Abstract
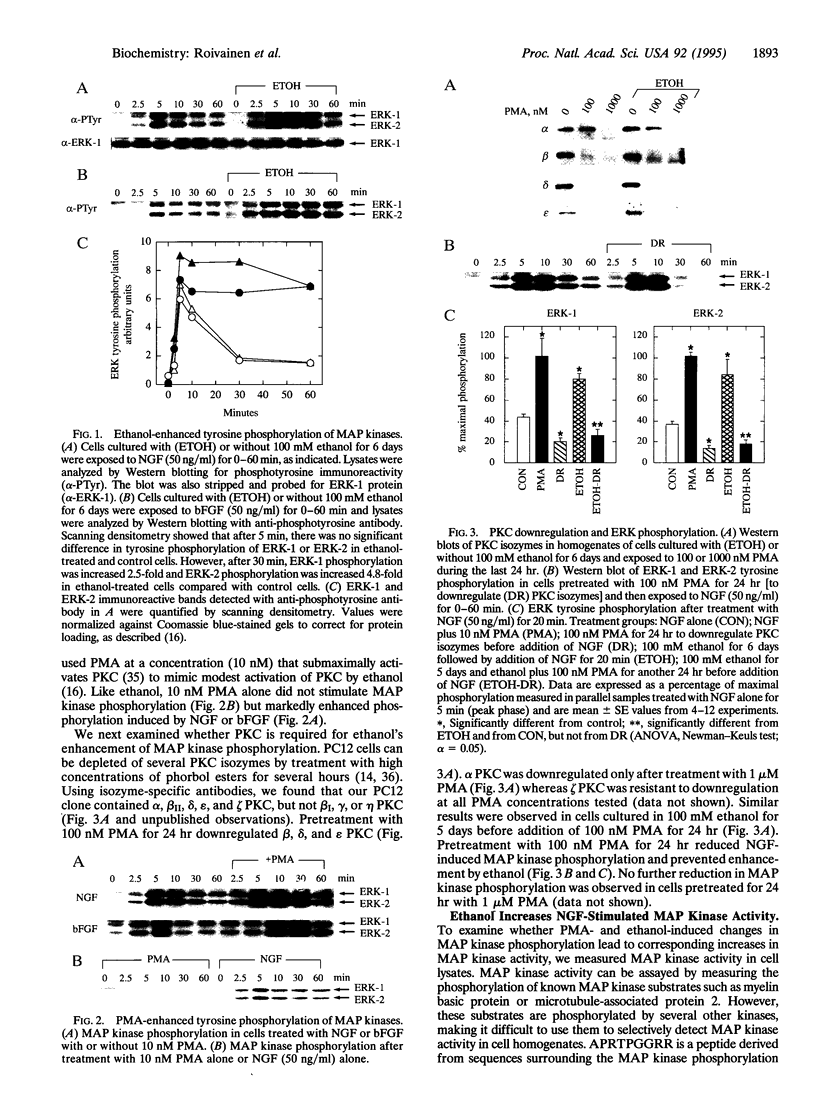
Excessive alcohol consumption alters neuronal growth and causes striking elongation of axons and dendrites in several brain regions. This could result from increased sensitivity to neurotrophic factors, since ethanol markedly enhances nerve growth factor (NGF)- and basic fibroblast growth factor (bFGF)-stimulated neurite outgrowth in the neural cell line PC12. The mechanism by which ethanol enhances growth factor responses was investigated by examining activation of mitogen-activated protein kinases (MAP kinases), a key event in growth factor signaling. Ethanol (100 mM) increased NGF- and bFGF-induced activation of MAP kinases. This increase, like ethanol-induced increases in neurite outgrowth, was prevented by down regulation of beta, delta, and epsilon protein kinase C (PKC) isozymes. Since chronic ethanol exposure specifically upregulates delta and epsilon PKC, these findings suggest that ethanol promotes neurite growth by enhancing growth factor signal transduction through a delta or epsilon PKC-regulated pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Robbins D. J., Haycock J. W., Seger R., Cobb M. H., Krebs E. G. Identification of an activator of the microtubule-associated protein 2 kinases ERK1 and ERK2 in PC12 cells stimulated with nerve growth factor or bradykinin. J Neurochem. 1992 Jul;59(1):147–156. doi: 10.1111/j.1471-4159.1992.tb08885.x. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A., Tavares M. A., Uylings H. B., Paula-Barbosa M. Granule cell loss and dendritic regrowth in the hippocampal dentate gyrus of the rat after chronic alcohol consumption. Brain Res. 1988 Nov 8;473(1):1–14. doi: 10.1016/0006-8993(88)90309-5. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Charles C. H., Abler A. S., Lau L. F. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992 Jan;7(1):187–190. [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Galofré E., Fábregues I., López-Tejero D. Effects of chronic ethanol consumption beginning at adolescence: increased numbers of dendritic spines on cortical pyramidal cells in the adulthood. Acta Neuropathol. 1989;78(5):528–532. doi: 10.1007/BF00687715. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Yamashita T., Hoshi M., Kawakami M., Sakai H. Microtubule-associated-protein (MAP) kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- Haycock J. W., Ahn N. G., Cobb M. H., Krebs E. G. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. A., Hunter B. E., Walker D. W. Alterations and recovery of dendritic spine density in rat hippocampus following long-term ethanol ingestion. Brain Res. 1988 Sep 6;459(2):381–385. doi: 10.1016/0006-8993(88)90656-7. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Landreth G. E., Smith D. S., McCabe C., Gittinger C. Characterization of a nerve growth factor-stimulated protein kinase in PC12 cells which phosphorylates microtubule-associated protein 2 and pp250. J Neurochem. 1990 Aug;55(2):514–523. doi: 10.1111/j.1471-4159.1990.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993 Jan 29;72(2):269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Messing R. O., Carpenter C. L., Diamond I., Greenberg D. A. Ethanol regulates calcium channels in clonal neural cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6213–6215. doi: 10.1073/pnas.83.16.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing R. O., Henteleff M., Park J. J. Ethanol enhances growth factor-induced neurite formation in PC12 cells. Brain Res. 1991 Nov 29;565(2):301–311. doi: 10.1016/0006-8993(91)91662-k. [DOI] [PubMed] [Google Scholar]
- Messing R. O., Petersen P. J., Henrich C. J. Chronic ethanol exposure increases levels of protein kinase C delta and epsilon and protein kinase C-mediated phosphorylation in cultured neural cells. J Biol Chem. 1991 Dec 5;266(34):23428–23432. [PubMed] [Google Scholar]
