Abstract
Background
Cryptococcosis due to Cryptococcus gattii is endemic in various parts of the world, affecting mostly immunocompetent patients. A national surveillance study of cryptococcosis, including demographical, clinical and microbiological data, has been ongoing since 1997 in Colombia, to provide insights into the epidemiology of this mycosis.
Methodology/Principal Findings
From 1,209 surveys analyzed between 1997–2011, 45 cases caused by C. gattii were reported (prevalence 3.7%; annual incidence 0.07 cases/million inhabitants/year). Norte de Santander had the highest incidence (0.81 cases/million/year), representing 33.3% of all cases. The male: female ratio was 3.3∶1. Mean age at diagnosis was 41±16 years. No specific risk factors were identified in 91.1% of patients. HIV infection was reported in 6.7% of patients, autoimmune disease and steroids use in 2.2%. Clinical features included headache (80.5%), nausea/vomiting (56.1%) and neurological derangements (48.8%). Chest radiographs were taken in 21 (46.7%) cases, with abnormal findings in 7 (33.3%). Cranial CT scans were obtained in 15 (33.3%) cases, with abnormalities detected in 10 (66.7%). Treatment was well documented in 30 cases, with most receiving amphotericin B. Direct sample examination was positive in 97.7% cases. Antigen detection was positive for all CSF specimens and for 75% of serum samples. C. gattii was recovered from CSF (93.3%) and respiratory specimens (6.6%). Serotype was determined in 42 isolates; 36 isolates were serotype B (85.7%), while 6 were C (14.3%). The breakdowns of molecular types were VGII (55.6%), VGIII (31.1%) and VGI (13.3%). Among 44 strains, 16 MLST sequence types (ST) were identified, 11 of them newly reported.
Conclusions/Significance
The results of this passive surveillance study demonstrate that cryptococcosis caused by C. gattii has a low prevalence in Colombia, with the exception of Norte de Santander. The predominance of molecular type VGII is of concern considering its association with high virulence and the potential to evolve into outbreaks.
Author Summary
Cryptococcosis is caused by Cryptococcus neoformans and C. gattii, with the most serious manifestation of disease being infection of the central nervous system (CNS). C. neoformans tends to cause disease in immunosuppressed patients, especially those infected with HIV, while C. gattii affects immunocompetent patients preferentially. C. gattii is usually endemic in tropical and subtropical areas. However, highly virulent strains have recently emerged in temperate areas, such as British Columbia in Canada and the Pacific Northwest of the United States. The Colombian national cryptococcal survey characterized the demographic, clinical manifestations and microbiological aspects of C. gattii cryptococcosis. An annual average incidence of infection of 0.07 cases/million inhabitants/year was determined. In contrast, in Norte de Santander the incidence reached 0.81 cases/million inhabitants/year. The national prevalence was 3.7% among all forms of cryptococcosis. Involvement of the CNS (88%) was the commonest clinical manifestation of cryptococcosis. Molecular type VGII, which is the same molecular type as described in the recent outbreaks of this mycosis, was the most prevalent. Overall, clinical C. gattii strains from Colombia showed great genetic diversity. This work contributes to knowledge of the global epidemiology of cryptococcosis and its clinical behavior in Colombian patients.
Introduction
Cryptococcosis is a fungal disease that affects humans and animals and is caused by two species, Cryptococcus neoformans and Cryptococcus gattii [1]. C. gattii has been recognized as a distinct species from C. neoformans due to differences in the morphology of the basidia, environmental niches, multiple gene genealogies, unique patterns generated by different molecular typing techniques, inefficient crossing of species with the production of sterile progeny and a lack of genetic recombination [2]. C. gattii can be easily and reliably differentiated from C. neoformans through a simple phenotypic procedure, growth on CGB (canavanine, glycine and bromothymol blue) culture medium [3]. C. gattii assimilates glycine, is resistant to canavanine and changes the color of the media due to an alteration in pH when creatinine is degraded into ammonia. C. neoformans is not able to assimilate glycine; therefore, it does not grow on this media [3].
The two species cause different clinical manifestations and have different biological characteristics [1]. C. neoformans is responsible for most cases of cryptococcosis worldwide [4]. Until recently C. gattii was considered rare, however cryptococcosis by C. gattii has gained importance because of its emergence in 1999, which resulted in an outbreak on Vancouver Island and other closely related regions in British Columbia, Canada [5] and the increasing number of cases since 2004 in the Pacific Northwest of the United States [6]. Recent studies based on Multilocus Microsatellite Type (MLMT) and Multilocus Sequence Type (MLST) analyses, as well as whole genome analysis, point towards South America as a potential origin for the outbreak strains [7], [8].
Previously, it was thought that C. gattii was restricted to tropical and subtropical regions [9], but the emergence of the outbreak events due to virulent strains in temperate areas of North America suggest a more global distribution of this yeast [5], [6]. In addition, independent cases have been reported from Mediterranean Europe [10].
The knowledge about the epidemiology of C. gattii is recent, although the first publications of meningeal cryptococcosis was in a child from the Congo in 1970 [11], while another proven infection occurred in a patient with a lumbar tumor as described by the French physician Ferdinand Curtis in 1896 [12].
C. gattii is a fungal pathogen globally set, with a potential primary ecological niche being associated in some way with decaying wood from a large range of tree species [13]. C. gattii has been associated with at least 54 species of trees native to tropical, subtropical and temperate regions [13], [14]. Currently, four major molecular types of C. gattii (VGI = AFLP4, VGII = AFLP6, VGIII = AFLP5 and VGIV = AFLP7) are accepted according to the characterization by PCR fingerprinting, Random Amplification of Polymorphic DNA (RAPD), Amplified Fragment Length Polymorphism (AFLP), MLMT and MLST analyses, which are different to the genotypes of C. neoformans (VNI to VNIV) [15]–[17]. In addition a number of hybrids, including inter-species (C. neoformans var. neoformans x C. gattii DB, C. neoformans var. grubii x C. gattii AB) and intra-species (C. neoformans var. grubii x C. neoformans var. neoformans AD) hybrids, have been identified [18], [19].
In Colombia, a national survey on cryptococcosis is ongoing since 1997, led by the Instituto Nacional de Salud in Bogotá (INS) and the Corporación para Investigaciones Biológicas in Medellin (CIB) [20], [21]. The objective of the present work was to analyze demographic, clinical and microbiological data concerning cryptococcosis caused by C. gattii received through the survey during the period of 1997–2011. Our overarching aim was to provide a broad brushstroke picture of this mycosis in Colombia.
Materials and Methods
Design
This is a descriptive study of the clinical, epidemiologic and microbiological characteristics of cases of cryptococcosis due to C. gattii in Colombia, identified through an ongoing national survey.
Survey
The information was obtained by a survey designed according to the guidelines of the European Confederation of Medical Mycology, and processed by health professionals in different public and private health care institutions in Colombia, which also sent the corresponding strains from each case to the INS for centralized genotyping.
The survey contained the following information: year of diagnosis of cryptococcosis, patient's demographic data, such as gender and age, department (Colombia political divisions) and place of residence, risk factors for cryptococcosis (HIV infection, use of corticosteroids, autoimmune disease, organ transplants, presence of malignant solid tumors and hematologic malignancies, diabetes mellitus, liver cirrhosis, chronic kidney disease and sarcoidosis). In cases associated with HIV infection, we asked if cryptococcosis defined AIDS; the date of diagnosis of HIV, the clinical manifestations of cryptococcosis and the type of initial treatment. Also, diagnostic tests performed viz. direct examinations, cultures and the determination of the capsular antigen in serum and cerebrospinal fluid (CSF) and the findings of diagnostic imaging (radiographs of the chest and cross-sectional neuroimaging). In addition to the aforementioned information, the evolution of the disease was determined for patients from Norte de Santander.
Ethical statement
The study was approved by the Ethics Committee of the CIB. Additionally, it had technical and ethical approval of the INS.
Analysis
With the information received, a database was created using Biolomics ver. 7.5.44 (BioAware SA., Belgium), while the data were analyzed statistically using Epiinfo ver. 6.1 (CDC, USA).
Case definition
A case was considered probable when there were clinical findings consistent with cryptococcosis. Cases were confirmed after isolation of a Cryptococcus spp from a normally sterile site, from sputum, bronchoalveolar lavage or biopsy.
Incidence
The mean annual incidence rate was determined using as denominator the Colombian population census done in 2003, an intermediate year of the surveillance, determined by DANE [22]. For Norte de Santander, the population for the year 2003 was also used [22].
Microbiology
Strain identification was done using conventional mycology techniques. Determination of species was performed by culturing the strains on CGB media [3]. In the first years of surveillance, serotype (B versus C) was determined using specific antisera available commercially (Iatron, Japan).
Antifungal susceptibility
The susceptibility profiles to amphotericin B (AMB) using the E-test (BioMerieux, France) and to fluconazole (FCZ) and voriconazole (VCZ) using disc diffusion method M44-A described by the Clinical Laboratory Standards Institute (CLSI) were determined at the CIB laboratory. Quality control was done by including the Candida albicans strain ATCC 90028, which shows an inhibition range between 32–43 mm.
Molecular type and mating type
Molecular type was determined in all strains using PCR fingerprinting with the primer (GTG)5 [15]. Mating type a or α (alpha) was determined using specific primers described previously [23].
