Abstract
Background
Standard treatment of pulmonary metastasis in patients with Wilms tumor (WT) includes 12 Gy radiation therapy (RT) to the entire chest. The risk of breast cancer (BC) in a large cohort of female survivors of WT has not previously been reported.
Methods
2,492 female participants in National Wilms Tumor Studies 1–4 (1969–1995) were followed from age 15 through mid 2013 for incident BC. Median age at last contact was 27.3 years. We calculated cumulative risk at age 40 (CR40), hazard ratios (HR) by Cox regression, standardized incidence ratios (SIR) relative to US population rates and [shown in brackets] 95% confidence intervals.
Results
Numbers of survivors with invasive BC divided by numbers at risk were 16/369 (CR40=14.8% [8.7–24.5]) for women who received chest RT for metastatic WT, 10/894 (CR40=3.1% [1.3–7.41]) for those who received only abdominal RT and 2/1,229 (CR40=0.3% [0.0–2.3]) for those who received no RT. The SIRs for these three groups were 27.6 [16.1–44.2] based on 5,010 person-years (PY) of follow-up, 6.0 [2.9,11.0] based on 13,185 PY, and 2.2 [0.3,7.8] based on 13,560 PY, respectively. The risk was high regardless of chest RT among women diagnosed with WT at age 10 or later, with 9/90 developing BC (CR40=13.5% [5.6–30.6], SIR=23.6 [10.8–44.8], PY=1,463).
Conclusion
Female WT survivors treated with chest RT had high risk of early BC, with nearly 15% developing invasive disease by age 40. Current guidelines that recommend screening only survivors receiving ≥20 Gy RT to the chest might be re-evaluated.
Introduction
Modern treatment of the childhood kidney cancer Wilms tumor (WT) has led to cure rates approaching 90%.1 Survivors have increased rates of secondary cancers,2 due in part to late effects of radiation therapy (RT) and chemotherapy. Among patients with unilateral WT enrolled on the first three protocols of the National Wilms Tumor Study (NWTS), approximately 11% had pulmonary metastases at the time of WT diagnosis3 and a further 9% developed them at relapse.4 Most such patients received an RT dose of 12 (the current standard) or 14 Gy to the entire chest.5–7
The Childhood Cancer Survivor Study (CCSS) estimated that childhood cancer survivors treated with and without chest RT had, respectively, 24.7 and 4.8 times the rates of breast cancer (BC) of the US population.8 Information on BC in childhood cancer survivors mainly comes from Hodgkin lymphoma (HL) patients,9 who comprised 65% of BC cases in the CCSS cohort.10 Chest RT in HL survivors was associated with a cumulative incidence of BC of 12.9% by age 40.8 HL patients receive varying quantities of radiation. Lee et al.11 reported a median of 35 Gy and range of 15–60 Gy. Unlike whole lung RT in WT, the radiation fields used for HL may not always include the entire volume of both breasts.12 Current guidelines from the Children’s Oncology Group recommend routine screening for BC in survivors of childhood cancer only if the chest RT dose is ≥20 Gy,13,14 which would exclude most patients with WT.
Information on BC in WT survivors is scarce. The CCSS and British Childhood Cancer Survivor Study (BCCSS) reported 3 and 8 cases, respectively.8,15 We report here on the largest number of BC cases observed in WT survivors thus far, focusing on the increased risk due to chest RT with a view towards evaluating the adequacy of current screening guidelines. The associations of BC risk with abdominal RT, use of doxorubicin, which had increased the risk of secondary tumors in an earlier study,16 and age at onset of WT were also examined.
Methods
Study cohort
The study population consisted of 2,492 female US and Canadian patients aged 0–19 years at WT onset who were enrolled on one of the first four NWTS protocols (1969–1995). All participants survived to age 15 or 5 years from WT onset, whichever came later, and were followed from that time forward until age of last contact or death. The closing date for follow-up was June 30, 2013. They were consented by their parent/guardian for enrollment in the NWTS and were re-consented as adults for continuing follow-up at age 18. Secondary malignancies were ascertained via clinical records and annual status reports; wherever possible, they were confirmed by medical records review.
