Abstract
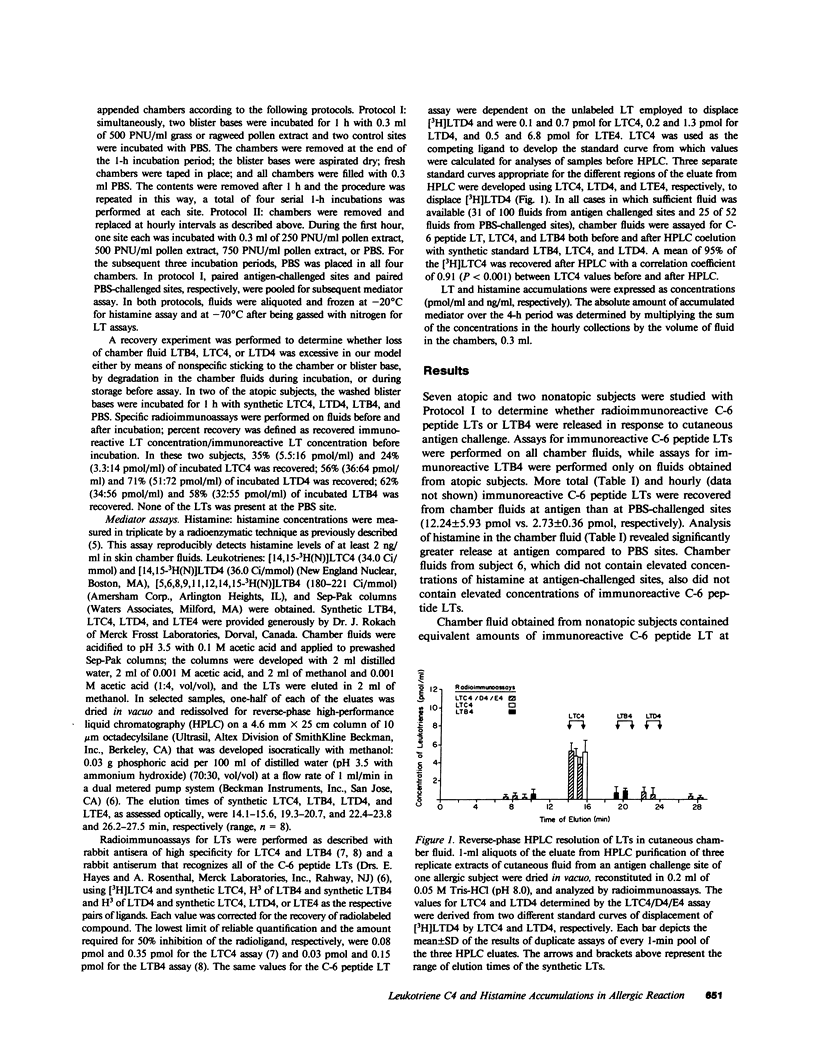
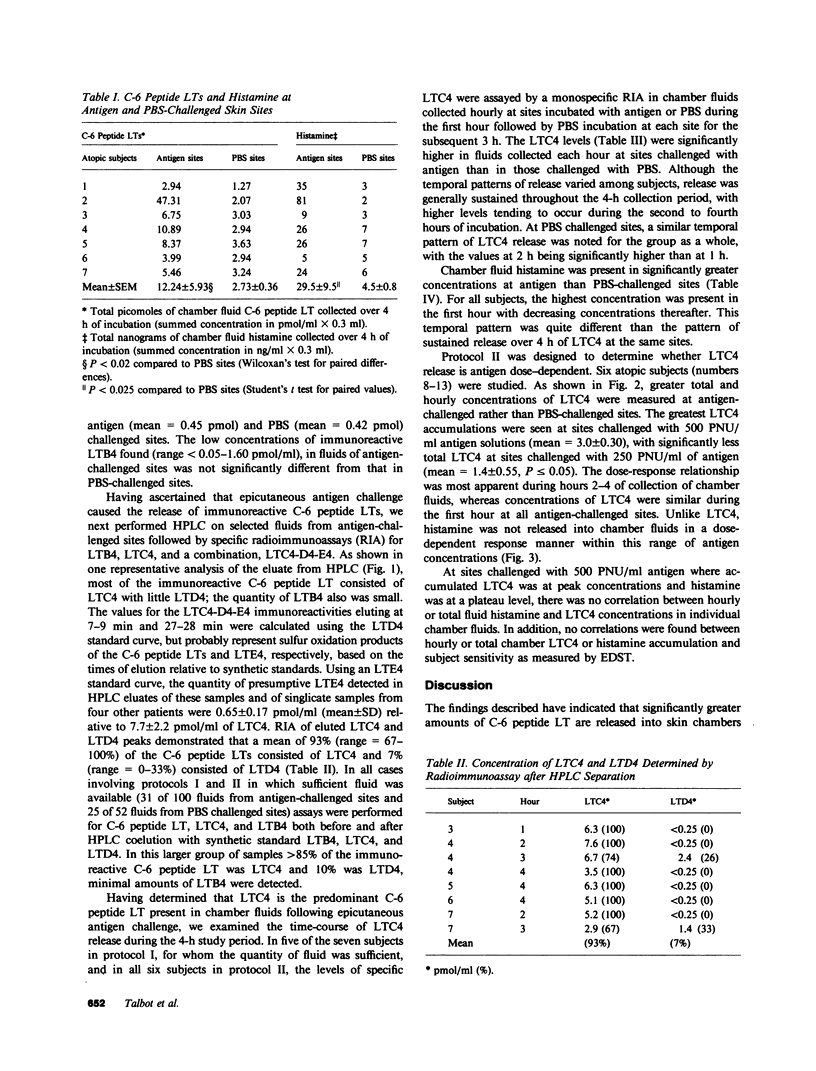
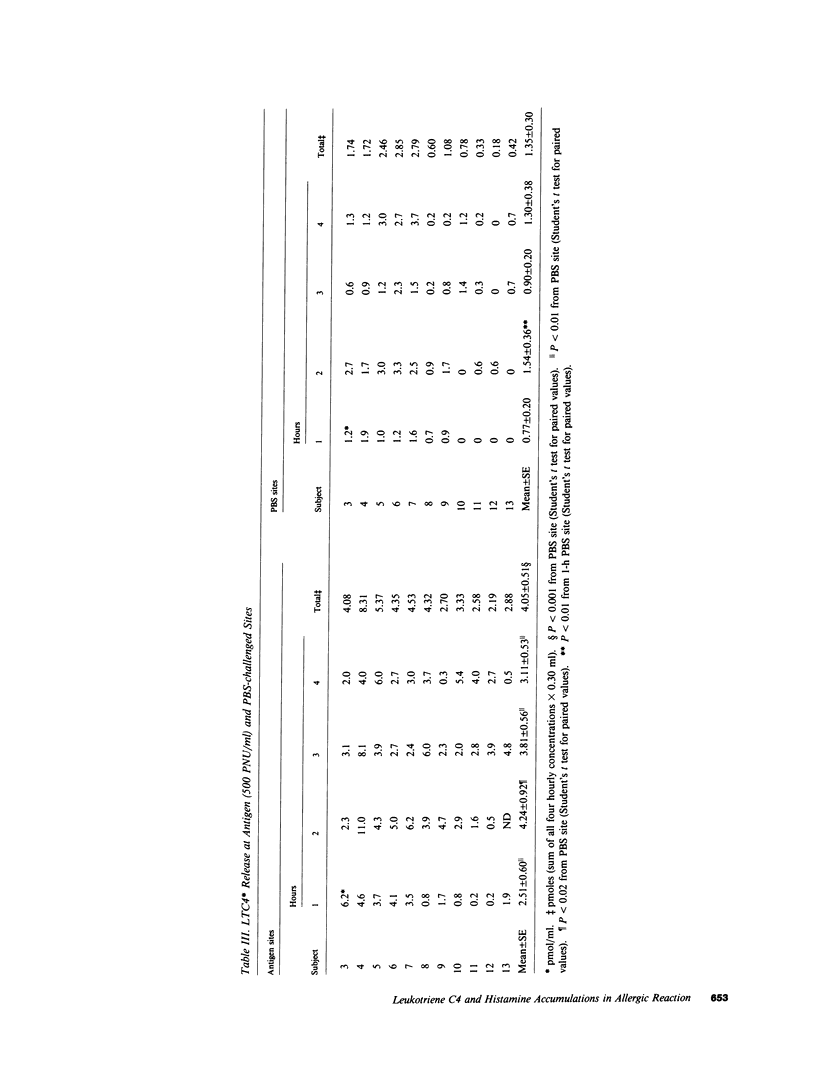
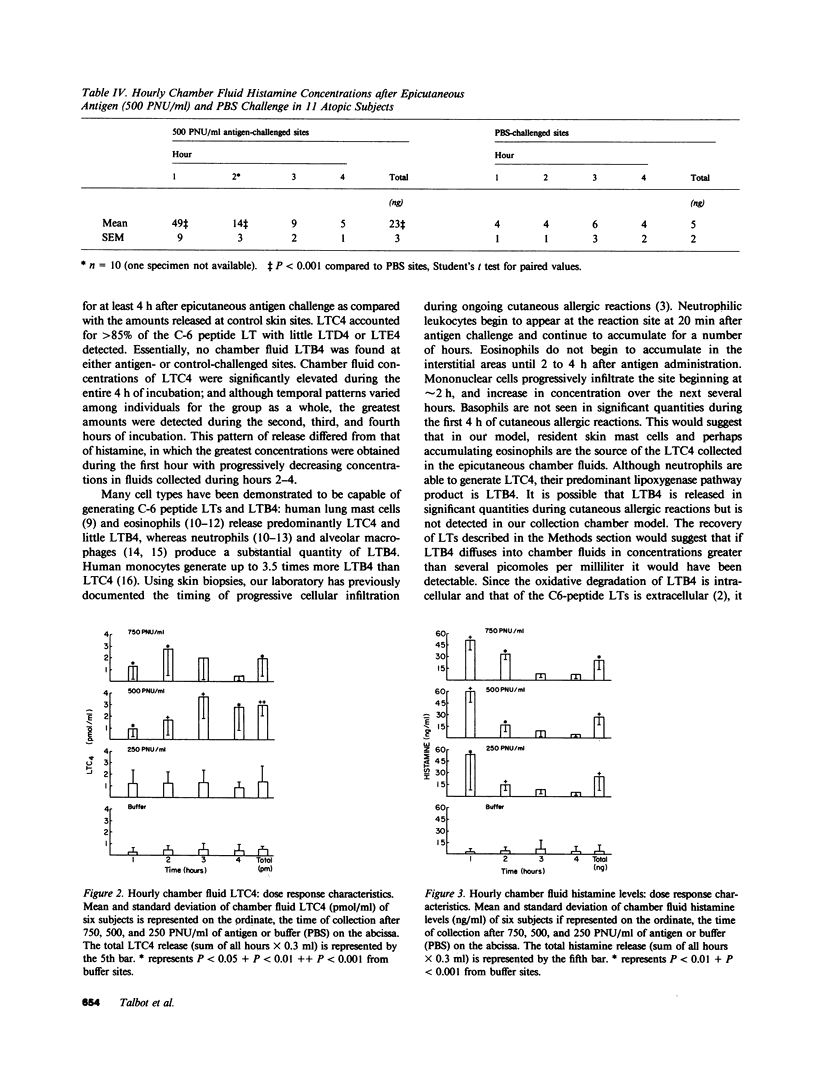
To determine whether lipoxygenase products of arachidonic acid metabolism are released in vivo during human allergic cutaneous reactions, we serially assayed chamber fluid placed over denuded skin sites for the presence of both C-6 peptide leukotrienes (e.g., LTC4, LTD4, and LTE4) and leukotriene B4 (LTB4), using radioimmune assay and HPLC separation, and compared it to histamine (assayed radioenzymatically) in 13 atopic and two nonatopic volunteers. Skin chamber sites challenged with ragweed or grass pollen antigen (250-750 protein nitrogen units/ml) for the first hour and phosphate-buffered saline (PBS) for the next 3 h were assayed hourly and compared to sites challenged with PBS alone. As assessed by HPLC, LTC4 composed greater than 85% of the C-6 peptide leukotriene released at any skin site, whereas little LTD4 or LTE4 was detected. LTC4 was present in significantly greater concentrations at antigen sites as compared to PBS-challenged sites throughout the 4-h period. Minimal concentrations of LTB4 were found throughout this time period and were not different at antigen or PBS sites. Histamine was present in significantly greater concentrations at antigen rather than PBS sites, but the pattern of release was different from that of LTC4. Peak histamine release invariably occurred during the