Multilocus Sequence Typing (MLST)
For 44 strains, seven unlinked genetic loci, including CAP59, GPD1, LAC1, PLB1, SOD1, URA5 and the IGS1 region, were amplified following the ISHAM consensus MLST typing scheme for C. neoformans and C. gattii [16]. Amplification of loci was carried out in the Molecular Mycology Research Laboratory, University of Sydney at Westmead Hospital, Westmead, Australia, and the sequences were obtained commercially (Macrogen Inc., Korea). The generated sequences were manually edited using the Sequencher ver. 5.2 (Gene Codes Corporation, USA) software. With the concatenated sequences, a dendrogram showing the genetic relationships between the strains was constructed with the program Mega version 5.05 [24], based on maximum likelihood analysis. Allele types and sequence types (ST) were identified using the ISHAM consensus MLST database at mlst.mycologylab.org. The reference strains for C. gattii WM 179 (VGI = AFLP4; B/alpha), WM 178 (VGII = AFLP6; B/alpha), WM 175 (VGIII = AFLP5; B/alpha) and WM 779 (VGIV = AFLP7; C/alpha), were included in the genetic analyses for verification of the four major molecular types of C. gattii [15]. Genetic diversity was assessed by calculating the Simpson's diversity index (D) [25].
Literature review
A search was performed using the Medline database about cryptococcosis by C. gattii, reported from January 1970 to 31 July 2014, its prevalence and the molecular types of clinical strains from the world using the following search terms in English: Cryptococcus gattii, epidemiology, meningitis, HIV, AIDS, children. Articles in Spanish and Portuguese were searched in the databases SciELO and Lilacs. Only available articles were included. In addition, some references cited in articles obtained in the primary search were included also.
Results
Prevalence, incidence of cases per year and place of origin
Over the period from January 1997 to December 2011, 1,209 surveys were completed, with corresponding cultured isolate, and were submitted from 76 centers in 25 departments of Colombia and Bogotá D.C. Of these, 45 (3.7%) corresponded to cases of cryptococcosis by C. gattii.
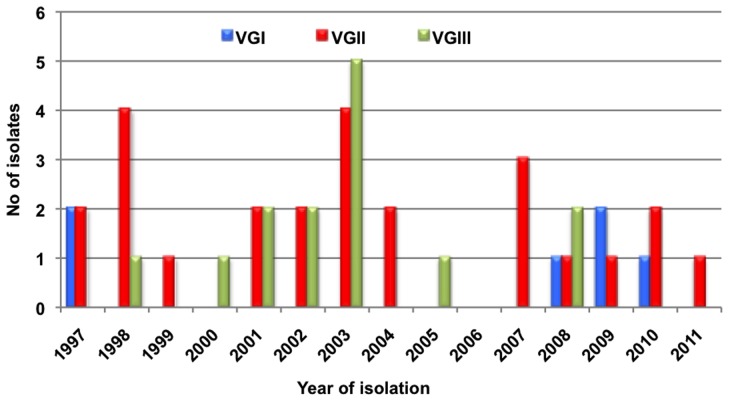
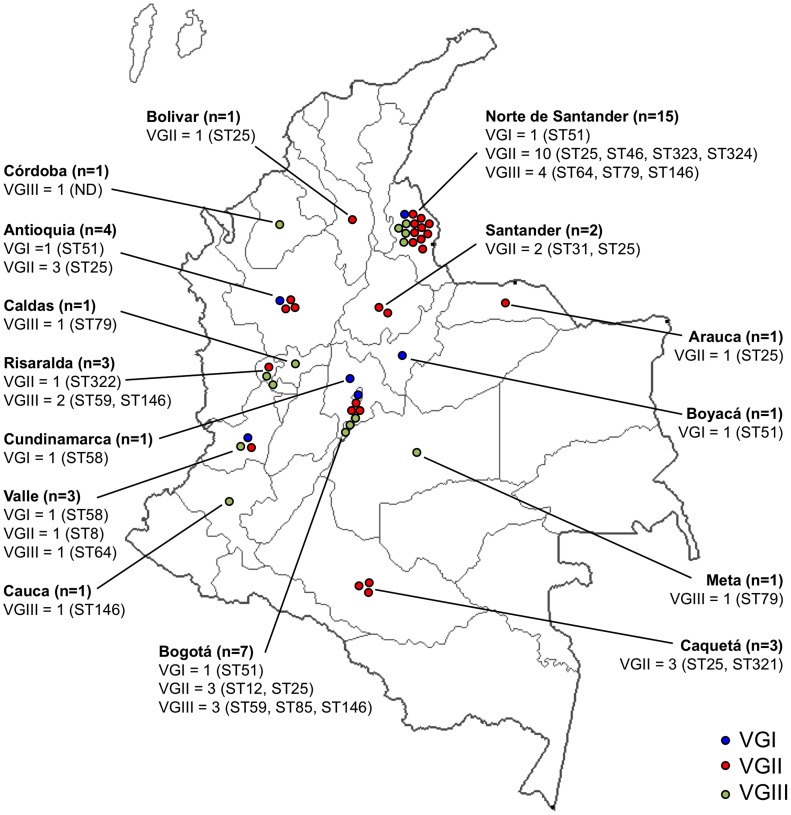
The number of cases received per year is shown in Figure 1 and Table S1 and the origin and number of cases per department is shown in Figure 2 and Table S1. It should be noted that almost a third of the patients (33.3%) resided in Norte de Santander. The average annual incidence of C. gattii in Colombia was 0.07 cases per million inhabitants per year, but in Norte de Santander, it was 0.81 cases per million inhabitants per year.
Figure 1. Distribution per year and molecular type of Cryptococcus gattii strains recovered in Colombia from 1997–2011.
Figure 2. Distribution per department of the number, molecular type and STs of Cryptococcus gattii strains recovered in Colombia from 1997 to 2011.
ND = ST was not determined.
Demographic information
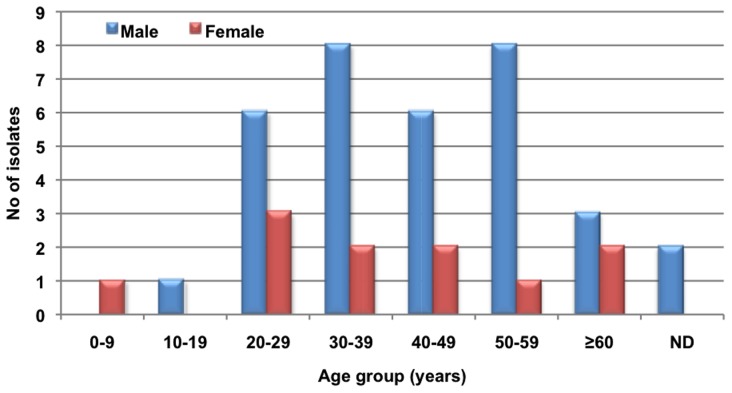
A preponderance of males 34 (75.5%) was found, with the male: female ratio being 3.3∶1. The average age of the patients was 40±16 years with a range of4 to 68 years. The age and gender distribution is shown in Figure 3. There were 2 (4.5%) cases in children under 16 years-of-age.
Figure 3. Distribution per age and gender of cryptococcosis cases reported in Colombia from 1997 to 2011.
Risk factors
No predisposing risk factors were reported in 41 (91.1%) of patients. From the cases in which the risk factors were known, the most frequent were: HIV infection (3; 6.7%) and autoimmune disease with the use of steroids (1; 2.2%). Analysis of the Norte de Santander patients revealed that 93% had no apparent risk factor.
Clinical findings
Clinical manifestations were available for 41 patients (Table 1). The most frequent abnormal physical findings were: headache (33; 80.5%), nausea and vomiting (23; 56.1%), mental changes (20; 48.8%) and visual alterations (18; 43.9%).
Table 1. Clinical manifestations of patients with Cryptococcus gattii cryptococcosis in Colombia, 1997–2011.
| Signs and symptoms | n/Total | % |
| Headache | 33/41 | 80.5 |
| Nausea and vomiting | 23/41 | 56.1 |
| Mental alterations | 20/41 | 48.8 |
| Visual alterations | 18/41 | 43.9 |
| Fever | 15/41 | 36.6 |
| Meningeal signs | 14/41 | 34.1 |
| Intracranial hypertension with or without hydrocephalus | 12/41 | 29.3 |
| Seizures | 7/41 | 17.1 |
| Focal neurological signs | 6/41 | 14.6 |
| Cough | 4/41 | 9.8 |
The most common clinical presentation was neurocryptococcosis (39 cases; 86.7%), followed by pulmonary cryptococcosis (3; 6.7%) and disseminated disease (2; 4.4%). Clinical presentation was not determined in 1 case (2.2%).
Diagnostic imaging
In 21 (46.7%) cases results of chest X-ray were available, with 7 (33.3%). showing abnormalities. Computed tomography (CT) of the head was conducted in 15 (33.3%) cases with abnormalities reported in 10 (66.7%).
Treatment
Initial antifungal therapy was reported in 30 (66.7%) surveys. AMB was used most often, in 29 (96.7%) patients.
Microbiology
Various diagnostic methods were carried out for cryptococcal identification, including direct microscopy, determination of the capsular antigen in serum and CSF and culture of CSF and respiratory samples. The results of these tests are described in Table 2. The serotype was determined in 42 (93.3%) strains: 36 (85.7%) were serotype B, while 6 (14.3%) were serotype C.