Radiation
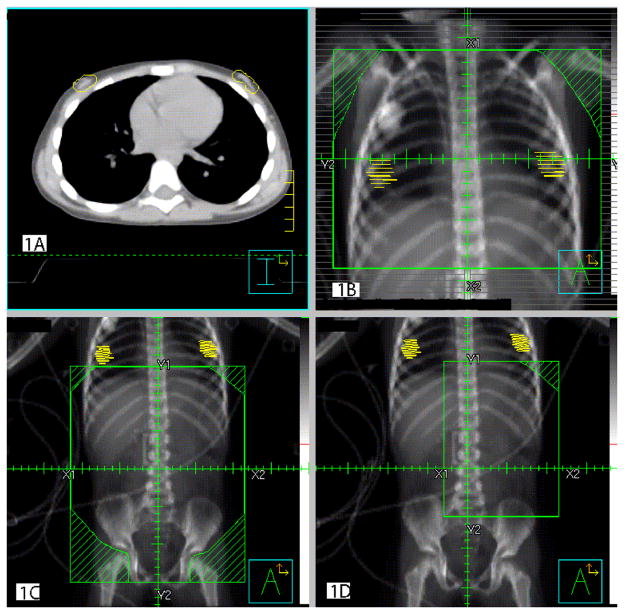
The first protocol (NWTS-1) specified uniform doses of 14 Gy for pulmonary metastasis at the time of WT diagnosis.7 The dose was reduced to 12 Gy during NWTS-2 and remained 12 Gy on NWTS-3,4.5,6,17 The entire chest, from approximately the clavicle to the L1 vertebra, regardless of the number and location of visible metastases, was included in the treatment volume. These studies lacked specific protocols for pulmonary metastasis at relapse, but most physicians used 12Gy whole chest RT for the initial metastasis and lower doses to smaller fields for subsequent ones. There was greater variation in the abdominal doses, with intensified chemotherapy replacing RT in later protocols. Most patients in NWTS-1 received 18–40 Gy RT to the renal fossa (flank), depending on age, with whole abdomen RT or boosts given for more extensive disease. By NWTS-4 only a minority of patients at “high risk” of relapse by virtue of stage or histology received any flank RT, and then in a standard dose of 10.8 Gy, with additional amounts for more extensive disease. Relapse treatment was largely at the discretion of the local investigator. Figure 1 shows standard fields for chest, whole abdomen and flank, respectively, for a female patient age 5. Actual fields varied with age and tumor location.
Figure 1.
Figures 1A–D: Axial CT scan and digitally reconstructed radiographs (DRR) with breast tissue contours in a 5 year old girl with Wilms tumor. A) Axial CT scan of the chest, B) DRR of a standard whole lung radiation field, C) DRR of a standard whole abdomen radiation field and D) DRR of a standard flank field.
Patients were classified as to whether or not they received chest RT, abdominal RT only or no RT and by the total dose to any portion of the chest (none, 1–12 Gy, and >12 Gy). Of those receiving chest RT, 87% had a single course of treatment to the entire chest. Regression analyses utilized the highest cumulative RT doses given to the entire or to any portion of the left (L) and right (R) chest and to the L and R flank.
Covariates
Covariates included age at WT diagnosis and whether or not doxorubicin was administered.
Statistical methods
We calculated cumulative risk of BC as a function of participant age, subtracting the Kaplan-Meier estimate from 1.18,19 Since only 3% of participants died of other causes, this was comparable to cumulative incidence where death is considered a competing risk.20 Standardized incidence ratios (SIRs), with tests, confidence intervals and inter-group comparisons based on Poisson statistics,18 compared rates of BC in WT survivors with age- and period-specific US population rates, both in yearly intervals.21 Participants were considered at risk of BC until the end of follow-up, even if a previous BC had occurred.
The effects on BC risk of RT variables and covariates after adjustment for each other were estimated as hazard ratios (HR) using Cox models for age at first BC. To investigate the separate effects of contralateral vs. ipsilateral RT, each patient generated two records, one for the L and one for the R breast. Participants with bilateral BC, whether synchronous or metachronous, contributed two events. Correlation between records for the same patient was accounted for by grouped jackknife (robust) standard errors; influence and tests for non-proportionality were assessed by standard methods.22 All calculations were performed in R.23 Ninety-five percent confidence intervals (CI) are shown in brackets.