first hour and decreased progressively thereafter, whereas the greatest amounts of LTC4 were detected during the 2nd to 4th hours. The amount of LTC4 accumulating at the site was dependent upon the dosage of antigen used in the epicutaneous challenge. We have demonstrated in this study that of the leukotrienes assessed LTC4 is released in the greatest quantity in situ during in vivo allergic cutaneous reactions and that it is present at such sites for at least 4 h after antigen challenge. Since intradermal injection of LTC4 in humans induces wheal and flare responses that persist for hours, our findings support the hypothesis that LTC4 is an important mediator of human allergic skin reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins P. C., Valenzano M., Zweiman B. Plasma concentrations of histamine measured by radioenzymatic assay: effects of histaminase incubations. J Allergy Clin Immunol. 1982 Jan;69(1 Pt 1):39–45. doi: 10.1016/0091-6749(82)90085-9. [DOI] [PubMed] [Google Scholar]
- Atkins P., Green G. R., Zweiman B. Histologic studies of human skin test responses to ragweed, compound 48-80, and histamine. J Allergy Clin Immunol. 1973 May;51(5):263–273. doi: 10.1016/0091-6749(73)90128-0. [DOI] [PubMed] [Google Scholar]
- Bedard P. M., Atkins P. C., Zweiman B. Antigen-induced local mediator release and cellular inflammatory responses in atopic subjects. J Allergy Clin Immunol. 