Abstract
Background
Postural tachycardia syndrome (POTS) is characterized clinically by an exaggerated increase in heart rate (HR) but an associated cognitive impairment disables many patients. Modafinil might be effective in improving the cognitive symptoms, but modafinil may stimulate the sympathetic nervous system and worsen tachycardia in POTS. We tested the hypothesis that modafinil would worsen tachycardia and orthostatic symptoms in POTS.
Methods
Patients with POTS (n=54) underwent a randomized crossover trial with modafinil 100mg vs. placebo. HR and systolic blood pressure (SBP) were measured seated and standing prior to modafinil or placebo administration, and then hourly for 4 hours.
Results
Over 4 hours, standing HR was not significantly different between modafinil and placebo (ANOVA Pdrug=0.328), but seated SBP was significantly higher in the modafinil group (109±12 mmHg vs. 104±10 mmHg; P=0.004). Modafinil also significantly increased both the seated SBP (ANOVA Pdrug=0.004) and standing SBP (ANOVA Pdrug=0.041) over time. There was no significant difference between modafinil and placebo over the 4 hour period with regard to POTS symptom burden scores (14 ± 12 vs. 14 ± 12; P=0.962).
Conclusions
Modafinil did not significantly worsen standing HR or acute orthostatic symptoms in POTS patients compared to placebo, and improved upright blood pressure. Therefore, modafinil could be tested as a potential treatment for the cognitive impairment in POTS.
Keywords: Modafinil, stimulant, postural tachycardia syndrome, blood pressure, heart rate
INTRODUCTION
Patients who suffer from postural tachycardia syndrome (POTS) have an excessive increase in heart rate (HR) upon standing that improves upon lying down. POTS is associated with significant functional disability, including diminished quality of life, cognitive impairment, daytime sleepiness, unrefreshing sleep, fatigue, lightheadedness, visual blurring, and anxiety, with resultant decreased quality of life in otherwise healthy individuals.1
Patients diagnosed with POTS often report declines in cognitive function described as “brain fog”. Previous studies have shown that patients with POTS have significant inattention that could contribute to cognitive dysfunction in this disease.2 Modafinil is a stimulant of potential interest in the management of POTS-related cognitive symptoms because of its ability to improve mental function and alertness in various patient populations.3, 4 However, modafinil may elevate the HR and blood pressure (BP) due to activation of the sympathetic nervous system 5, and this could limit tolerability of modafinil in POTS patients
In this study, we tested the hypothesis that modafinil would increase standing HR and therefore worsen the symptoms found in POTS patients.
METHODS
Participants
POTS patients (n=54; 48 female) who met POTS diagnostic criteria (increase in HR ≥ 30 bpm with standing in the absence of orthostatic hypotension) were enrolled in this study between January 2004 and September 2012.6–8 Written informed consent was obtained from each subject before initiating the study, and the Vanderbilt University Institutional Review Board approved this study. The data reported are parts of “The Treatment of Orthostatic Intolerance” study, a series of acute treatment trials involving various pharmacologic agents to empirically treat POTS (http://www.clinicaltrials.gov/ct2/show/NCT00262470). None of the modafinil data have been previously published.
Study Diet and Posture Study
For at least 3 days before testing, subjects consumed a low monoamine, caffeine-free diet containing 150 mEq/day Na+ and 60–80 mEq/day K+. Long-term medications were discontinued 5 half-life periods before the study. A “posture study” was performed to diagnose patients with POTS and for baseline characterization. HR, systolic BP (SBP), diastolic BP (DBP), and fractionated plasma catecholamines were measured after overnight rest with the patient in the supine position and again after standing for up to 30 minutes (as tolerated) as part of the posture study. Concentrations of norepinephrine and epinephrine were obtained based on protocol previously described by our group.9
Medication Trials
The medication trials were started in the morning ≥ 2 hours after a light breakfast and in a post-void state. Modafinil is rapidly absorbed with a peak drug level of 2–4 hours.10 Patients were given modafinil 100 mg (Cephalon, Frazer, PA) and placebo (“Cebocaps”; Forest Pharmaceuticals, New York, NY) in a randomized crossover fashion on separate days, with 31 patients receiving placebo on the first day. The patients were seated in a chair during the data collection except during prescribed periods of standing. BP and HR were measured with an automated arm cuff vital signs monitor (Dinamap Vital Signs Monitor; Critikon Company, Tampa, FL) and digitally acquired into a custom-designed database (Microsoft Access, Microsoft Corporation, Redmond, WA). Before study drug administration, and then hourly for 4 hours after study drug administration, the HR and BP were measured for every patient while seated. They were then asked to stand up, and HR and BP measurements were repeated after they have been standing for 10 minutes. The study was single-blinded, with the patient blinded to drug or placebo administration. Only the nurse administering the study drug was aware of its contents; she was not involved in either recording or interpreting the patients’ response to therapy.