Imputation of missing data
We imputed missing information on RT doses as described.24 Two patients missing information on chest and 20 on flank RT for primary disease were assigned the protocol dose. Multiple imputation was used for missing information on 16 patients eligible for chest and 3 treated with abdominal RT for relapse.25 Twenty filled-in datasets were generated based on random draws from complete records, stratified on prior treatment history and side of relapse. Nineteen yielded the same classification of subjects by radiation category (none, chest, abdominal only), the other differing only for a single subject, and a single consensus classification was used for analysis. Results using the quantitative RT variables differed slightly by imputed dataset, and standard methods were used to combine them.26
Results
Description of cohort
The 2,492 participants had a median age at last follow-up of 27.3 years (interquartile range 21.5–33.2, maximum 55.2 years). Twenty-nine cases of invasive BC were ascertained among 28 participants (Table 1). Twenty were confirmed by pathology report, 1 by publication, 2 by death certificate, 2 by clinical records and 4 from detailed annual status report or other patient contact. Patient #1, diagnosed with WT at age 7, developed a phylloides tumor in the R breast at age 15 and a ductal carcinoma in the L breast at age 34. The median age at first BC diagnosis was 34.3 years (range 15.5–48.4 years) and the median time from WT diagnosis to first BC diagnosis was 27.1 years (range 7.9–35.7 years). At diagnosis, 15 BC were on the L side, 12 on the R and 2 were bilateral.
Table 1.
Selected characteristics of breast cancer cases
| ID | Age (yrs) @ | Side of BC | Cumulative RT dose (Gy) | Doxorubicin | ||||
|---|---|---|---|---|---|---|---|---|
| Chest | Flank | |||||||
| BC | WT | L | R | L | R | |||
| 1 | 15.5 | 7.6 | R | 12.0 | 12.0 | 0 | 19.5 | Y |
| 2 | 23.0 | 3.0 | R | 12.0 | 12.0 | 10.5 | 0 | Y |
| 3 | 24.4 | 3.7 | B | 12.0 | 12.0 | 21.6 | 0 | Y |
| 4 | 26.8 | 7.6 | R | 13.5 | 13.5 | 35.0 | 0 | Y |
| 5 | 27.2 | 1.6 | L | 0 | 0 | 10.5 | 10.5 | N |
| 6 | 27.8 | 0.8 | R | 0 | 0 | 10.0 | 10.0 | N |
| 7 | 30.2 | 12.4 | L | 40.0 | 14.0 | 40.7 | 0 | N |
| 8 | 30.6 | 7.8 | L | 12.0 | 12.0 | 10.5 | 10.5 | Y |
| 9 | 30.9 | 3.7 | R | 14.0 | 14.0 | 0 | 0 | N |
| 10 | 31.0 | 1.9 | R | 0 | 0 | 0 | 24 | Y |
| 11 | 31.1 | 17.8 | L | 0 | 0 | 0 | 0 | N |
| 12 | 32.1 | 5.4 | R | 15.0 | 15.0 | 26.0 | 0 | Y |
| 13 | 33.4 | 8.5 | L | 12.0 | 12.0 | 20.0 | 20 | Y |
| 14 | 34.0 | 4.1 | L | 21.0 | 21.0 | 0 | 0 | N |
| 1 | 34.4 | 7.6 | L | 12.0 | 12.0 | 0 | 19.5 | Y |
| 15 | 34.7 | 9.9 | L | 12.0 | 12.0 | 10.5 | Y | |
| 16 | 34.9 | 6.9 | L | 12.0 | 12.0 | 21.4 | 12 | Y |
| 17 | 35.6 | 4.3 | R | 12.0 | 12.0 | 20.1 | 20.1 | Y |
| 18 | 36.1 | 12.4 | L | 0 | 0 | 21.1 | 0 | Y |
| 19 | 36.4 | 4.1 | R | 0 | 12.3 | 0 | 0 | N |
| 20 | 36.9 | 4.1 | L | 0 | 0 | 30.0 | 0 | Y |
| 21 | 37.2 | 9.9 | L | 14.0 | 14.0 | 26.0 | 0 | N |
| 22 | 37.6 | 12.8 | L | 0 | 0 | 20.0 | 0 | Y |
| 23 | 38.8 | 3.1 | R | 14.0 | 14.0 | 34.5 | 0 | Y |
| 24 | 39.9 | 11.3 | R | 0 | 0 | 0 | 21.4 | N |
| 25 | 41.2 | 13.3 | B | 0 | 0 | 20 | 0 | Y |
| 26 | 42.2 | 13.9 | L | 0 | 0 | 32.7 | 0 | N |
| 27 | 44.3 | 14.5 | L | 0 | 0 | 20.0 | 0 | Y |
| 28 | 48.4 | 12.9 | L | 0 | 0 | 0 | 0 | N |
Six patients reported ductal carcinoma in situ (DCIS) without invasive disease, all confirmed by pathology report. These 6 events were included only in a brief sensitivity analysis. They may have been particularly susceptible to detection bias resulting from more intensive screening of patients who received chest RT.