1983 Apr;71(4):394–398. doi: 10.1016/0091-6749(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Bisgaard H., Ford-Hutchinson A. W., Charleson S., Taudorf E. Detection of leukotriene C4-liked immunoreactivity in tear fluid from subjects challenged with specific allergen. Prostaglandins. 1984 Mar;27(3):369–374. doi: 10.1016/0090-6980(84)90196-5. [DOI] [PubMed] [Google Scholar]
- Bisgaard H., Kristensen J., Søndergaard J. The effect of leukotriene C4 and D4 on cutaneous blood flow in humans. Prostaglandins. 1982 Jun;23(6):797–801. doi: 10.1016/0090-6980(82)90124-1. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Fruteau de Laclos B., Rabinovitch H., Picard S., Braquet P., Hébert J., Laviolette M. Eosinophil-rich human polymorphonuclear leukocyte preparations characteristically release leukotriene C4 on ionophore A23187 challenge. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):310–315. doi: 10.1016/0091-6749(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Creticos P. S., Peters S. P., Adkinson N. F., Jr, Naclerio R. M., Hayes E. C., Norman P. S., Lichtenstein L. M. Peptide leukotriene release after antigen challenge in patients sensitive to ragweed. N Engl J Med. 1984 Jun 21;310(25):1626–1630. doi: 10.1056/NEJM198406213102502. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Pawlowski N. A., Cramer E. B., King T. K., Cohn Z. A., Scott W. A. Human alveolar macrophages produce leukotriene B4. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7866–7870. doi: 10.1073/pnas.79.24.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Payan D. G., Goldman D. W. Immunopathogenetic roles of leukotrienes in human diseases. J Clin Immunol. 1984 Mar;4(2):79–84. doi: 10.1007/BF00915039. [DOI] [PubMed] [Google Scholar]