Table 2. Clinical, microbiological and molecular data of Cryptococcus gattii cryptococcosis cases in Colombia, 1997–2011.
| Type of exam | n/total | % |
| Positive direct examination | 42/43 | 97.7 |
| Capsular antigen detection | 14/18 | 77.8 |
| Serum reactive | 6/8 | 75.0 |
| CSF reactive | 14/14 | 100.0 |
| Positive culture | 45/45 | 100.0 |
| Origin of strains | ||
| CSF | 40/45 | 88.9 |
| CSF and blood | 2/45 | 4.4 |
| Bronchoalveolar lavage | 1/45 | 2.2 |
| Lung biopsy | 1/45 | 2.2 |
| Sputum | 1/45 | 2.2 |
| Serotype | ||
| B | 36/42 | 85.7 |
| C | 6/42 | 14.3 |
| Molecular type | ||
| VGI | 6/45 | 13.3 |
| VGII | 25/45 | 55.6 |
| VGIII | 14/45 | 31.1 |
| Mating type | ||
| a | 21/45 | 46.7 |
| alpha | 24/45 | 53.3 |
Antifungal susceptibility
Susceptibility to antifungals was determined for 42/45 C. gattii strains. All strains were susceptible to AMB (MIC <2 µg/ml). Thirteen (31%) were susceptible (MIC ≤8 µg/ml), 14 (33.3%) susceptible dose-dependent (SDD) (MIC 16–32 µg/ml) and 15 (35.7%) resistant (MIC ≥64 µg/ml) to FCZ. With regard to VCZ, 39 (92.9%) were susceptible (MIC ≤1 µg/ml), 1 (2.4%) SDD (MIC 2 µg/ml) and 2 (4.8%) resistant (MIC ≥4 µg/ml).
Molecular type and mating type
The molecular types of the 45 VG isolates in order of frequency were: VGII 25 (55.6%), VGIII 14 (31.1%), and VGI 6 (13.3%) (Table 2). The distribution of isolates per molecular type per department is shown in Figure 2. In regard to mating type of the strains, 24 (53.3%) were mating type α and 21 (46.7%) were mating type a (Table 2).
Multilocus sequence typing (MLST)
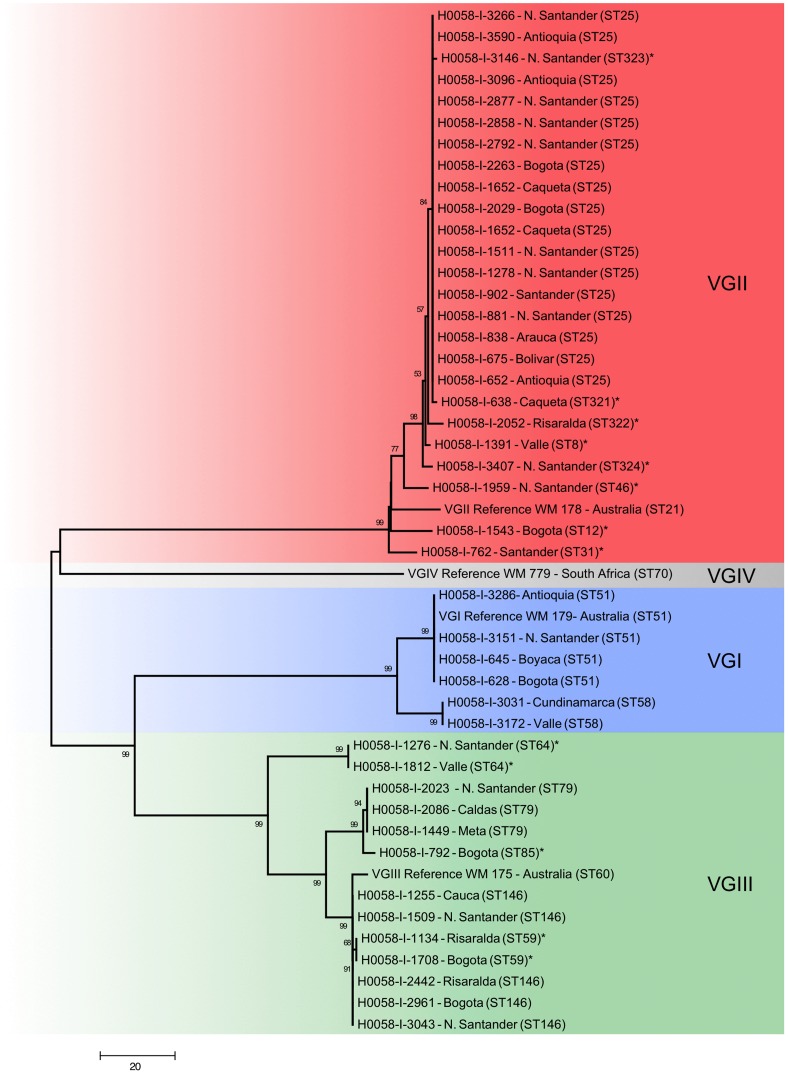
Among the 44 isolates genotyped, 16 sequence types (ST) were identified: two STs amongst the VGI strains, nine STs amongst the VGII strains (with ST25 being the most common; 17 (68%) strains) and five STs amongst the VGIII strains (Table 3). Eight STs of the VGII strains (ST8, ST12, ST31, ST46, ST321, ST322, ST323 and ST324) and three of the VGIII strains (ST59, ST64 and ST85) were identified for the first time in this study. The genetic relationship of the studied strains is shown in Figure 4, while the distribution of the STs per department is shown in Figure 2. The MLST data was incorporated into the C. neoformans and C. gattii MLST databases accessible at http://mlst.mycologylab.org and the sequences of identified alleles were deposited in GenBank (Table S2).
Table 3. Molecular type, mating type, allele types and sequence types of Colombian Cryptococcus gattii clinical strains.
| Molecular type RFLP/AFLP | Strain number | Other collection number | State | Mating type | CAP59 | GPD1 | IGS1 | LAC1 | PLB1 | SOD1 | URA5 | ST |
| VGI/AFLP4 | H0058-I-628 | WM 2039 | Bogotá | a | 16 | 5 | 3 | 5 | 5 | 32 | 12 | 51 |
| H0058-I-645 | Boyacá | alpha | 16 | 5 | 3 | 5 | 5 | 32 | 12 | 51 | ||
| H0058-I-3151 | Norte de Santander | alpha | 16 | 5 | 3 | 5 | 5 | 32 | 12 | 51 | ||
| H0058-I-3286 | Antioquia | alpha | 16 | 5 | 3 | 5 | 5 | 32 | 12 | 51 | ||
| H0058-I-3031 | Cundinamarca | alpha | 16 | 11 | 13 | 19 | 15 | 34 | 14 | 58 | ||
| H0058-I-3172 | Valle | alpha | 16 | 11 | 13 | 19 | 15 | 34 | 14 | 58 | ||
| VGII/AFLP6 | H0058-I-652 | WM 2043 | Antioquia | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 |
| H0058-I-675 | WM 04.77, LA584 | Bolívar | alpha | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-838 | WM 2065, LA596 | Arauca | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-881 | WM 08.295, LA225 | Norte de Santander | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-902 | WM 2069, LA 603 | Santander | alpha | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-1278 | WM 05.275 | Norte de Santander | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-1511 | WM 05.399 | Norte de Santander | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-1652 | Caquetá | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | ||
| H0058-I-2029 | WM 05.394 | Bogotá | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-2151 | Caquetá | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | ||
| H0058-I-2263 | Bogotá | alpha | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | ||
| H0058-I-2792 | WM 08.297 | Norte de Santander | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-2858 | WM 08.298 | Norte de Santander | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-2877 | WM 08.299 | Norte de Santander | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-3096 | WM 11.119 | Antioquia | alpha | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | |
| H0058-I-3266 | Norte de Santander | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | ||
| H0058-I-3590 | Antioquia | a | 2 | 6 | 25 | 4 | 18 | 12 | 10 | 25 | ||
| H0058-I-1391 | WM 05.349 | Valle | a | 2 | 6 | 10 | 4 | 18 | 12 | 2 | 8* | |
| H0058-I-638 | WM 04.76, LA 221 | Caquetá | alpha | 2 | 6 | 45* | 4 | 18 | 12 | 10 | 321* | |
| H0058-I-3146 | Norte de Santander | a | 2 | 6 | 95* | 4 | 18 | 12 | 10 | 323* | ||
| H0058-I-3407 | Norte de Santander | a | 2 | 21 | 25 | 4 | 41* | 12 | 2 | 324* | ||
| H0058-I-1543 | WM 05.397 | Bogotá | alpha | 11* | 21 | 6 | 4 | 16 | 5* | 2 | 12* | |
| H0058-I-2052 | Risaralda | a | 14 | 21 | 94* | 4 | 18 | 107* | 7 | 322* | ||
| H0058-I-762 | WM 04.78, LA224 | Santander | alpha | 4 | 6 | 6 | 4 | 18 | 22* | 2 | 31* | |
| H0058-I-1959 | WM 05.272 | Norte de Santander | a | 26* | 6 | 4 | 28 | 2 | 27* | 2 | 46* | |
| VGIII/AFLP5 | H0058-I-792 | WM 2063, LA594 | Bogotá | alpha | 29 | 9 | 5 | 9 | 4 | 28 | 21 | 85* |
| H0058-I-1442 | WM 11.102 | Meta | alpha | 29 | 7 | 5 | 2 | 4 | 28 | 21 | 79 | |
| H0058-I-2023 | WM 11.105 | Norte de Santander | alpha | 29 | 7 | 5 | 2 | 4 | 28 | 21 | 79 | |
| H0058-I-2086 | WM 11.106 | Caldas | alpha | 29 | 7 | 5 | 2 | 4 | 28 | 21 | 79 | |
| H0058-I-1255 | Cauca | alpha | 18 | 3 | 1 | 3 | 17 | 28 | 19 | 146 | ||
| H0058-I-1509 | Norte de Santander | alpha | 18 | 3 | 1 | 3 | 17 | 28 | 19 | 146 | ||
| H0058-I-2442 | WM 11.112 | Risaralda | alpha | 18 | 3 | 1 | 3 | 17 | 28 | 19 | 146 | |
| H0058-I-2961 | WM 11.118 | Bogotá | alpha | 18 | 3 | 1 | 3 | 17 | 28 | 19 | 146 | |
| H0058-I-3043 | Norte de Santander | alpha | 18 | 3 | 1 | 3 | 17 | 28 | 19 | 146 | ||
| H0058-I-1134 | WM 2088 | Risaralda | a | 18 | 3 | 1 | 3 | 17 | 38 | 19 | 59* | |
| H0058-I-1708 | WM 11.104 | Bogotá | a | 18 | 3 | 1 | 3 | 17 | 38 | 19 | 59* | |
| H0058-I-1276 | WM 2114, LA705 | Norte de Santander | alpha | 43 | 31 | 63* | 43 | 32 | 39 | 18 | 64* | |
| H0058-I-1812 | WM 05.371 | Valle | alpha | 43 | 31 | 63* | 43 | 32 | 39 | 18 | 64* |
Note: *New allele or sequence type.
