Abstract
An amplification of tandem CAG trinucleotide sequences in DNA due to errors in DNA replication is involved in at least four hereditary neurodegenerative diseases. The CAG triplet repeats when translated into protein give rise to tracts of glutamine residues, which are a prominent feature of many transcription factors, including the TATA-binding protein of transcription factor TFIID. We have used a biotin-labeled, complementary peptide nucleic acid (PNA) to invade the CAG repeats in intact chromatin and then employed a method for the selective isolation of transcriptionally active chromatin restriction fragments containing the PNA.DNA hybrids. The PNA-containing chromatin fragments were captured on streptavidin-agarose magnetic beads and shown to contain all the CAG.PNA hybrids of the active chromatin fraction. DNA hybridization experiments using a DNA probe specific for unique sequences downstream of the CAG-tandem repeats confirmed that the PNA.DNA hybrids contained the transcribed gene for the TATA-binding protein. In contrast, no hybridization signal was detected with a DNA probe specific for the c-myc protooncogene, which is amplified and transcriptionally active in COLO 320DM cells but lacks CAG tandem repeats.
Full text
PDF

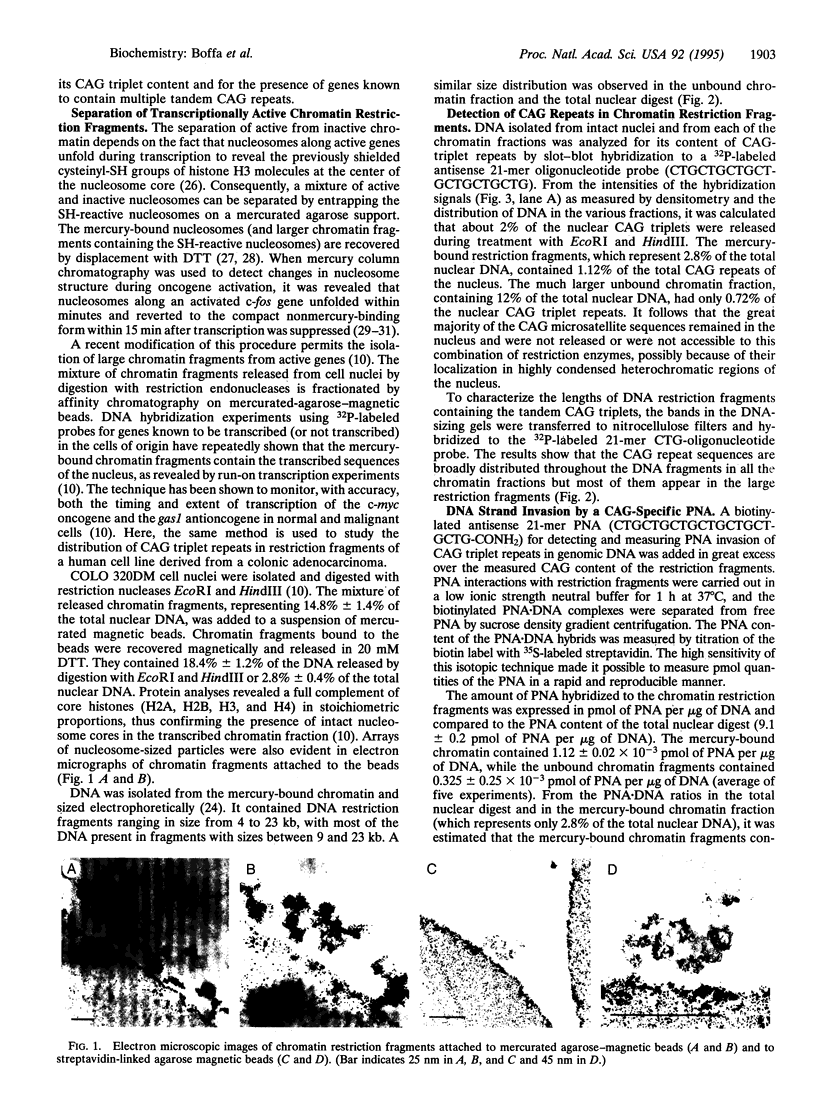


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N., Bienz M. Functional dissection of Drosophila abdominal-B protein. Mech Dev. 1991 Aug;35(1):55–64. doi: 10.1016/0925-4773(91)90041-4. [DOI] [PubMed] [Google Scholar]
- Allfrey V. G., Chen T. A. Nucleosomes of transcriptionally active chromatin: isolation of template-active nucleosomes by affinity chromatography. Methods Cell Biol. 1991;35:315–335. doi: 10.1016/s0091-679x(08)60578-6. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Walker J., Chen T. A., Sterner R., Mariani M. R., Allfrey V. G. Factors affecting nucleosome structure in transcriptionally active chromatin. Histone acetylation, nascent RNA and inhibitors of RNA synthesis. Eur J Biochem. 1990 Dec 27;194(3):811–823. doi: 10.1111/j.1432-1033.1990.tb19474.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. L., Driver E. D., Miesfeld R. L. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994 Aug 11;22(15):3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Cleland T. A., Boffa L. C., Carpaneto E. M., Mariani M. R., Valentin E., Mendez E., Allfrey V. G. Recovery of transcriptionally active chromatin restriction fragments by binding to organomercurial-agarose magnetic beads. A rapid and sensitive method for monitoring changes in higher order chromatin structure during gene activation and repression. J Biol Chem. 1993 Nov 5;268(31):23409–23416. [PubMed] [Google Scholar]
- Chen T. A., Allfrey V. G. Rapid and reversible changes in nucleosome structure accompany the activation, repression, and superinduction of murine fibroblast protooncogenes c-fos and c-myc. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5252–5256. doi: 10.1073/pnas.84.15.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. A., Sterner R., Cozzolino A., Allfrey V. G. Reversible and irreversible changes in nucleosome structure along the c-fos and c-myc oncogenes following inhibition of transcription. J Mol Biol. 1990 Apr 5;212(3):481–493. doi: 10.1016/0022-2836(90)90327-I. [DOI] [PubMed] [Google Scholar]
- Cherny D. Y., Belotserkovskii B. P., Frank-Kamenetskii M. D., Egholm M., Buchardt O., Berg R. H., Nielsen P. E. DNA unwinding upon strand-displacement binding of a thymine-substituted polyamide to double-stranded DNA. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1667–1670. doi: 10.1073/pnas.90.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egholm M., Buchardt O., Christensen L., Behrens C., Freier S. M., Driver D. A., Berg R. H., Kim S. K., Norden B., Nielsen P. E. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993 Oct 7;365(6446):566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- Gaspar M. L., Meo T., Tosi M. Structure and size distribution of the androgen receptor mRNA in wild-type and Tfm/Y mutant mice. Mol Endocrinol. 1990 Oct;4(10):1600–1610. doi: 10.1210/mend-4-10-1600. [DOI] [PubMed] [Google Scholar]