Of the 18 BC cases with information on estrogen (ER) and progesterone (PR) receptors, 14 were ER+/PR+, 2 were ER+/PR−, and 2 were ER−/PR−. Three of 16 cases having known human epidermal growth factor 2 receptor status were positive. Eight cases had a known family history of BC. Five died, all due to metastatic BC. The 5- and 10- year Kaplan-Meier survival probabilities following first BC diagnosis were 91% (95% CI [67,98]) and 74% [41,90], respectively.
Overall, 3.20 cases of BC were expected from US population rates based on 31,755 person-years (PY) of observation. (Table 2) The SIR was 29/3.20=9.1 (95% CI [6.0,13.0]) and the estimated cumulative risk at age 40 (CR40) was 4.5% [2.8,7.2].
Table 2.
Patient numbers, person-years of follow-up, standardized incidence ratios, and cumulative risks of breast cancer at age 40, by major risk categories
| Subgroup | No. patients | PY | SIR | No. BC | BC Exp. | BC risk @ age 40 (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| All patients | 2,492 | 31,755 | 9.1 | 29 | 3.20 | 4.5 | 2.8–7.2 |
| RT Group* | |||||||
| No RT | 1,229 | 13,560 | 2.2 | 2 | 0.92 | 0.3 | 0.0–2.3 |
| Abdominal RT only | 894 | 13,185 | 6.0 | 10 | 1.67 | 3.1 | 1.3–7.1 |
| Chest RT | 369 | 5,010 | 27.6 | 17 | 0.62 | 14.8 | 8.7–24.5 |
| Chest dose* | |||||||
| 0 Gy | 2,122.9 | 26,746 | 4.6 | 12 | 2.59 | 2.3 | 1.0–5.1 |
| 1–12 Gy | 246.9 | 2,857 | 46.8 | 9 | 0.19 | 14.4 | 7.6–30.1 |
| >12 Gy | 122.2 | 2,153 | 18.9 | 8 | 0.42 | 14.2 | 7.1–29.3 |
| Age at WT (years) | |||||||
| 0–4 | 1,856 | 22,326 | 6.0 | 11 | 1.84 | 3.1 | 1.5–6.3 |
| 5–9 | 546 | 7,967 | 9.2 | 9 | 0.98 | 4.4 | 2.0–9.1 |
| 10–19 | 90 | 1,463 | 23.6 | 9 | 0.38 | 13.5 | 5.6–30.6 |
| Doxorubicin | |||||||
| No | 1,518 | 19,742 | 4.8 | 11 | 2.29 | 2.6 | 1.2–5.4 |
| Yes | 974 | 12,013 | 19.7 | 18 | 0.91 | 9.4 | 4.9–17.4 |
| Current age (years) | |||||||
| 15–24 | 2,492 | 19,979 | 21.3 | 3 | 0.14 | NA | NA |
| 25–29 | 1,491 | 6,086 | 6.4 | 3 | 0.47 | ||
| 30–35 | 942 | 3,527 | 11.1 | 10 | 0.90 | ||
| 35–39 | 477 | 1,580 | 9.6 | 9 | 0.94 | ||
| 40+ | 184 | 582 | 5.3 | 4 | 0.75 |
Chest irradiation
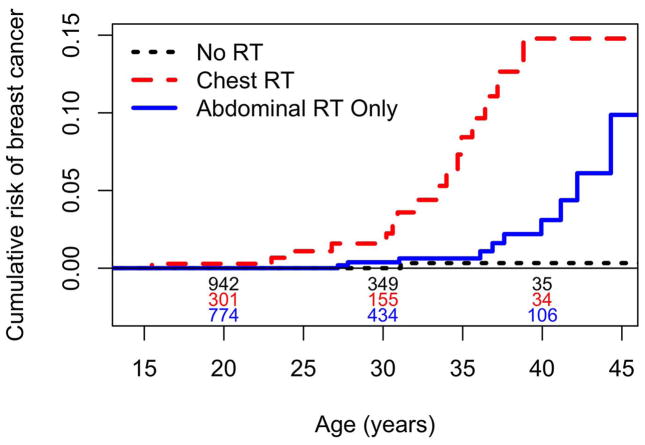
Sixteen of 369 patients who received chest RT developed BC, one of them twice. The SIR was 27.6 [16.1,44.2] and the CR40 was 14.8%. (Table 2 and Figure 1) Among the BC cases, RT was administered to the bilateral chest at doses of 12 Gy (9 patients), 14 Gy (3), 15 Gy (1) and 21 Gy (1), respectively. (Table 1) Numbers were assigned to patients in Table 1 in order of age at (first) BC diagnosis, and cannot be directly linked to the patient database. Patient #7 received 14 Gy to the bilateral field plus a boost of 26 Gy to the left lung, whereas patient #20 was treated with 12.3 Gy to the R lung only.