Figure 4. Genetic relationship of the Cryptococcus gattii strains causing cryptococcosis in Colombia from 1997 to 2011.
Sequence types identified for the first time in this study are indicated with an asterisk (*).
To estimate the genetic diversity of the isolates studied, the Simpson's Diversity index (D) was calculated. In general, C. gattii strains from Colombia were highly diverse (D = 0.20). The greatest diversity was identified in the molecular type VGIII (D = 0.26), followed by VGII (D = 0.48) and VGI (D = 0.56).
Follow-up
For 11 patients treated in Norte de Santander, follow-up was undertaken. Nine (81.8%) were discharged alive, while 2 (18.2%) died during hospitalization.
Literature review
Table S3 reflects the prevalence of cryptococcosis by C. gattii around the world, as well as their molecular types, when reported [26]–[112].
Discussion
The incidence of cryptococcosis caused by C. gattii globally is generally low overall [113]. The mean annual incidence found in this study for Colombia, of 0.07 cases per million inhabitants per year reflects this rarity. Similar findings (0.09 cases per million per year) have been described from New Zealand [111]. However, some regions and population groups of the world have very high incidence, such as Papua New Guinea (43 cases per million per year) [114], the Australian aborigines domiciled in the Northern Territory (6.3 cases per million per year) [115] and British Columbia, Canada (5.8 cases per million per year) [62]. It is striking that the incidence of this disease in the department Norte de Santander is eleven times higher than the national (0.81 cases per million per year), which puts it at the level described for Australia as a continent(0.61 cases per million per year) [115].
Regarding the prevalence of C. gattii infections, the value of 3.7% found in this survey reflects Colombia is a country of overall low prevalence, despite being in the torrid zone of the planet. However, Norte de Santander has a high prevalence of 33.3% (60% in patients without AIDS) [59], placing this region of Colombia on level with other countries of high prevalence such as Papua New Guinea [107], [108], the Northern Territory of Australia [110], and Brazil [27] (Table S3). In Brazil, there is a clear difference between the North and South. In the area that covers the North and Northeast regions, the prevalence is very high, with values above 20% [28]–[33], while in the regions Center West, Southeast and South, the prevalence is low [34]–[48]. Interestingly, a high proportion of cases of cryptococcosis due to C. gattii in the northern region of Brazil have been present in HIV-negative children [28]. A high prevalence of disease has also been reported from Venezuela [15], [52]–[54], French Guiana [55] Vietnam [100] and Hong Kong (China) [87]. Similar findings have been reported in some African countries, especially Botswana and Malawi, where a prevalence of 13% of C. gattii cryptococcosis was found in patients with AIDS, a high value for this type of population [76]. Another study reported a prevalence of 30% of C. gattii in hospitalized AIDS patients in Botswana [77] (Table S3). On the other hand, the overall prevalence in Europe is low, and many of the cases are from people immigrating coming from other regions in the world where cryptococcosis is more common[10], [68], [69]. Against this trend, an apparently endemic strain from an environmental source in the Netherlands was recently reported [116]. A low prevalence of cryptococcosis by C. gattii was also reported from South Africa, where AIDS-related cryptococcosis due to C. neoformans is epidemic [82]. Similar findings have been reported from other countries of the African continent [79]–[81], [84], in Mexico [50], [51], Argentina [15], [56], [57] and Asia including China (without Hong Kong) [85], [86], India [93]–[95] and in Southeast Asia [96]–[99] but excluding Vietnam and Thailand (Table S3).
As in cryptococcosis patients infected with C. neoformans, disease referable to C. gattii is more frequent in men [117], typically young adults [115], although in this study we found children also and older patients.
The presence of cryptococcosis by C. gattii in children domiciled in tropical areas in Brazil [28], [34], French Guiana [55], and parts of Australia (Meyer, unpublished) in a remarkable finding that invites further enquiry and research.
The majority (91.1%) of the Colombian patients described in the current study did not report any apparent risk factor. There were, however, some cases in patients with AIDS or immunosuppressed by another means. The behavior of Colombian patients is in accord with the descriptions of previous studies [111], [118], [119]. The epidemiology of cryptococcosis by C. gattii has changed in the last decade, with the recognition that disease affects immunocompetent and immunosuppressed patients, including patients with AIDS, in regions of the world outside of the tropical and subtropical areas [14], [120], [121]. In Australia, where this trend is also observed, the number of immunosuppressed patients without AIDS increased from 9% to 28% [115]. In British Columbia, Canada the risk factors for infection with C. gattii were the use of oral steroids, the presence of pneumonia and other lung diseases [120]. Also, cryptococcosis was more frequent in patients older than 50 years, active smokers, HIV-positive individuals and a history of invasive cancer [120]. In the Pacific Northwest of the United States, patients infected with the outbreak strains of C. gattii, when compared with patients infected with different strains, were significantly more likely to have predisposing conditions and respiratory symptoms and a lower probability of having CNS involvement [121]. In Colombian patients, the most common clinical presentation was the CNS involvement with predominance of cryptococcal meningitis and intracranial hypertension. These clinical manifestations have been described in Latin America [39], Asia [96], Africa [82], [83], Australia [115] and Papua New Guinea [107], [108]. This contrasts with the patients described in Vancouver, the Pacific Northwest of the United States and the Northern Territory of Australia where primary lung disease with granuloma formation predominates [120], [121].
It is believed that C. gattii is clinically more virulent than C. neoformans, as well as being more likely to be a primary pathogen, with a propensity to cause multiple cryptococcal granulomas in the lungs and the brain of affected patients [111], [118]. In a mouse model, clinical isolates of C. gattii from the outbreak in British Columbia induced an inflammatory response less protective because the organisms somehow inhibit the migration of neutrophils towards the sites of infection. Additionally, in contrast to C. neoformans, they fail to induce the production of protective cytokines [122]. In vitro studies of the same isolates showed that dendritic cells are able to destroy cryptococci, but C. gattii evades adaptive immunity by preventing maturation of those cells and causing an inadequate activation and proliferation of T cells [123]. Recently, it has been shown in mice that infection by C. gattii decreases the effective response of Th1/Th17 mediated by dendritic cells and regulates the low expression of lung cytokines, resulting in an inability to produce a protective immunity in immunocompetent hosts [124].
Compared to patient with C neoformans infections, meningoencephalitis caused by C. gattii responds more slowly to antifungal therapy, and patients require a longer duration of treatment [111], [119]. Diagnostic imaging of the lung and brain are typically abnormal in patients infected with C. gattii. In the current study a third of the patients presented with gross lung abnormalities.
In immunocompetent patients affected by C. gattii, pulmonary nodules or masses with diameters ranging from 5 to 52 mm in diameter and focal areas of consolidation have been described [125]. Some clinical considerations in the differentiation of the infections caused by C. gattii and C. neoformans were recently revised [126].
The diagnosis of the vast majority of the Colombian patients reported in the study was obtained by analysis of CSF samples. Direct microscopic examination showed high positivity (97.7%), compared with much lower rates reported for this test in patients without AIDS [87], [127]. Also, capsular antigen in the CSF was reactive in all cases as measured by latex agglutination. Definitive diagnosis was established in all patients using culture, and the use of CBG agar established which cases were caused by C. gattii. The most frequently identified serotype in Colombian clinical strains was serotype B (85.7%), which is the most prevalent serotype in clinical and environmental samples. C. gattii serotype C was less common although has been associated with AIDS and with immunocompetent patients [13].
In Colombian patients, three of the four molecular types of C. gattii were found with a clear predominance of VGII, followed by VGIII and to a lesser extent VGI. The preponderance of the molecular type VGII in Colombia is similar to that reported in Western Australia [109], [112], Brazil (especially in the Northern region) [27] and Venezuela [15]. Equally, the molecular type VGII is the main biotype responsible for outbreaks in British Columbia, Canada [63] and the Pacific Northwest of the United States [64]. Hence the importance of determining the taxonomy of C. gattii strains because of the epidemic potential associated with the VGII molecular type.
The emergence of specific genotypes of endemic and epidemic disease has been reflected in the large global effort being made to increase knowledge about the population genetics of C. neoformans and C. gattii [15], [112]. MLST data has demonstrated that Colombian C. gattii strains are genetically diverse. In spite of the small number of isolates studied, several genotypes were identified belonging to the full range of molecular types, in contrast to the less diverse and rather clonal C. gattii populations reported in other countries such as Canada, USA, eastern Australia and Thailand, where few genotypes have been identified amongst a much larger number of strains [5], [6], [23], [63], [99]. The identification of the same STs in different departments (Figure 2), for example the prevalent VGII ST25 found in 7 different departments, the VGI ST51 and ST58, and the VGIII ST64, ST79 and ST146, suggests the circulation of genotypes in the country.