Patients were asked to rate their symptom burden immediately before and at 2 and 4 hours after study drug administration using the Vanderbilt Orthostatic Symptom Score (VOSS).11 The scores could range from 0–90, with higher scores representing worse symptoms.
Statistical Analysis
The primary end point was the 10-minute standing HR 4 hours after study drug administration. Paired t-tests were used to compare single time point data between interventions, and repeated-measures analyses of variance (ANOVA) with a Greenhouse-Geisser correction were used to compare data over time Two-tailed P ≤ 0.05 were considered statistically significant. Statistical analyses were performed with SPSS for Windows (version 21.0, IBM Corporation, Armonk, NY). Prism for Windows 5 (version 5.02, GraphPad Software, Inc, La Jolla, CA) was used for graphical presentation.
RESULTS
Demographics and Baseline Patient Characterization
HR increased significantly with standing (73±10 bpm to 118±22 bpm, P<0.001) and without significant decrease in BP, consistent with the diagnosis of POTS (Table 1). The mean supine plasma norepinephrine and epinephrine values were within the normal range (norepinephrine <475 pg/mL and epinephrine <75 pg/mL), but both upright norepinephrine (223±114 pg/ml vs. 831±435 pg/mL; P<0.001) and epinephrine (23±30 pg/ml vs. 83±110 pg/ml; P=0.001) increased significantly with standing.
Table 1.
Demographics and Posture Study
| Total Subjects (n) | 54 |
| Woman, (n) | 48 (89%) |
| Age (years) | 32 ± 10 |
| Supine | |
| Heart Rate (beats/min) | 73 ± 10 |
| Systolic Blood Pressure (mmHg) | 108 ± 11 |
| Diastolic Blood Pressure (mmHg) | 67 ± 10 |
| Norepinephrine (pg/mL) | 223 ± 114 |
| Epinephrine (pg/mL) | 23 ± 30 |
| Standing | |
| Heart Rate (beats/min) | 118 ± 22 * |
| Systolic Blood Pressure (mmHg) | 113 ± 23 |
| Diastolic Blood Pressure (mmHg) | 71 ± 14 |
| Norepinephrine (pg/mL) | 832 ± 435 * |
| Epinephrine (pg/mL) | 83 ± 110 * |
| Change from Supine to Standing | |
| Heart Rate (beats/min) | 45 ± 21 |
| Systolic Blood Pressure (mmHg) | 4 ± 20 |
| Diastolic Blood Pressure (mmHg) | 4 ± 13 |
| Norepinephrine (pg/mL) | 615 ± 380 |
| Epinephrine (pg/mL) | 60 ± 112 |
Data are presented as mean ± standard deviation. Reported P values are for paired t-test comparing supine and upright parameters.
P < 0.001
Seated and Standing HR with Modafinil and Placebo
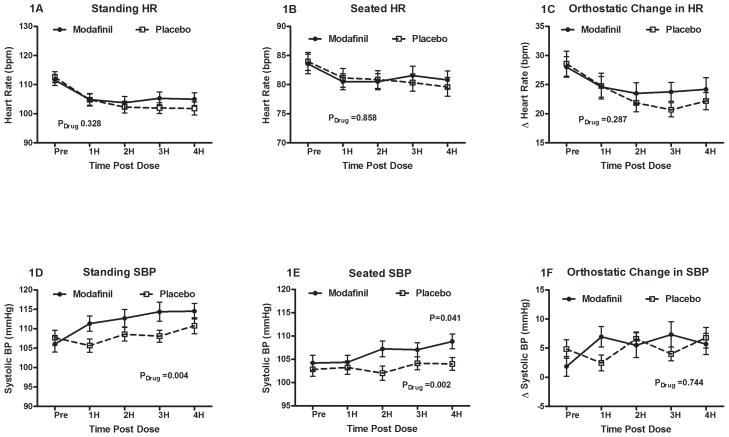
All 54 subjects underwent the paired administration of placebo and modafinil on different randomized days. The standing HR before study drug administration was similar between modafinil and placebo groups (112±14 vs. 113±14 bpm; P=0.575) with a significant decrease in standing HR over the 4 hour period across both groups (ANOVA Ptime < 0.001). This did not significantly differ between modafinil and placebo over time (ANOVA Pdrug = 0.328). The standing HR (105±16 vs. 101±16 bpm; P=0.139, Figure 1A) and seated HR (83±12 vs. 84±11 bpm; P=0.763; Figure 1B) at 4 hours post administration were both similar between the modafinil and the placebo group.