RT above 12 Gy did not increase risk compared to 12 Gy or less, but the range of doses was narrow. Among all patients who received chest RT, to the nearest Gy, 4% received a cumulative pulmonary dose of <12 Gy, 64% received 12 Gy, 19% between 13 and 15 Gy and 13% above 15 Gy. Most of the higher doses were to a portion of the lung.
Abdominal irradiation
Ten of 894 patients who received abdominal RT only developed BC. The SIR was 10/1.67=6.0 [2.9,11.0], p<0.0001 for the null hypothesis that SIR=1.0, and the CR40 was 3.1%. (Table 2 and Figure 1). However, the test comparing the SIR of 6.0 for patients who received abdominal RT only with the SIR of 2.2 for those who received no RT was not statistically significant (p=0.23). Nor was there a difference in the SIRs between 202 patients who received whole abdomen RT (2/0.28=7.2) and 682 who received RT only to the flank or other portions of the abdomen (8/1.39=5.8, p=0.68).
Seven of the 10 BC cases who received abdominal but no chest RT were treated for a WT on the L (5) or R (2) side only and developed unilateral BC. In all 7 instances, the BC developed on the same side as the WT (p=0.02). One of us (JAK) examined the radiation fields for the 10 cases and estimated the likely range of doses to the L and R breast. Patients #5 and #6, who received whole abdomen RT, had an estimated dose of 5.3–10.5 Gy and 3.0–10.0 Gy to the L and of 8.9–10.5 and 3.0–10.0 Gy to the R breast, respectively. Patients #20, #22, and #26 had estimated ranges of 3.0–12.0, 4.0–20.0, and 29.4–32.7 Gy, all to the L breast. For these 5 patients, significant amounts of RT were delivered to at least a portion of the breast where the cancer occurred.
Other subgroups
Nine cases of BC were observed among 90 patients whose WT was diagnosed after their 10th birthday: SIR=23.6 [10.8,44.8], CR40=13.5% (Table 2). Five of the 9 accounted for all BC cases ascertained at age 39 and above, of which 4 received flank RT (Table 1). Eighteen of 974 patients treated with doxorubicin developed BC: SIR=19.7 [11.7,31.1], CR40=9.4%. Both subgroups contained disproportionately higher risk patients more likely also to receive RT. The SIR declined with increasing current age (Table 2), as might be expected due to the corresponding increase in background rates, but the test for trend was not statistically significant (p = 0.23).
Multiple regression analyses
Cox regression models, where tumors in the L and R breast were considered as separate events, were fit to compare ipsilateral vs. contralateral RT and to determine whether covariate effects remained statistically significant after adjustment for RT. For quantitative analysis we chose natural logarithms (started at 1) of the RT doses, rather than the doses themselves, for 2 reasons. First, the Cox model then implies that baseline rates are multiplied by a power of dose, which is a more plausible dose-response relationship than an exponential increase.18 Second, variation among coefficient estimates over the imputed datasets was much less with the log transform due to the reduced influence of “outliers”.