The finding of STs previously reported in other parts of the world, shows a wider geographic dispersion of some C. gattii genotypes, especially amongst the VGI isolates, for which the herein identified STs have been reported from several countries; ST51 was previously found in Australia, China, India, Mexico, Papua New Guinea and USA [10], [16], [128], while ST58 had already been described from the Netherlands, Germany and USA [68]. Among the VGII isolates, the commonest ST identified in this study, ST25, was already reported in one isolate from Aruba [129], while the STs among the VGIII isolates, ST79 and ST146, were reported in two and one isolates from Mexico and the USA, respectively [129].
In Colombia, the majority of the strains of C. gattii are mating type a [130], in contradistinction to what was found in British Columbia, Canada [5] and of the Pacific coast of the United States [6], and southwestern Western Australia where the main mating type is α, which could suggest the possibility of genetic exchange that could have had an impact on the origin of the outbreaks. C. gattii has been recovered frequently from the environment in Colombia and in the city of Cúcuta (Norte de Santander), with both serotypes B and C having been cultured [131], [132].
The initial treatment of cryptococcosis in almost all patients from Colombian was done with AMB, with or without FCZ, which is in agreement with the results of susceptibility testing of all strains of C. gattii to these antifungals and according with the international guidelines for countries with limited resources [133]. The resistance of approx. half of the Colombian strains to fluconazole is a phenomenon that is being studied by the group of the CIB (C de Bedout, personal communication).
Supporting Information
STROBE checklist.
(DOCX)
Number of Cryptococcus gattii cryptococcosis cases in Colombia, per state, year and molecular type. Number of cases in brackets.
(DOCX)
Gene Bank accession numbers for the allele types of Colombian Cryptococcus gattii clinical strains.
(DOCX)
Cryptococcus gattii: Prevalence and molecular type of clinical isolates reported worldwide.
(DOCX)
Acknowledgments
Colombian Group for the Study of Cryptococcosis
Antioquia: Catalina de Bedout, Angela Tobón, Angela Restrepo, Myrtha Arango, Corporación para Investigaciones Biológicas, Medellín. Dora Rivas, Luz Marina Melquizoz, Victoria García, Hospital General de Medellín. Ana María Restrepo, Clínica CES. Maryan Vásquez, Magda Cárdenas, Mayiber Henao, Edna Vásquez, Clínica Saludcoop Medellín. Carlos Agudelo, Alejandro Vélez, Carlos Ignacio Gómez, Hospital Pablo Tobón Uribe. Hilda Álvarez, Hospital Marco Fidel Suárez. Marcelo Gaviria, Clínica Medellín. Bogotá: María Isabel Medina, Luz Maldonado, Hospital Simón Bolívar. Carlos Álvarez, Nidia Torres, Judy Andrea Puerta, Claudia Linares, Maritza Rojas, Hospital San Ignacio. Claudia Clavijo, Clínica San Rafael. Martha Isabel Garzón, Hospital el Tunal. Claudia Clavijo, Clínica San Rafael. Sandra Nuñez, Gloria Inés Gallo, LSP Bogotá. Luz Mery Jiménez, Hospital La Victoria. Andrea López Guachetá, Hospital Meissen. Nubia Escobar, Hospital de Kennedy. Rossana Mejía, Clínica Nueva. Norte de Santander: Yeni Peña, Hospital Universitario Erasmo Meoz. Valle: Claudia Rocío Castañeda, Mónica Recalde, Juan Diego Vélez, Juan Carlos Alvir, Álvaro Iván Muñoz, Fernando Rozo, Fundación Valle del Lilí, Nancy Villamarín, Hospital Universitario del Valle. Caldas: Martha Cecilia Kogson, Gilberto Manjarres, Hospital Santa Sofía. Huila: Luis Fernando Duran, Hospital Universitario de Neiva. Meta: Sandra Hurtado, Hospital Departamental de Villavicencio. Risaralda: Myriam Gómez, Hospital San Jorge.
We also acknowledge the anonymous reviewers for critical comments on manuscript and Richard Malik for reviewing the manuscript.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. All strains are deposited in the culture collection of the national Institute of Health in Bogota (Contact Elizabeth Castañeda, email: ecastaneda21@gmail.com) and are available following the regulations of the International Biodiversity Convention (1993) and the subsequent laws of the South American states, including Colombia. The Biodiversity convention states: Article 15 access to Genetic Resources: paragraph 1: “Recognizing the sovereign rights of States over their natural resources, the authority to determine access to genetic resources rests with the national governments and is subject to national legislation”. The laws, specifically the Colombian Decree 391 of 1996, Law 165 of 1994 states: that the genetic resources of Plants, Animals and Microorganisms belong to the country of origin. As such, microbial strains can only be provided for specific projects signing material transfer agreements for the use of a specific project, with an agreed co-authorship on all publications resulting from the use of the provided microbial cultures. The microbial strains that were obtained through a material transfer agreement have to be destroyed after completion of the study, and cannot be passed on to third parties. Any additional researcher who wants to work with these strains needs to obtain the strains directly from the culture collection of the National Institute of Health, Instituto Nacional de Salud, Bogotá after signing a new material transfer agreement.
Funding Statement
The National Health and Medical Research Council grant APP1031943 to WM. CF was supported by a PhD scholarship “Becas Francisco José Caldas” provided by COLCIENCIAS, funding agency in Colombia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, et al. (2005) Deciphering the model pathogenic fungus Cryptococcus neoformans . Nat Rev Microbiol 3: 753–764. [DOI] [PubMed] [Google Scholar]
- 2. Kwon-Chung KJ, Boekhout T, Fell JW, Diaz M (2002) Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae). Taxon 51: 804–806. [Google Scholar]
- 3. Kwon-Chung KJ, Polacheck I, Bennett JE (1982) Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J Clin Microbiol 15: 535–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525–530. [DOI] [PubMed] [Google Scholar]
- 5. Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, et al. (2004) A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A 101: 17258–17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byrnes EJ 3rd, Li W, Lewit Y, Ma H, Voelz K, et al. (2010) Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 6: e1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hagen F, Ceresini PC, Polacheck I, Ma H, van Nieuwerburgh F, et al. (2013) Ancient dispersal of the human fungal pathogen Cryptococcus gattii from the Amazon rainforest. PLoS One 7: e71148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engelthaler DM, Hicks ND, Gillece JD, Roe CC, Schupp JM, et al. (2014) Cryptococcus gattii in North American Pacific Northwest: whole-population genome analysis provides insights into species evolution and dispersal. mBio 5: e01464–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwon-Chung KJ, Bennett JE (1984) Epidemiologic differences between the two varieties of Cryptococcus neoformans . Am J Epidemiol 120: 123–130. [DOI] [PubMed] [Google Scholar]
- 10. Hagen F, Colom MF, Swinne D, Tintelnot K, Iatta R, et al. (2012) Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerg Infect Dis 18: 1618–1624.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gattii F, Eeckels R (1970) An atypical strain of Cryptococcus neoformans (San Felice) Vuillemin 1894. Part I. Description of the disease and of the strain. Ann Soc Belges Med Trop Parasitol Mycol 50: 689–694. [PubMed] [Google Scholar]
- 12. Barnett JA (2010) A history of research on yeasts 14: medical yeasts part 2, Cryptococcus neoformans. . Yeast 27: 875–904. [DOI] [PubMed] [Google Scholar]
- 13. Springer DJ, Chaturvedi V (2010) Projecting global occurrence of Cryptococcus gattii. . Emerg Infect Dis 16: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaturvedi V, Chaturvedi S (2011) Cryptococcus gattii: a resurgent fungal pathogen. Trends Microbiol 19: 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E, et al. (2003) Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis 9: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer W, Aanensen DM, Boekhout T, Cogliati M, Diaz MR, et al. (2009) Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. . Med Mycol 47: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer W, Gilgado F, Ngamskulrungroj P, Trilles L, Hagen F, et al. Molecular typing of the Cryptococcus neoformans/Cryptococcus gattii species complex (2011) In: Heitman J, Kozel TR, Kwon-Chung J, Perfect JR, Casadevall A. Cryptococcus: form human pathogen to model yeast. Washington D.C.: ASM Press. pp 327–357. [Google Scholar]
- 18. Bovers M, Hagen F, Kuramae EE, Diaz MR, Spanjaard L, et al. (2006) Unique hybrids between fungal pathogens Cryptococcus neoformans and Cryptococcus gattii . FEMS Yeast Res 6: 599–607. [DOI] [PubMed] [Google Scholar]
- 19. Aminnejad M, Diaz M, Arabatzis M, Castañeda E, Lazera M, et al. (2012) Identification of novel hybrids between Cryptococcus neoformans var. grubii VNI and Cryptococcus gattii VGII. Mycopathologia 173: 337–46.. [DOI] [PubMed] [Google Scholar]
- 20. Lizarazo J, Linares M, de Bedout C, Restrepo A, Agudelo CI, et al. (2007) Estudio clínico y epidemiológico de la criptococosis en Colombia: resultados de nueve años de la encuesta nacional, 1997–2005. Biomédica 27: 94–109. [PubMed] [Google Scholar]
- 21. Escandón P, de Bedout C, Lizarazo J, Agudelo CI, Tobón A, et al. (2012) Criptococosis en Colombia: resultados de la encuesta nacional, 2006–2010. Biomédica 32: 386–398.23715187 [Google Scholar]
- 22.Departamento Administrativo Nacional de Estadística (DANE) (2012) http://www.dane.gov.co/index.php?option = com_content&view = article&id = 75&Itemid = 72 consulted June 11, 2012.
