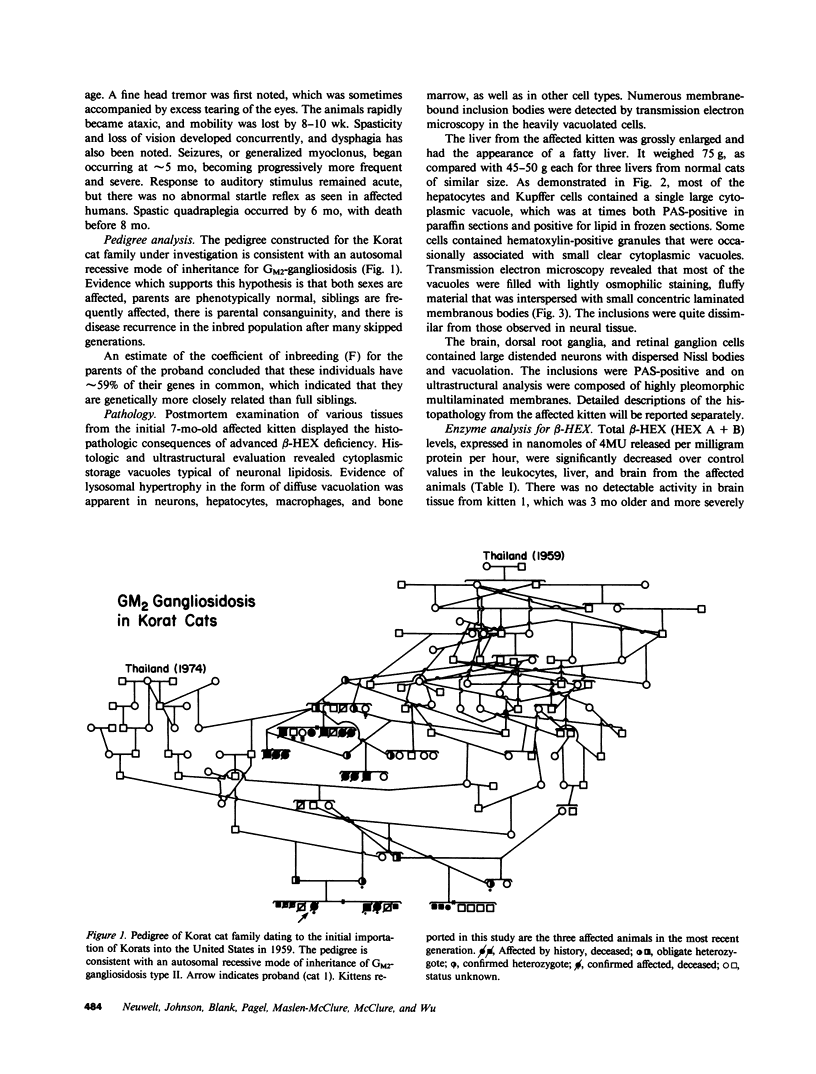



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. J., Mole J. A., Lindsey J. R., Creel R. M. Animal models of human ganglioside storage diseases. Fed Proc. 1976 Apr;35(5):1193–1201. [PubMed] [Google Scholar]
- Baker H. J., Reynolds G. D., Walkley S. U., Cox N. R., Baker G. H. The gangliosidoses: comparative features and research applications. Vet Pathol. 1979 Nov;16(6):635–649. doi: 10.1177/030098587901600602. [DOI] [PubMed] [Google Scholar]
- Bell C. E., Jr, Sly W. S., Brot F. E. Human beta-glucuronidase deficiency mucopolysaccharidosis: identification of cross-reactive antigen in cultured fibroblasts of deficient patients by enzyme immunoassay. J Clin Invest. 1977 Jan;59(1):97–105. doi: 10.1172/JCI108627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. E., Hoffman L. M., Adachi M., Amsterdam D., Schneck L. Enzyme replacement treatment for Tay-Sachs disease brain cells in culture utilizing concanavalin A-mediated hexosaminidase A uptake: biochemical and morphological evidence of GM2 mobilization. Acta Neuropathol. 1980;50(1):9–17. doi: 10.1007/BF00688529. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Munnell J. F., Lorenz M. D., Murphy J. V., Baker H. J., Rattazzi M. C. GM2 ganglioside lysosomal storage disease in cats with beta-hexosaminidase deficiency. Science. 1977 May 27;196(4293):1014–1017. doi: 10.1126/science.404709. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gahm N. H., Russman B. S., Cerciello R. L., Fiorentino M. R., McGrath D. M. Chronic cerebellar stimulation for cerebral palsy: a double-blind study. Neurology. 1981 Jan;31(1):87–90. doi: 10.1212/wnl.31.1.87. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Siddiqui B. Isolation and characterization of glycosphingolipid from animal cells and their membranes. Methods Enzymol. 1974;32:345–367. doi: 10.1016/0076-6879(74)32036-8. [DOI] [PubMed] [Google Scholar]
- Hickman S., Shapiro L. J., Neufeld E. F. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun. 1974 Mar 15;57(1):55–61. doi: 10.1016/s0006-291x(74)80356-6. [DOI] [PubMed] [Google Scholar]
- Johnson W. G., Desnick R. J., Long D. M., Sharp H. L., Krivit W., Brady B., Brady R. O. Intravenous injection of purified hexosaminidase A into a patient with Tay-Sachs disease. Birth Defects Orig Artic Ser. 1973 Mar;9(2):120–124. [PubMed] [Google Scholar]
- Kaback M. M., Shapiro L. J., Hirsch P., Roy C. Tay-Sachs disease heterozygote detection: a quality control study. Prog Clin Biol Res. 1977;18:267–279. [PubMed] [Google Scholar]