Figure 1. Measured effects of modafinil and placebo on patients with POTS over time.
Modafinil used resulted in no change in the standing heart rate (HR; Panel 1A), seated HR (Panel 1B) or orthostatic tachycardia (Panel 1C) over time. In contrast, modafinil increased standing systolic blood pressure (SBP; Panel 1D), seated SBP (Panel 1E), but not the orthostatic increase in SBP (Panel 1F). *P<0.05 was considered significant.
The orthostatic delta HR (Figure 1C) was similar at baseline between both groups (28±13 vs. 29±15 bpm; P=0.769), and was not significantly different after 4 hours (24±14 vs. 22±11 bpm; P=0.316). There were no differences detected with respect to modafinil versus placebo over time (Pdrug= 0.287).
Seated and Standing BP with Modafinil and Placebo
There were no significant differences between the modafinil and placebo days in seated SBP, seated DBP, or seated MAP at baseline prior to the study. Modafinil significantly increased both the seated and standing SBP (Figure 1D) over time compared to placebo (standing SBP ANOVA Pdrug = 0.004; seated SBP ANOVA Pdrug= 0.041). By 4 hours, BP was significantly higher in the modafinil group than placebo for seated MAP (84±9 vs. 82±9 mmHg; P=0.004), seated SBP (109±12 vs. 104±10 mmHg; Figure 1E), and seated DBP (71±8 vs. 68±8 mmHg; P=0.007). Orthostatic SBP for the modafinil and placebo groups was not significantly different at baseline (2±12 vs. 5±11 mmHg; P=0.173), or at 4 hours (6±13 vs. 7±13 mmHg; P=0.650). There was also no significant difference over time in the orthostatic SBP between groups (ANOVA Pdrug = 0.744; Figure 1F).
Symptoms with Modafinil and Placebo
The VOSS scores were completed by 37 study subjects. There were no significant differences between the symptom scores of the modafinil and placebo groups at baseline (15±10 vs. 18±16; P=0.208) and at 4 hours (14±12 vs. 14±12; P=0.962). Notably, the mental clouding component of VOSS was neither significantly different at baseline between modafinil and placebo (1.7±1.8 vs. 1.9±2.6; P=0.614), nor at 4 hours after study drug administration (1.5±1.9 vs. 1.7±2.5; P=0.781).
DISCUSSION
POTS is a chronic disorder of the autonomic nervous system affecting 500,000–3 million Americans with a strong female predominance.1, 7, 12 The cardinal feature of this disorder is a substantial and persistent rise in HR of patients upon standing.1 Many POTS patients complain of “mental clouding,” which contributes significantly to disability.2, 12, 13 This is a broad term that likely encompasses sleep disturbance, diminished attention, reduced concentration, lightheadedness, and fatigue.12, 13 Medications known to improve mental function are mostly stimulants. There is concern that stimulant medications may worsen the tachycardia associated with POTS and may have a limited benefit in POTS treatment.14 We assessed whether modafinil would increase the standing HR and worsen symptoms in POTS, thus limiting its tolerability for treatment of these patients.
HR with Modafinil in POTS
From a pathophysiological perspective, tachycardia is thought to be a strong driver of POTS symptoms.15 Tachycardia can lead to decreased cardiac filling and compromised cardiac output despite increased HR, and may result in decreased cerebral perfusion.15 Therefore, stimulants could potentially worsen symptoms of POTS by increasing the tachycardia.