- 23. Halliday CL, Carter DA (2003) Clonal reproduction and limited dispersal in an environmental population of Cryptococcus neoformans var. gattii isolates from Australia. J Clin Microbiol 41: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simpson EH (1949) Measurement of diversity. Nature 163: 688. [Google Scholar]
- 26. Rozenbaum R, Gonçalves AJ (1994) Clinical epidemiological study of 171 cases of cryptococcosis. Clin Infect Dis 18: 369–380. [DOI] [PubMed] [Google Scholar]
- 27. Trilles L, Lazéra Mdos S, Wanke B, Oliveira RV, Barbosa GG, et al. (2008) Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcus gattii in Brazil. Mem Inst Oswaldo Cruz 103: 455–462. [DOI] [PubMed] [Google Scholar]
- 28. Corrêa Mdo P, Oliveira EC, Duarte RR, Pardal PP, Oliveira Fde M, et al. (1999) Cryptococcosis in children in the State of Pará, Brazil. Rev Soc Bras Med Trop 32: 505–508. [PubMed] [Google Scholar]
- 29. Santos WR, Meyer W, Wanke B, Costa SP, Trilles L, et al. (2008) Primary endemic Cryptococcosis gattii by molecular type VGII in the state of Pará, Brazil. Mem Inst Oswaldo Cruz 103: 813–818. [DOI] [PubMed] [Google Scholar]
- 30. Freire AK, dos Santos Bentes A, de Lima Sampaio I, Matsuura AB, Ogusku MM, et al. (2012) Molecular characterization of the causative agents of cryptococcosis in patients of a tertiary healthcare facility in the state of Amazonas-Brazil. Mycoses 55: e145–50 doi: 10.1111/j.1439-0507.2012.02173.x [DOI] [PubMed] [Google Scholar]
- 31. Da Silva BK, Freire AK, Bentes Ados S, Sampaio Ide L, Santos LO, et al. (2012) Characterization of clinical isolates of the Cryptococcus neoformans-Cryptococcus gattii species complex from the Amazonas State in Brazil. Rev Iberoam Micol 29: 40–43. [DOI] [PubMed] [Google Scholar]
- 32. Martins LM, Wanke B, Lazéra Mdos S, Trilles L, Barbosa GG, et al. (2011) Genotypes of Cryptococcus neoformans and Cryptococcus gattii as agents of endemic cryptococcosis in Teresina, Piauí (Northeastern Brazil). Mem Inst Oswaldo Cruz 106: 725–730. [DOI] [PubMed] [Google Scholar]
- 33. Matos CS, de Souza Andrade A, Oliveira NS, Barros TF (2012) Microbiological characteristics of clinical isolates of Cryptococcus spp. in Bahia, Brazil: molecular types and antifungal susceptibilities. Eur J Clin Microbiol Infect Dis 31: 1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsujisaki RA, Paniago AM, Lima Júnior MS, Alencar Dde S, Spositto FL, et al. (2013) First molecular typing of cryptococcemia-causing Cryptococcus in central-west Brazil. Mycopathologia 176: 267–272. [DOI] [PubMed] [Google Scholar]
- 35. Souza LK, Fernandes O de F, Kobayashi CC, Passos XS, Costa CR, et al. (2005) Antifungal susceptibilities of clinical and environmental isolates of Cryptococcus neoformans in Goiânia city, Goiás, Brazil. Rev Inst Med Trop Sao Paulo 47: 253–256. [DOI] [PubMed] [Google Scholar]
- 36. Souza LK, Souza Junior AH, Costa CR, Faganello J, Vainstein MH, et al. (2010) Molecular typing and antifungal susceptibility of clinical and environmental Cryptococcus neoformans species complex isolates in Goiania, Brazil. Mycoses 53: 62–67. [DOI] [PubMed] [Google Scholar]
- 37. Hasimoto e Souza LK, Costa CR, Fernandes Ode F, Abrão FY, Silva TC, et al. (2013) Clinical and microbiological features of cryptococcal meningitis. Rev Soc Bras Med Trop 46: 343–347. [DOI] [PubMed] [Google Scholar]
- 38.Favalessa OC, Lazéra Mdos S, Wanke B, Trilles L, Takahara DT, et al. (2014) Fatal Cryptococcus gattii genotype AFLP6/VGII infection in a HIV-negative patient: case report and a literature review. Mycoses Jun 30 : doi: 10.1111/myc.12210 [DOI] [PubMed] [Google Scholar]
- 39. Lindenberg Ade S, Chang MR, Paniago AM, Lazéra Mdos S, Moncada PM, et al. (2008) Clinical and epidemiological features of 123 cases of cryptococcosis in Mato Grosso do Sul, Brazil. Rev Inst Med Trop Sao Paulo 50: 75–78. [DOI] [PubMed] [Google Scholar]
- 40. Favalessa OC, Ribeiro LC, Tadano T, Fontes CJ, Dias FB, et al. (2009) First description of phenotypic profile and in vitro drug susceptibility of Cryptococcus spp. yeast isolated from HIV-positive and HIV-negative patients in State of Mato Grosso. Rev Soc Bras Med Trop 42: 661–665. [DOI] [PubMed] [Google Scholar]
- 41. Ohkusu M, Tangonan N, Takeo K, Kishida E, Ohkubo M, et al. (2002) Serotype, mating type and ploidy of Cryptococcus neoformans strains isolated from patients in Brazil. Rev Inst Med Trop Sao Paulo 44: 299–302. [DOI] [PubMed] [Google Scholar]
- 42. Almeida AM, Matsumoto MT, Baeza LC, de Oliveira E Silva RB, Kleiner AA, et al. (2007) Molecular typing and antifungal susceptibility of clinical sequential isolates of Cryptococcus neoformans from Sao Paulo State, Brazil. FEMS Yeast Res 7: 152–164. [DOI] [PubMed] [Google Scholar]
- 43. Motta A, Almeida GD, Almeida J, Pinto MI, Onorio S, Rossi F (2010) Cryptococcus: species distribution and susceptibility profile of isolates in a teaching hospital from São Paulo-Brazil. Int J Infect Dis 14: e126 doi:10.1016/j.ijid.2010.02.1764 [Google Scholar]
- 44. Nascimento E, Bonifácio da Silva ME, Martinez R, von Zeska Kress MR (2014) Primary cutaneous cryptococcosis in an immunocompetent patient due to Cryptococcus gattii molecular type VGI in Brazil: a case report and review of literature. Mycoses 57: 442–447. [DOI] [PubMed] [Google Scholar]
- 45. Moreira Tde A, Ferreira MS, Ribas RM, Borges AS (2006) Cryptococcosis: clinical epidemiological laboratorial study and fungi varieties in 96 patients. Rev Soc Bras Med Trop 39: 255–258. [DOI] [PubMed] [Google Scholar]
- 46. Silva PR, Rabelo RA, Terra AP, Teixeira DN (2008) Susceptibility to antifungal agents among Cryptococcus neoformans varieties isolated from patients at a university hospital. Rev Soc Bras Med Trop 41: 158–162. [DOI] [PubMed] [Google Scholar]
- 47. Mora DJ, da Cunha Colombo ER, Ferreira-Paim K, Andrade-Silva LE, Nascentes GA, et al. (2012) Clinical, epidemiological and outcome features of patients with cryptococcosis in Uberaba, Minas Gerais, Brazil. Mycopathologia 173: 321–327. [DOI] [PubMed] [Google Scholar]
- 48. Casali AK, Goulart L, Rosa e Silva LK, Ribeiro AM, Amaral AA, et al. (2003) Molecular typing of clinical and environmental Cryptococcus neoformans isolates in the Brazilian state Rio Grande do Sul. FEMS Yeast Res 3: 405–415. [DOI] [PubMed] [Google Scholar]
- 49. López-Martínez R, Soto-Hernández JL, Ostrosky-Zeichner L, Castanón-Olivares LR, Angeles-Morales V, et al. (1996) Cryptococcus neoformans var. gattii among patients with cryptococcal meningitis in Mexico. First observations. Mycopathologia 134: 61–64. [DOI] [PubMed] [Google Scholar]
- 50. Castañón-Olivares LR, Arreguín-Espinosa R, Ruiz-Palacios SG, López-Martínez R (2000) Frequency of Cryptococcus species and varieties in Mexico and their comparison with some Latin American countries. Rev Latinoam Microbiol 42: 35–40. [PubMed] [Google Scholar]
- 51. Castañón-Olivares LR, Martínez KM, Cruz RM, Rivera MA, Meyer W, et al. (2009) Genotyping of Mexican Cryptococcus neoformans and C. gattii isolates by PCR-fingerprinting. Med Mycol 47: 713–721. [DOI] [PubMed] [Google Scholar]
- 52. Villanueva E, Mendoza M, Torres E, Albornoz MB (1989) Serotipificación de 27 cepas de Cryptococcus neoformans aisladas en Venezuela. Acta Científica Venezolana 40: 151–154. [PubMed] [Google Scholar]
- 53. Pérez C, Dolande M, Moya M, Roselló A, de Capriles CR, et al. (2008) Cryptococcus neoformans, Cryptococcus gattii: serotypes in Venezuela. Mycopathologia 166: 149–153. [DOI] [PubMed] [Google Scholar]
- 54. Pérez C, Hernández Y, Guzmán ME, Arias F, Nweihed L, et al. (2009) Estudio clínico-epidemiológico de la criptococosis en Venezuela, años 1994-2003. Kasmera 37: 140–147. [Google Scholar]
- 55. Debourgogne A, Iriart X, Blanchet D, Veron V, Boukhari R, et al. (2011) Characteristics and specificities of Cryptococcus infections in French Guiana, 1998-2008. Med Mycol 49: 864–871. [DOI] [PubMed] [Google Scholar]
- 56. Bava AJ, Negroni R (1992) Características epidemiológicas de 105 casos de criptococosis diagnosticados en la República Argentina entre 1981–1990. Rev Inst Med trop Sao Paulo 34: 335–340. [PubMed] [Google Scholar]
- 57. Bava AJ, Robles AM, Negroni R, Arechavala A, Bianchi M (1997) Estudio de algunos aspectos epidemiológicos de 253 casos de criptococosis. Rev Iberoam Micol 14: 111–114. [PubMed] [Google Scholar]
- 58. Bustamante B, Swinne D (1998) Aislamiento de Cryptococcus neoformans variedad gattii en dos pacientes peruanos. Rev Iberoam Micol 15: 22–24. [PubMed] [Google Scholar]
- 59. Lizarazo J, Chaves O, Peña Y, Escandón P, Agudelo CI, et al. (2012) Comparación de los hallazgos clínicos y de supervivencia entre pacientes VIH positivos y VIH negativos con criptococosis meníngea en un hospital del tercer nivel. Acta Med Colomb 37: 49–61. [Google Scholar]