Preliminary analysis (results not shown), using a model that included all 4 quantitative RT variables, yielded HRs above 1 for ipsilateral and below 1 for contralateral RT whether to the chest or flank. Confidence intervals were wide, however, and the fit highly dependent on 2 cases. Model I (Table 3) was fit under the assumption that only ipsilateral RT mattered. This confirmed the importance of chest RT but suggested little or no effect for abdominal RT. Treatment with doxorubicin was estimated to double the BC risk after accounting for other variables, but was not statistically significant (p=0.12). By contrast, the HR for age at WT onset above 10 was 4.6 and highly statistically significant. There was some evidence for non-proportionality (p=0.02); the HR for chest RT decreased with increasing age.
Table 3.
Results of Cox multiple regression analyses with the individual breast (R or L) as the unit of analysis and current age as time
| Explanatory variable | Model I | Model II* | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| log(1+chest dose)-ipsilateral | 1.96 | 1.45–2.69 | <0.001 | |||
| log(1+flank dose)-ipsilateral | 1.09 | 0.88–1.35 | 0.438 | 1.51 | 1.13–2.03 | 0.005 |
| Doxorubicin | 2.24 | 0.80–6.22 | 0.123 | 1.49 | 0.36–6.19 | 0.581 |
| Age@WT≥10 years | 4.59 | 1.88–11.2 | 0.001 | 14.61 | 4.20–50.1 | <0.001 |
Subgroup of 2,123 participants who did NOT receive chest RT
When analysis was restricted to patients who had not received chest RT (Model II), an increase in risk with flank RT was apparent. The HR associated with older age at onset increased markedly. No individual observation had exceptional influence on the regression coefficients in Models I and II, and contributions to their variance from differences between imputed data sets were negligible.
Ductal carcinoma in situ
A sensitivity analysis included among the BC events the 6 DCIS cases, 5 of whom received chest and 1 abdominal RT only. This increased the estimated risks by age 40 to 20.4% for the chest and 4.0% for the abdominal RT groups. The overall SIR was virtually unchanged at 9.2=35/3.81 since DCIS cases from SEER were used in the comparison. The component SIRs were 29.6=22/0.74 for chest, 5.5=11/2.0 for abdominal and 1.9=2/1.1 for no RT.
Discussion
This study identified 29 cases of invasive BC in WT survivors, the largest number reported thus far in a single study. All but 4 occurred before age 40. Overall, WT survivors had 9.1 times the rate of BC of similarly aged women in the US population and a cumulative risk of 4.5% at 40 years of age. WT patients treated for pulmonary metastasis with chest RT had a 14.8% cumulative risk of BC at age 40 and rates 27.6 times background. These findings are consistent with ranges of SIRs (13.3–55.5) and 40–45 year cumulative incidence (13–20%) reported in a recent review of studies of BC in survivors of pediatric cancer who had chest RT as part of their primary treatment.14 They were not materially altered by inclusion of DCIS among the BC cases.
The protocol chest RT dose for pulmonary metastasis was 14 Gy in NWTS-1 but was reduced to 12 Gy in NWTS-2 following unacceptable acute pulmonary toxicity and remained at this level in NWTS-3,4. The vast majority of WT patients with chest metastases received the protocol dose. CCSS reported that each additional Gy of radiation increased the excess odds ratio of BC by 0.27.10 Approximating HR by odds ratio, this corresponds to a HR of 4.2 (95% CI [2.2, 9.0]) for 12 Gy chest RT, relative to none. From Table 3, we estimate a HR of 5.7 [2.6,12.7] for 12 Gy. These results are remarkably consistent, in spite of differences between studies. The CCSS case-control study used dosimetry to calculate radiation exposure to cancerous breast tissue in cases and analogous breast tissue in matched controls. The HR estimated for 12 Gy was based on linear extrapolation from results for substantially higher doses administered especially to HL patients, many of whom had RT only to involved fields.12 By contrast, the HR estimated here was based largely on results for treatment of the entire chest with 12 Gy. The clustering of doses around 12 Gy, however, limited our ability to detect a dose-response relationship.
Sixteen (89%) of 18 patients for whom receptor status was known were ER+/PR+. This percentage is higher than for SEER cases of comparable age, and more characteristic of post-menopausal cases. It is compatible with the notion that radiogenic cancers are typical of those that occur in the general population, but are induced to appear earlier.