- Miller M. W., Chiaia N. L., Rhoades R. W. Intracellular recording and injection study of corticospinal neurons in the rat somatosensory cortex: effect of prenatal exposure to ethanol. J Comp Neurol. 1990 Jul 1;297(1):91–105. doi: 10.1002/cne.902970107. [DOI] [PubMed] [Google Scholar]
- Nemenoff R. A., Winitz S., Qian N. X., Van Putten V., Johnson G. L., Heasley L. E. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993 Jan 25;268(3):1960–1964. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Pentney R. J., Quackenbush L. J. Dendritic hypertrophy in Purkinje neurons of old Fischer 344 rats after long-term ethanol treatment. Alcohol Clin Exp Res. 1990 Dec;14(6):878–886. doi: 10.1111/j.1530-0277.1990.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Qui M. S., Green S. H. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992 Oct;9(4):705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- Rohan P. J., Davis P., Moskaluk C. A., Kearns M., Krutzsch H., Siebenlist U., Kelly K. PAC-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science. 1993 Mar 19;259(5102):1763–1766. doi: 10.1126/science.7681221. [DOI] [PubMed] [Google Scholar]
- Roivainen R., McMahon T., Messing R. O. Protein kinase C isozymes that mediate enhancement of neurite outgrowth by ethanol and phorbol esters in PC12 cells. Brain Res. 1993 Oct 8;624(1-2):85–93. doi: 10.1016/0006-8993(93)90063-s. [DOI] [PubMed] [Google Scholar]
- Roivainen R., Messing R. O. The phorbol derivatives thymeleatoxin and 12-deoxyphorbol-13-O-phenylacetate-10-acetate cause translocation and down-regulation of multiple protein kinase C isozymes. FEBS Lett. 1993 Mar 15;319(1-2):31–34. doi: 10.1016/0014-5793(93)80031-o. [DOI] [PubMed] [Google Scholar]
- Sanghera J. S., Peter M., Nigg E. A., Pelech S. L. Immunological characterization of avian MAP kinases: evidence for nuclear localization. Mol Biol Cell. 1992 Jul;3(7):775–787. doi: 10.1091/mbc.3.7.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Kaziro Y. Function of Ras as a molecular switch in signal transduction. J Biol Chem. 1992 Dec 5;267(34):24149–24152. [PubMed] [Google Scholar]
- Seth A., Gonzalez F. A., Gupta S., Raden D. L., Davis R. J. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992 Dec 5;267(34):24796–24804. [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993 Nov 5;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]
- Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992 Mar 20;68(6):1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- Troppmair J., Bruder J. T., App H., Cai H., Liptak L., Szeberényi J., Cooper G. M., Rapp U. R. Ras controls coupling of growth factor receptors and protein kinase C in the membrane to Raf-1 and B-Raf protein serine kinases in the cytosol. Oncogene. 1992 Sep;7(9):1867–1873. [PubMed] [Google Scholar]
- Volonté C., Rukenstein A., Loeb D. M., Greene L. A. Differential inhibition of nerve growth factor responses by purine analogues: correlation with inhibition of a nerve growth factor-activated protein kinase. J Cell Biol. 1989 Nov;109(5):2395–2403. doi: 10.1083/jcb.109.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. W., Hunter B. E., Abraham W. C. Neuroanatomical and functional deficits subsequent to chronic ethanol administration in animals. Alcohol Clin Exp Res. 1981 Spring;5(2):267–282. doi: 10.1111/j.1530-0277.1981.tb04901.x. [DOI] [PubMed] [Google Scholar]
- West J. R., Hodges C. A., Black A. C., Jr Prenatal exposure to ethanol alters the organization of hippocampal mossy fibers in rats. Science. 1981 Feb 27;211(4485):957–959. doi: 10.1126/science.7466371. [DOI] [PubMed] [Google Scholar]
- Wooten M. W., Ewald S. J. Alcohols synergize with NGF to induce early differentiation of PC12 cells. Brain Res. 1991 Jun 7;550(2):333–339. doi: 10.1016/0006-8993(91)91337-z. [DOI] [PubMed] [Google Scholar]
- Zou J., Rabin R. A., Pentney R. J. Ethanol enhances neurite outgrowth in primary cultures of rat cerebellar macroneurons. Brain Res Dev Brain Res. 1993 Mar 19;72(1):75–84. doi: 10.1016/0165-3806(93)90161-3. [DOI] [PubMed] [Google Scholar]