- 60. Cattana ME, Tracogna MF, Fernández MS, Carol Rey MC, Sosa MA, et al. (2013) Genotipificación de aislamientos clínicos del complejo Cryptococcus neoformans/Cryptococcus gattii obtenidos en el Hospital «Dr. Julio C. Perrando», de la ciudad de Resistencia (Chaco, Argentina). Rev Argent Microbiol 45: 89–92. [DOI] [PubMed] [Google Scholar]
- 61. Illnait-Zaragozí MT, Ortega-Gonzalez LM, Hagen F, Martínez-Machin GF, Meis JF (2013) Fatal Cryptococcus gattii genotype AFLP5 infection in an immunocompetent Cuban patient. Med Mycol Case Rep 9: 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoang LM, Maguire JA, Doyle P, Fyfe M, Roscoe DL (2004) Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997–2002): epidemiology, microbiology and histopathology. J Med Microbiol 53: 935–940. [DOI] [PubMed] [Google Scholar]
- 63. Galanis E, Macdougall L (2010) Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis 16: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lockhart SR, Iqbal N, Harris JR, Grossman NT, DeBess E, et al. (2013) Cryptococcus gattii in the United States: genotypic diversity of human and veterinary isolates. PLoS One 8: e74737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Byrnes EJ 3rd, Li W, Lewit Y, Ma H, Voelz K, et al. (2010) Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 6: e1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Byrnes EJ 3rd, Li W, Ren P, Lewit Y, Voelz K, et al. (2011) A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog 7: e1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chaturvedi S, Dyavaiah M, Larsen RA, Chaturvedi V (2005) Cryptococcus gattii in AIDS patients, southern California. Emerg Infect Dis 11: 1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tintelnot K, Lemmer K, Losert H, Schär G, Polak A (2004) Follow-up of epidemiological data of cryptococcosis in Austria, Germany and Switzerland with special focus on the characterization of clinical isolates. Mycoses 47: 455–464. [DOI] [PubMed] [Google Scholar]
- 69. Viviani MA, Cogliati M, Esposto MC, Lemmer K, Tintelnot K, et al. (2006) Molecular analysis of 311 Cryptococcus neoformans isolates from a 30-month ECMM survey of cryptococcosis in Europe. FEMS Yeast Res 6: 614–619. [DOI] [PubMed] [Google Scholar]
- 70. Dromer F, Mathoulin S, Dupont B, Laporte A (1996) Epidemiology of cryptococcosis in France: a 9-year survey (1985–1993). French Cryptococcosis Study Group. Clin Infect Dis 23: 82–90. [DOI] [PubMed] [Google Scholar]
- 71.Maduro AP, Mansinho K, Teles F, Silva I, Meyer W, et al. (2013) Insights on the genotype distribution among Cryptococcus neoformans and C. gattii Portuguese clinical isolates. Curr Microbiol DOI 10.1007/s00284-013-0452-0. [DOI] [PubMed]
- 72. Velegraki A, Kiosses VG, Pitsouni H, Toukas D, Daniilidis VD, et al. (2001) First report of Cryptococcus neoformans var. gattii serotype B from Greece. Med Mycol 39: 419–422. [DOI] [PubMed] [Google Scholar]
- 73. Colom MF, Frasés S, Ferrer C, Jover A, Andreu M, et al. (2005) First case of human cryptococcosis due to Cryptococcus neoformans var. gattii in Spain. J Clin Microbiol 43: 3548–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hagen F, van Assen S, Luijckx GJ, Boekhout T, Kampinga GA (2010) Activated dormant Cryptococcus gattii infection in a Dutch tourist who visited Vancouver Island (Canada): a molecular epidemiological approach. Med Mycol 48: 528–531. [DOI] [PubMed] [Google Scholar]
- 75. Iatta R, Hagen F, Fico C, Lopatriello N, Boekhout T, et al. (2012) Cryptococcus gattii infection in an immunocompetent patient from Southern Italy. Mycopathologia 174: 87–92. [DOI] [PubMed] [Google Scholar]
- 76. Litvintseva AP, Thakur R, Reller LB, Mitchell TG (2005) Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in Sub-Saharan Africa. J Infect Dis 192: 888–892. [DOI] [PubMed] [Google Scholar]
- 77. Steele KT, Thakur R, Nthobatsang R, Steenhoff AP, Bisson GP (2010) In-hospital mortality of HIV-infected cryptococcal meningitis patients with C. gattii and C. neoformans infection in Gaborone, Botswana. Med Mycol 48: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 78. Swinne D, Nkurikiyinfura JB, Muyembe TL, Swinne D (1986) Clinical isolates of Cryptococcus neoformans from Zaire. Eur J Clin Microbiol 5: 50–51. [DOI] [PubMed] [Google Scholar]
- 79. Bii CC, Makimura K, Abe S, Taguchi H, Mugasia OM, et al. (2007) Antifungal drug susceptibility of Cryptococcus neoformans from clinical sources in Nairobi, Kenya. Mycoses 50: 25–30. [DOI] [PubMed] [Google Scholar]
- 80. Mdodo R, Moser SA, Jaoko W, Baddley J, Pappas P, et al. (2011) Antifungal susceptibilities of Cryptococcus neoformans cerebrospinal fluid isolates from AIDS patients in Kenya. Mycoses 54: e438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bogaerts J, Rouvroy D, Taelman H, Kagame A, Aziz MA, et al. (1999) AIDS-associated cryptococcal meningitis in Rwanda (1983–1992): epidemiologic and diagnostic features. J Infect 39: 32–37. [DOI] [PubMed] [Google Scholar]
- 82. Morgan J, McCarthy KM, Gould S, Fan K, Arthington-Skaggs B, et al. (2006) Cryptococcus gattii infection: characteristics and epidemiology of cases identified in a South African province with high HIV seroprevalence, 2002–2004. Clin Infect Dis 43: 1077–1080. [DOI] [PubMed] [Google Scholar]
- 83. Meiring ST, Quan VC, Cohen C, Dawood H, Karstaedt AS, et al. (2012) A comparison of cases of paediatric-onset and adult-onset cryptococcosis detected through population-based surveillance, 2005–2007. AIDS 26: 2307–2314. [DOI] [PubMed] [Google Scholar]
- 84. Heyderman RS, Gangaidzo IT, Hakim JG, Mielke J, Taziwa A, et al. (1998) Cryptococcal meningitis in human immunodeficiency virus-infected patients in Harare, Zimbabwe. Clin Infect Dis 26: 284–289. [DOI] [PubMed] [Google Scholar]
- 85. Chen J, Varma A, Diaz MR, Litvintseva AP, Wollenberg KK, et al. (2008) Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg Infect Dis 14: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li M, Liao Y, Chen M, Pan W, Weng L (2012) Antifungal susceptibilities of Cryptococcus species complex isolates from AIDS and non-AIDS patients in Southeast China. Braz J Infect Dis 16: 175–179. [DOI] [PubMed] [Google Scholar]
- 87. Lui G, Lee N, Ip M, Choi KW, Tso YK, et al. (2006) Cryptococcosis in apparently immunocompetent patients. QJM 99: 143–151. [DOI] [PubMed] [Google Scholar]
- 88. Liaw SJ, Wu HC, Hsueh PR (2010) Microbiological characteristics of clinical isolates of Cryptococcus neoformans in Taiwan: serotypes, mating types, molecular types, virulence factors, and antifungal susceptibility. Clin Microbiol Infect 16: 696–703. [DOI] [PubMed] [Google Scholar]
- 89. Tseng HK, Liu CP, Ho MW, Lu PL, Lo HJ, et al. (2013) Microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. PLoS One 17;8: e61921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Choi YH, Ngamskulrungroj P, Varma A, Sionov E, Hwang SM, et al. (2010) Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res 10: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hwang SM (2012) Molecular typing of clinical Cryptococcus gattii isolates in Korea. J of Bacteriol Virol 42: 152–155. [Google Scholar]
- 92. Padhye AA, Chakrabarti A, Chander J, Kaufman L (1993) Cryptococcus neoformans var. gattii in India. J Med Vet Mycol 31: 165–168. [PubMed] [Google Scholar]
- 93. Banerjee U, Datta K, Casadevall A (2004) Serotype distribution of Cryptococcus neoformans in patients in a tertiary care center in India. Med Mycol 42: 181–186. [DOI] [PubMed] [Google Scholar]
- 94. Jain N, Wickes BL, Keller SM, Fu J, Casadevall A, et al. (2005) Molecular epidemiology of clinical Cryptococcus neoformans strains from India. J Clin Microbiol 43: 5733–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nagarathna S, Veena Kumari HB, Arvind N, Divyalakshmi A, Chandramuki A, et al. (2010) Prevalence of Cryptococcus gattii causing meningitis in a tertiary neurocare center from south India: A pilot study. Indian J Pathol Microbiol 53: 855–856. [DOI] [PubMed] [Google Scholar]
- 96. Tay ST, Rohani MY, Hoo TS, Hamimah H (2010) Epidemiology of cryptococcosis in Malaysia. Mycoses 53: 509–514. [DOI] [PubMed] [Google Scholar]
- 97. Chan M, Lye D, Win MK, Chow A, Barkham T (2014) Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii . Int J Infect Dis 11: 110–115. [DOI] [PubMed] [Google Scholar]
- 98. Poonwan N, Mikami Y, Poosuwan S, Boon-Long J, Mekha N, et al. (1997) Serotyping of Cryptococcus neoformans strains isolated from clinical specimens in Thailand and their susceptibility to various antifungal agents. Eur J Epidemiol 13: 335–340. [DOI] [PubMed] [Google Scholar]