- Holroyde M. C., Altounyan R. E., Cole M., Dixon M., Elliott E. V. Bronchoconstriction produced in man by leukotrienes C and D. Lancet. 1981 Jul 4;2(8236):17–18. doi: 10.1016/s0140-6736(81)90254-3. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Chernov T., Goetzl E. J. Characteristics of the epitope of leukotriene B4 recognized by a highly specific mouse monoclonal antibody. Biochem Biophys Res Commun. 1984 Sep 28;123(3):944–950. doi: 10.1016/s0006-291x(84)80225-9. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren J. A., Hammarström S., Goetzl E. J. A sensitive and specific radioimmunoassay for leukotriene C4. FEBS Lett. 1983 Feb 7;152(1):83–88. doi: 10.1016/0014-5793(83)80487-6. [DOI] [PubMed] [Google Scholar]
- Matthay M. A., Eschenbacher W. L., Goetzl E. J. Elevated concentrations of leukotriene D4 in pulmonary edema fluid of patients with the adult respiratory distress syndrome. J Clin Immunol. 1984 Nov;4(6):479–483. doi: 10.1007/BF00916578. [DOI] [PubMed] [Google Scholar]
- Pawlowski N. A., Abraham E., Hamill A., Scott W. A. The cyclooxygenase and lipoxygenase activities of platelet-depleted human monocytes. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):324–330. doi: 10.1016/0091-6749(84)90124-6. [DOI] [PubMed] [Google Scholar]