WT survivors who did not receive chest RT also had an excess of breast cancer: SIR=12/2.59=4.6 [2.4,8.1], p<0.0001 (Table 2). While the excess could well be due to abdominal RT, this has not been proven. The SIR for those who received abdominal RT was even higher (6.0), but not significantly so in comparison with the 2.2 observed for those who received no RT. Some BC cases received flank RT affecting breast tissue at doses that could plausibly explain their occurrence and there was evidence of a correlation between the side of WT and side of BC. Our quantitative analyses, however, demonstrated an effect for flank RT only when restricted to patients who received no chest RT (Model II, Table 3). The uncertainty is due in part to the very small number (2) of BC cases who received no RT and to measurement error, i.e., the possibly weak correlation between the recorded ipsilateral flank dose and the actual breast dose. Also, some patients who received whole abdomen RT later developed ovarian failure, which could lessen their BC risk. Flank fields occupy a greater portion of the chest in younger whereas breasts are larger in older children, and there is a question as to which receives the higher dose. Radiation field parameters and techniques remained remarkably consistent throughout the NWTS. However, higher energy radiation from Cobalt-60 machines and linear accelerators in recent protocols would have resulted in a lower breast exposure compared to orthovoltage x-rays used previously. A larger case-control study that uses dosimetry to estimate the breast doses, especially from flank RT, is needed to resolve these issues.
Another explanation for an excess of BC in non-irradiated WT survivors is the possibility of a common origin. WT is associated with a large number of syndromes and constitutional chromosomal abnormalities, some of which could also predispose to BC.27 In particular, breast carcinoma, phylloides tumor and WT have all been observed with the Li-Fraumeni syndrome.28 The recent discovery of a WT survivor who had a familial BRCA1 mutation and developed DCIS at age 21 raises the possibility that both tumors were influenced by the mutation.29 Many WT occur in association with overexpression of the IGF2 gene, which might also be involved in the etiology of BC.30
Although the SIR for participants treated with doxorubicin was 19.7 (Table 2), 4 times higher than that for patients not so treated (p=0.0002), this comparison ignored the fact that doxorubicin is used for “high risk” patients who usually also receive RT. The adjusted HR was only 2.2 and failed to achieve statistical significance, though a HR of 6 could not be ruled out at 95% confidence (Table 3). The effects of doxorubicin and RT are often difficult to separate due to the high correlation between them. CCSS found an adjusted OR for the association of doxorubicin and BC of 1.9, but this too lacked statistical significance.10 Doxorubicin possibly increases BC risk, but this is by no means established.
Older age at WT diagnosis was associated, surprisingly, with a 4–5 fold higher rate of BC (Table 3). Because our Cox model used age as the time scale, this does not merely reflect increasing BC rates with age.19 Nor does it reflect an increased sensitivity to chest RT among pubescent girls. Eight of 9 BC cases with WT diagnosed after age 10, including the 5 ascertained at the oldest ages (39–48), received no chest RT (Table 1) so that the HR in this subgroup was even higher (Model II, Table 3). While this may well be a chance finding, or attributable to unmeasured confounders, it is also possible that there are genetic or host factors associated with older age at WT diagnosis that lead to increased risk of later BC.
The major limitations of our study were the still small number of BC cases and the lack of dosimetry, which affected particularly our assessment of abdominal RT. Screening for BC was possibly more intense, or reporting of BC cases more timely, among patients receiving chest irradiation. Nonetheless, evidence was reasonably solid that irradiation of the entire chest with 12 Gy RT carried a significant hazard of early BC. WT patients treated for pulmonary metastases with 12 Gy of whole chest RT are strong candidates for BC surveillance prior to age 40, after which routine mammographic surveillance for women in the general population is recommended.31 Current guidelines,13,14 which suggest yearly mammography and breast MRI starting at age 25 or 8 years after treatment culmination only for those who received ≥20 Gy chest or mantle radiation, would need to be revised to facilitate the early diagnosis and prompt treatment of BC among WT survivors.
Figure 2.
Cumulative risk of breast cancer by radiation category. Numbers above abscissa are of participants still under observation at ages 20, 30, 40 in each category.