- 99. Kaocharoen S, Ngamskulrungroj P, Firacative C, Trilles L, Piyabongkarn D, et al. (2013) Molecular epidemiology reveals genetic diversity amongst isolates of the Cryptococcus neoformans/C. gattii species complex in Thailand. PLoS Negl Trop Dis 7: e2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chau TT, Mai NH, Phu NH, Nghia HD, Chuong LV, et al. (2010) A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam - high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis 10: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Day JN1, Hoang TN, Duong AV, Hong CT, Diep PT, et al. (2011) (2011) Most cases of cryptococcal meningitis in HIV-uninfected patients in Vietnam are due to a distinct amplified fragment length polymorphism-defined cluster of Cryptococcus neoformans var. grubii VN1. J Clin Microbiol 49: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tsunemi T, Kamata T, Fumimura Y, Watanabe M, Yamawaki M, et al. (2001) Immunohistochemical diagnosis of Cryptococcus neoformans var. gattii infection in chronic meningoencephalitis: the first case in Japan. Intern Med 40: 1241–1244. [DOI] [PubMed] [Google Scholar]
- 103. Okamoto K, Hatakeyama S, Itoyama S, Nukui Y, Yoshino Y, et al. (2010) Cryptococcus gattii genotype VGIIa infection in man, Japan, 2007. Emerg Infect Dis 16: 1155–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Taylor MB, Chadwick D, Barkham T (2002) First reported isolation of Cryptococcus neoformans var. gattii from a patient in Singapore. J Clin Microbiol 40: 3098–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Koh TH, Tan AL, Lo YL, Oh H (2002) Cryptococcus neoformans var. gattii meningitis in Singapore. Med Mycol 40: 221–223. [DOI] [PubMed] [Google Scholar]
- 106. Lingegowda BP, Koh TH, Ong HS, Tan TT (2011) Primary cutaneous cryptococcosis due to Cryptococcus gattii in Singapore. Singapore Med J 52: e160–162. [PubMed] [Google Scholar]
- 107. Laurenson IF, Trevett AJ, Lalloo DG, Nwokolo N, Naraqi S, et al. (1996) Meningitis caused by Cryptococcus neoformans var. gattii and var. neoformans in Papua New Guinea. Trans R Soc Trop Med Hyg 90: 57–60. [DOI] [PubMed] [Google Scholar]
- 108. Seaton RA, Verma N, Naraqi S, Wembri JP, Warrell DA (1997) Visual loss in immunocompetent patients with Cryptococcus neoformans var. gattii meningitis. Trans R Soc Trop Med Hyg 91: 44–49. [DOI] [PubMed] [Google Scholar]
- 109. Campbell LT, Fraser JA, Nichols CB, Dietrich FS, Carter D, et al. (2005) Clinical and environmental isolates of Cryptococcus gattii from Australia that retain sexual fecundity. Eukaryot Cell 4: 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ellis DH (1987) Cryptococcus neoformans var. gattii in Australia. Clin Microbiol 25: 430–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen S, Sorrell T, Nimmo G, Speed B, Currie B, et al. (2000) Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 31: 499–508. [DOI] [PubMed] [Google Scholar]
- 112. Ngamskulrungroj P, Gilgado F, Faganello J, Litvintseva AP, Leal AL, et al. (2009) Genetic diversity of the Cryptococcus species complex suggests that Cryptococcus gattii deserves to have varieties. PLoS One 4: e5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Harris J, Lockhart S, Chiller T (2012) Cryptococcus gattii: where do we go from here? Med Mycol 50: 113–129. [DOI] [PubMed] [Google Scholar]
- 114. Laurenson IF, Lalloo DG, Naraqi S, Seaton RA, Trevett AJ, et al. (1997) Cryptococcus neoformans in Papua New Guinea: a common pathogen but an elusive source. J Med Vet Mycol 35: 437–440. [DOI] [PubMed] [Google Scholar]
- 115. Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, et al. (2012) Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis 55: 789–798. [DOI] [PubMed] [Google Scholar]
- 116. Chowdhary A, Randhawa HS, Boekhout T, Hagen F, Klaassen CH, et al. (2012) Temperate climate niche for Cryptococcus gattii in Northern Europe. Emerg Infect Dis 18: 172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lizarazo J, Mendoza M, Palacios D, Vallejo A, Bustamante A, et al. (2000) Criptococosis ocasionada por Cryptococcus neoformans variedad gattii. . Acta Med Colomb 25: 171–178. [Google Scholar]
- 118. Mitchell DH, Sorrell TC, Allworth AM, Heath CH, McGregor AR, et al. (1995) Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis 20: 611–616. [DOI] [PubMed] [Google Scholar]
- 119. Speed B, Dunt D (1995) Clinical and host differences between infections with the two varieties of Cryptococcus neoformans . Clin Infect Dis 21: 28–34. [DOI] [PubMed] [Google Scholar]
- 120. MacDougall L, Fyfe M, Romney M, Starr M, Galanis E (2011) Risk factors for Cryptococcus gattii infection, British Columbia, Canada. Emerg Infect Dis 17: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Harris JR, Lockhart SR, Debess E, Marsden-Haug N, Goldoft M, et al. (2011) Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis 53: 1188–1195. [DOI] [PubMed] [Google Scholar]
- 122. Cheng PY, Sham A, Kronstad JW (2009) Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans . Infect Immun 77: 4284–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huston SM, Li SS, Stack D, Timm-McCann M, Jones GJ, et al. (2013) Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J Immunol 191: 249–261. [DOI] [PubMed] [Google Scholar]
- 124.Angkasekwinai P, Sringkarin N, Supasorn O, Fungkrajai M, Wang YH, et al. (2014) Cryptococcus gattii infection dampens Th1/Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect Immun pii: IAI.01773–14. [DOI] [PMC free article] [PubMed]
- 125. Fox DL, Müller NL (2005) Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. Am J Roentgenol 185: 622–626. [DOI] [PubMed] [Google Scholar]
- 126.Sorrell TC, Chen SC, Phillips P, Marr KA (2011) Clinical perspectives on Cryptococcus neoformans and Cryptococcus gattii: implications for diagnosis and management. In: Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A. Cryptococcus: from human pathogen to model yeast. ASM Press, Washington DC, 595–606. [Google Scholar]
- 127. Lee YC, Wang JT, Sun HY, Chen YC (2011) Comparisons of clinical features and mortality of cryptococcal meningitis between patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect 44: 338–345. [DOI] [PubMed] [Google Scholar]
- 128. Walraven CJ, Gerstein W, Hardison SE, Wormley F, Lockhart SR, et al. (2011) Fatal disseminated Cryptococcus gattii infection in New Mexico. PLoS One 6: e28625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Carriconde F, Gilgado F, Arthur I, Ellis D, Malik R, et al. (2011) Clonality and α-a recombination in the Australian Cryptococcus gattii VGII population–an emerging outbreak in Australia. PLoS One 6: e16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Escandón P, Sánchez A, Martínez M, Meyer W, Castañeda E (2006) Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res 6: 625–635. [DOI] [PubMed] [Google Scholar]
- 131. Callejas A, Ordoñez N, Rodriguez MC, Castañeda E (1998) First isolation of Cryptococcus neoformans var. gattii, serotype C, from the environment in Colombia. Med Mycol 36: 341–344. [PubMed] [Google Scholar]
- 132. Firacative C, Torres G, Rodríguez MC, Escandón P (2011) First environmental isolation of Cryptococcus gattii serotype B, from Cúcuta, Colombia. Biomédica 31: 118–123. [DOI] [PubMed] [Google Scholar]
- 133. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, et al. (2010) Clinical practice guidelines for the management of cryptococcal disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 50: 291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE checklist.
(DOCX)
Number of Cryptococcus gattii cryptococcosis cases in Colombia, per state, year and molecular type. Number of cases in brackets.
(DOCX)
Gene Bank accession numbers for the allele types of Colombian Cryptococcus gattii clinical strains.
(DOCX)
Cryptococcus gattii: Prevalence and molecular type of clinical isolates reported worldwide.
(DOCX)
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. All strains are deposited in the culture collection of the national Institute of Health in Bogota (Contact Elizabeth Castañeda, email: ecastaneda21@gmail.com) and are available following the regulations of the International Biodiversity Convention (1993) and the subsequent laws of the South American states, including Colombia. The Biodiversity convention states: Article 15 access to Genetic Resources: paragraph 1: “Recognizing the sovereign rights of States over their natural resources, the authority to determine access to genetic resources rests with the national governments and is subject to national legislation”. The laws, specifically the Colombian Decree 391 of 1996, Law 165 of 1994 states: that the genetic resources of Plants, Animals and Microorganisms belong to the country of origin. As such, microbial strains can only be provided for specific projects signing material transfer agreements for the use of a specific project, with an agreed co-authorship on all publications resulting from the use of the provided microbial cultures. The microbial strains that were obtained through a material transfer agreement have to be destroyed after completion of the study, and cannot be passed on to third parties. Any additional researcher who wants to work with these strains needs to obtain the strains directly from the culture collection of the National Institute of Health, Instituto Nacional de Salud, Bogotá after signing a new material transfer agreement.