- Hanvey J. C., Peffer N. J., Bisi J. E., Thomson S. A., Cadilla R., Josey J. A., Ricca D. J., Hassman C. F., Bonham M. A., Au K. G. Antisense and antigene properties of peptide nucleic acids. Science. 1992 Nov 27;258(5087):1481–1485. doi: 10.1126/science.1279811. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Sinn E., Yamamoto T., Wang J., Roy A., Horikoshi M., Roeder R. G. Highly conserved core domain and unique N terminus with presumptive regulatory motifs in a human TATA factor (TFIID). Nature. 1990 Jul 26;346(6282):387–390. doi: 10.1038/346387a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mhatre A. N., Trifiro M. A., Kaufman M., Kazemi-Esfarjani P., Figlewicz D., Rouleau G., Pinsky L. Reduced transcriptional regulatory competence of the androgen receptor in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1993 Oct;5(2):184–188. doi: 10.1038/ng1093-184. [DOI] [PubMed] [Google Scholar]
- Møllegaard N. E., Buchardt O., Egholm M., Nielsen P. E. Peptide nucleic acid.DNA strand displacement loops as artificial transcription promoters. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3892–3895. doi: 10.1073/pnas.91.9.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence specific inhibition of DNA restriction enzyme cleavage by PNA. Nucleic Acids Res. 1993 Jan 25;21(2):197–200. doi: 10.1093/nar/21.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991 Dec 6;254(5037):1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Peffer N. J., Hanvey J. C., Bisi J. E., Thomson S. A., Hassman C. F., Noble S. A., Babiss L. E. Strand-invasion of duplex DNA by peptide nucleic acid oligomers. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10648–10652. doi: 10.1073/pnas.90.22.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Johnson T., Suzuki M., Finch J. T. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Prior C. P., Cantor C. R., Johnson E. M., Littau V. C., Allfrey V. G. Reversible changes in nucleosome structure and histone H3 accessibility in transcriptionally active and inactive states of rDNA chromatin. Cell. 1983 Oct;34(3):1033–1042. doi: 10.1016/0092-8674(83)90561-5. [DOI] [PubMed] [Google Scholar]
- Soper T. S., Jones W. M., Manning J. M. Effects of substrates on the selective modification of the cysteinyl residues of D-amino acid transaminase. J Biol Chem. 1979 Nov 10;254(21):10901–10905. [PubMed] [Google Scholar]
- Tan J. A., Joseph D. R., Quarmby V. E., Lubahn D. B., Sar M., French F. S., Wilson E. M. The rat androgen receptor: primary structure, autoregulation of its messenger ribonucleic acid, and immunocytochemical localization of the receptor protein. Mol Endocrinol. 1988 Dec;2(12):1276–1285. doi: 10.1210/mend-2-12-1276. [DOI] [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J., Chen T. A., Sterner R., Berger M., Winston F., Allfrey V. G. Affinity chromatography of mammalian and yeast nucleosomes. Two modes of binding of transcriptionally active mammalian nucleosomes to organomercurial-agarose columns, and contrasting behavior of the active nucleosomes of yeast. J Biol Chem. 1990 Apr 5;265(10):5736–5746. [PubMed] [Google Scholar]
- Zhou Q., Boyer T. G., Berk A. J. Factors (TAFs) required for activated transcription interact with TATA box-binding protein conserved core domain. Genes Dev. 1993 Feb;7(2):180–187. doi: 10.1101/gad.7.2.180. [DOI] [PubMed] [Google Scholar]