- Peters S. P., MacGlashan D. W., Jr, Schulman E. S., Schleimer R. P., Hayes E. C., Rokach J., Adkinson N. F., Jr, Lichtenstein L. M. Arachidonic acid metabolism in purified human lung mast cells. J Immunol. 1984 Apr;132(4):1972–1979. [PubMed] [Google Scholar]
- Soter N. A., Lewis R. A., Corey E. J., Austen K. F. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983 Feb;80(2):115–119. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- Talbot S. F., Atkins P. C., Valenzano M., Zweiman B. Correlations of in vivo mediator release with late cutaneous allergic responses in humans. I. Kinetics of histamine release. J Allergy Clin Immunol. 1984 Dec;74(6):819–826. doi: 10.1016/0091-6749(84)90185-4. [DOI] [PubMed] [Google Scholar]
- Weiss J. W., Drazen J. M., Coles N., McFadden E. R., Jr, Weller P. F., Corey E. J., Lewis R. A., Austen K. F. Bronchoconstrictor effects of leukotriene C in humans. Science. 1982 Apr 9;216(4542):196–198. doi: 10.1126/science.7063880. [DOI] [PubMed] [Google Scholar]
- Weiss J. W., Drazen J. M., McFadden E. R., Jr, Weller P., Corey E. J., Lewis R. A., Austen K. F. Airway constriction in normal humans produced by inhalation of leukotriene D. Potency, time course, and effect of aspirin therapy. JAMA. 1983 May 27;249(20):2814–2817. [PubMed] [Google Scholar]
- Weller P. F., Lee C. W., Foster D. W., Corey E. J., Austen K. F., Lewis R. A. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7626–7630. doi: 10.1073/pnas.80.24.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]