Our primary finding is that acute administration of modafinil at a dose of 100mg did not significantly increase the standing HR of patients with POTS (P = 0.328). While it is a low dose, modafinil 100mg PO was used as it is the clinically relevant dose based upon prior reports of modafinil use in POTS.16
SBP with Modafinil in POTS
Modafinil significantly increased the seated (P=0.002) and standing SBP (P=0.004) in POTS patients compared to placebo. The increase in seated and standing SBP with modafinil in POTS patients, although not clinically significant, may be due to the stimulant effects of this drug.5
POTS Symptoms with Modafinil
Modafinil did not significantly worsen symptoms in POTS patients. Our use of self-rated tools, and not mental clouding specific tools, to assess mental clouding symptoms may have limited our ability to objectively measure this outcome.
Study Limitations
Our sample size was relatively small, but compares favorably to other studies in the same field. Modafinil was only compared to placebo and not to other stimulants. All participants in this study were female, reflecting the dominant POTS demographic, but limiting the generalizability of conclusions. The primary limitation of our study is that our symptoms assessment tool was not specifically designed to detect improvements in cognitive function. The severity and prevalence of inattention has previously been studied in POTS patients using well-validated psychological measures.2 Future studies should use validated cognitive measures to assess the degree of improvement in cognitive symptoms with modafinil. Further, this was an acute study, and chronic modafinil administration may have different effects. Armed with these safety data of modafinil in POTS patients, we can further press the investigation into long term therapy with modafinil.
Conclusions
Modafinil did not significantly increase the standing HR or symptoms and was well tolerated in POTS patients, suggesting that modafinil may be a viable option in treating POTS symptoms. Future studies examining long-term tolerability and benefits of modafinil in POTS patients are needed.
Acknowledgments
RESEARCH FUNDING
Supported in part by NIH grants R01 HL102387, U54 NS065736, P01 HL56693, UL1 TR000445 (Clinical and Translational Science Award).
Footnotes
Clinical Trials Registration: NCT00262470.
COMPETING INTERESTS
None
Reference List
- 1.Low PA, Sandroni P, Joyner M, et al. Postural tachycardia syndrome (POTS) J Cardiovasc Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raj V, Haman KL, Raj SR, et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009;80:339–344. doi: 10.1136/jnnp.2008.144360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taneja I, Haman K, Shelton RC, et al. A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol. 2007;27:76–79. doi: 10.1097/jcp.0b013e31802eb7ea. [DOI] [PubMed] [Google Scholar]
- 4.Turner DC, Robbins TW, Clark L, et al. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- 5.Taneja I, Diedrich A, Black BK, et al. Modafinil elicits sympathomedullary activation. Hypertension. 2005;45:612–618. doi: 10.1161/01.HYP.0000158267.66763.63. [DOI] [PubMed] [Google Scholar]
- 6.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 7.Raj SR. Postural Tachycardia Syndrome (POTS) Circulation. 2013;127:2336–2342. doi: 10.1161/CIRCULATIONAHA.112.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–580. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- 10.Robertson P, Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42:123–137. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Raj SR, Black BK, Biaggioni I, et al. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 12.Ocon AJ, Messer ZR, Medow MS, et al. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond) 2012;122:227–238. doi: 10.1042/CS20110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack KJ, Johnson JN, Rowe PC. Orthostatic intolerance and the headache patient. Semin Pediatr Neurol. 2010;17:109–116. doi: 10.1016/j.spen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Barnett KJ, Cooper NJ. The effects of a poor night sleep on mood, cognitive, autonomic and electrophysiological measures. J Integr Neurosci. 2008;7:405–420. doi: 10.1142/s0219635208001903. [DOI] [PubMed] [Google Scholar]
- 15.Masuki S, Eisenach JH, Schrage WG, et al. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol. 2007;103:1128–1135. doi: 10.1152/japplphysiol.00175.2007. [DOI] [PubMed] [Google Scholar]
- 16.Kanjwal K, Saeed B, Karabin B, et al. Preliminary observations suggesting that treatment with modafinil improves fatigue in patients with orthostatic intolerance. Am J Ther. 2011;18:449–452. doi: 10.1097/MJT.0b013e3181da0763. [DOI] [PubMed] [Google Scholar]