Acknowledgments
Support for research: NIH grant 2 R01 CA054498
Footnotes
Financial disclosures: None
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA-Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Breslow NE, Lange JM, Friedman DL, et al. Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer. 2010;127:657–66. doi: 10.1002/ijc.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breslow NE, Churchill G, Nesmith B, et al. Clinicopathological features and prognosis for Wilms-tumor patients with metastases at diagnosis. Cancer. 1986;58:2501–11. doi: 10.1002/1097-0142(19861201)58:11<2501::aid-cncr2820581125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Green DM, Breslow NE, Li YI, et al. The role of surgical excision in the management of relapsed Wilms-tumor patients with pulmonary metastases - a report from the National Wilms Tumor Study. J Pediatr Surg. 1991;26:728–33. doi: 10.1016/0022-3468(91)90021-k. [DOI] [PubMed] [Google Scholar]
- 5.D’Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms tumor - results of the 3rd National Wilms Tumor Study. Cancer. 1989;64:349–60. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.D’Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms tumor - results of the 2nd National Wilms Tumor Study. Cancer. 1981;47:2302–11. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.D’Angio GJ, Evans AE, Breslow N, et al. Treatment of Wilms tumor - results of the National Wilms Tumor Study. Cancer. 1976;38:633–46. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–7. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97:1428–37. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 10.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:3901–7. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee L, Pintilie M, Hodgson DC, Goss PE, Crump M. Screening mammography for young women treated with supradiaphragmatic radiation for Hodgkin’s lymphoma. Ann Oncol. 2008;19:62–7. doi: 10.1093/annonc/mdm440. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematol. 2011:323–9. doi: 10.1182/asheducation-2011.1.323. [DOI] [PubMed] [Google Scholar]
- 13.Children’s Oncology Group. Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers. Arcadia, CA: Children’s Oncology Group; 2008. [accessed Dec 28, 2013]. Available from URL: http://www.survivorshipguidelines.org/pdf/LTFUGuidelines.pdf. [Google Scholar]
- 14.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152:444–W154. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reulen RC, Taylor AJ, Winter DL, et al. Long-term population-based risks of breast cancer after childhood cancer. Int J Cancer. 2008;123:2156–63. doi: 10.1002/ijc.23743. [DOI] [PubMed] [Google Scholar]
- 16.Breslow NE, Takashima JR, Whitton JA, Moksness J, Dangio GJ, Green DM. 2nd malignant neoplasms following treatment for Wilms-tumor - a report from the National Wilms Tumor Study Group. J Clin Oncol. 1995;13:1851–9. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 17.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms’ tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1998;16:237–45. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 18.Breslow NE, Day NE. Statistical Methods in Cancer Research II: The Design and Analysis of Cohort Studies. Lyon, France: IARC; 1987. [PubMed] [Google Scholar]
- 19.Yasui Y. A Methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–13. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 20.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statist Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Surveillance Epdemiology and End Results (SEER) Program. [Accessed Oct 26, 2013];Research Data. 1973–2010 Available from URL: http://www.seer.cancer.gov.
- 22.Therneau TMG, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 23.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; [accessed Nov 20, 2011]. Available from URL: http://www.R-project.org. [Google Scholar]
- 24.Green DM, Lange JM, Qu AN, et al. Pulmonary disease after treatment for Wilms tumor: A report from the National Wilms Tumor Long-term Follow-up Study. Pediatr Blood Cancer. 2013;60:1721–6. doi: 10.1002/pbc.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin DB. The Bayesian bootstrap. Ann Statist. 1981;9:130–4. [Google Scholar]
- 26.Little RJA. Missing-data adjustments in large surveys. J Bus Econ Statist. 1988;6:287–96. [Google Scholar]
- 27.Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43:705–15. doi: 10.1136/jmg.2006.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birch JM, Alston RD, McNally RJQ, et al. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20:4621–8. doi: 10.1038/sj.onc.1204621. [DOI] [PubMed] [Google Scholar]
- 29.Dulude AM, D’Souza J, Harrison N, Ramanathan RK. Development of breast cancer in a 21-year-old childhood Wilms’ tumor survivor with a BRCA1 2634delC mutation. Clin Breast Cancer. 2011;11:268–9. doi: 10.1016/j.clbc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Bergman D, Halje M, Nordin M, Engstrom W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontol. 2013;59:240–9. doi: 10.1159/000343995. [DOI] [PubMed] [Google Scholar]
- 31.Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013 A review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA-Cancer J Clin. 2013;63:88–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]