- Kusiak J. W., Toney J. H., Quirk J. M., Brady R. O. Specific binding of 125I-labeled beta-hexosaminidase A to rat brain synaptosomes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):982–985. doi: 10.1073/pnas.76.2.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E. A., Baker D. E., Pagel M. A., Blank N. K. Cerebrovascular permeability and delivery of gentamicin to normal brain and experimental brain abscess in rats. J Neurosurg. 1984 Sep;61(3):430–439. doi: 10.3171/jns.1984.61.3.0430. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Barranger J. A., Brady R. O., Pagel M., Furbish F. S., Quirk J. M., Mook G. E., Frenkel E. Delivery of hexosaminidase A to the cerebrum after osmotic modification of the blood--brain barrier. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5838–5841. doi: 10.1073/pnas.78.9.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E. A., Barranger J. A., Pagel M. A., Quirk J. M., Brady R. O., Frenkel E. P. Delivery of active hexosaminidase across the blood-brain barrier in rats. Neurology. 1984 Aug;34(8):1012–1019. doi: 10.1212/wnl.34.8.1012. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Frenkel E. P. Is there a therapeutic role for blood-brain barrier disruption? Ann Intern Med. 1980 Jul;93(1):137–139. doi: 10.7326/0003-4819-93-1-137. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Rapoport S. I. Modification of the blood-brain barrier in the chemotherapy of malignant brain tumors. Fed Proc. 1984 Feb;43(2):214–219. [PubMed] [Google Scholar]
- Neuwelt E. A. Use of antibodies and the primary enzyme immunoassay (PEIA) to study enzymes: the arylsulfatase A--anti-arylsulfatase A system. Methods Enzymol. 1981;73(Pt B):550–578. [PubMed] [Google Scholar]
- Neuwelt E., Kohler P. F., Austin J. Primary enzyme immunoassay (PEIA): studies of the mutant enzyme in metachromatic leukodystrophy (primary enzyme immunoassay of arylsulfatase-A). Immunochemistry. 1973 Nov;10(11):767–773. doi: 10.1016/0019-2791(73)90179-1. [DOI] [PubMed] [Google Scholar]
- Penick R. J., Meisler M. H., McCluer R. H. Thin-layer chromatographic studies of human brain gangliosides. Biochim Biophys Acta. 1966 Apr 4;116(2):279–287. doi: 10.1016/0005-2760(66)90010-5. [DOI] [PubMed] [Google Scholar]
- Poenaru L., Dreyfus J. C. Electrophoretic study of hexosaminidases. Hexosaminidase C. Clin Chim Acta. 1973 Feb 12;43(3):439–442. doi: 10.1016/0009-8981(73)90486-5. [DOI] [PubMed] [Google Scholar]
- Proia R. L., d'Azzo A., Neufeld E. F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J Biol Chem. 1984 Mar 10;259(5):3350–3354. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. 3. Biochemical genetics of Tay-Sachs and Sandhoff's diseases. J Biol Chem. 1974 Apr 10;249(7):2054–2057. [PubMed] [Google Scholar]
- Suzuki K. The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem. 1965 Jul;12(7):629–638. doi: 10.1111/j.1471-4159.1965.tb04256.x. [DOI] [PubMed] [Google Scholar]
- Yu R. K., Itoh T., Yohe H. C., Macala L. J. Characterization of some minor gangliosides in Tay-Sachs brains. Brain Res. 1983 Sep 19;275(1):47–52. doi: 10.1016/0006-8993(83)90415-8. [DOI] [PubMed] [Google Scholar]
- von Specht B. U., Geiger B., Arnon R., Passwell J., Keren G., Goldman B., Padeh B. Enzyme replacement in Tay-Sachs disease. Neurology. 1979 Jun;29(6):848–854. doi: 10.1212/wnl.29.6.848. [DOI] [PubMed] [Google Scholar]