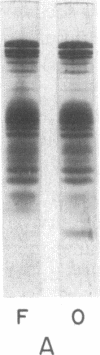
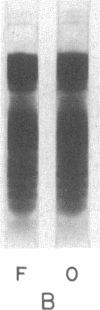
Abstract
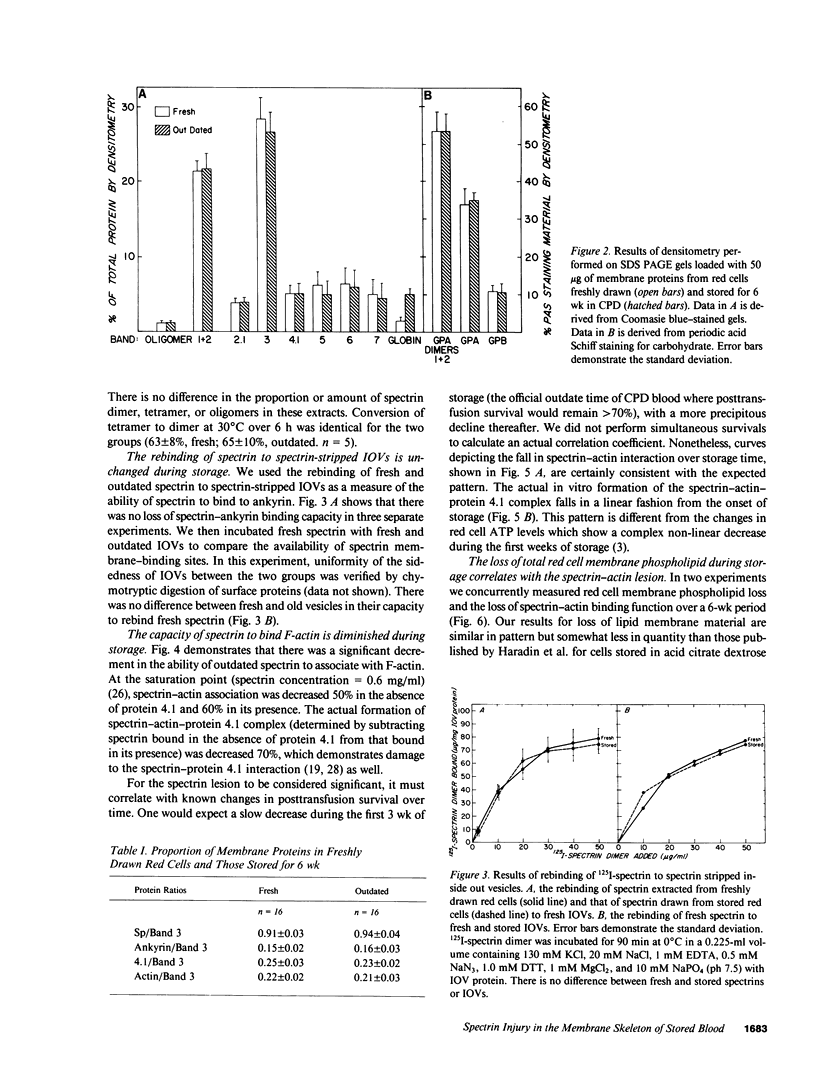
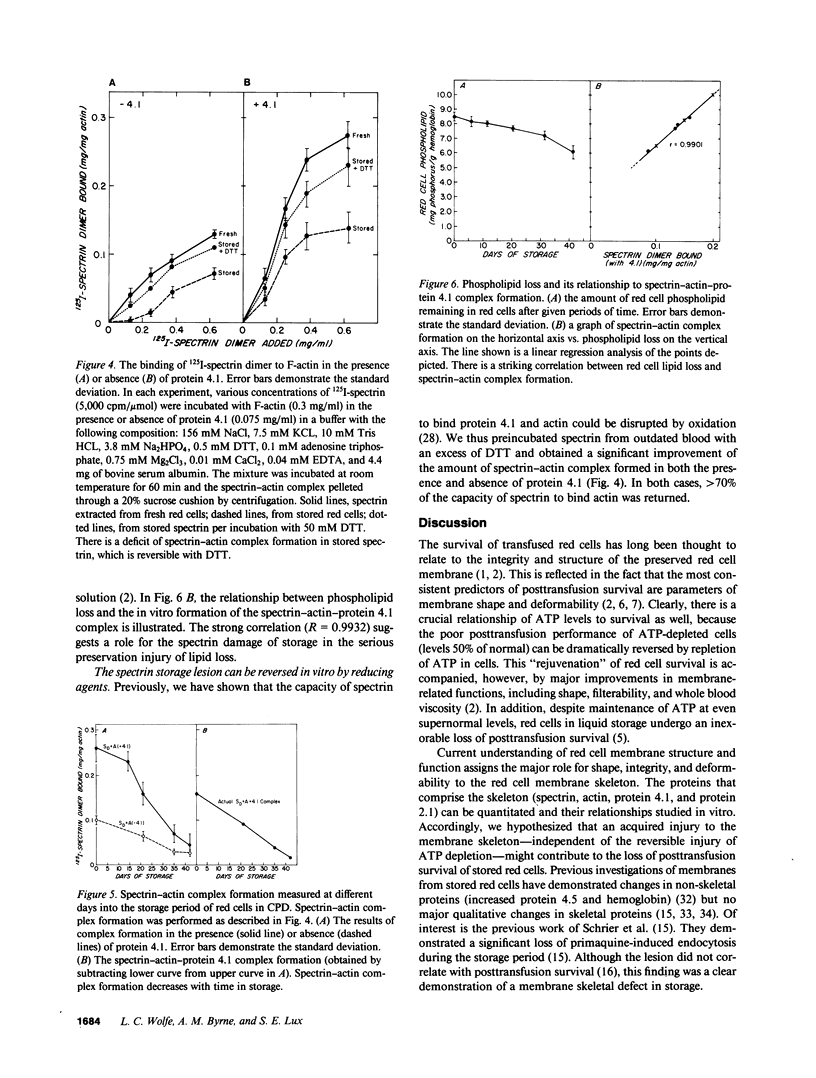
During liquid preservation under blood bank conditions, red cell membranes inexorably undergo damage that decreases erythrocyte survival after transfusion. Accordingly, we have surveyed membrane skeletal protein interactions during storage. We uncovered a decrease in the in vitro formation of spectrin-actin complex in the absence (50%) or presence (60%) of protein 4.1. Actual formation of the spectrin-actin-protein 4.1 complex fell in a linear fashion during the storage period. This fall in spectrin-actin interaction tightly correlated with the decline in total red cell phospholipid (R = 0.9932) measured simultaneously. This decrement of spectrin-actin association could be restored to greater than 70% of normal values by preincubation of stored spectrin with 50 mM dithiothreitol. This storage injury to spectrin-actin interaction might weaken the membrane skeleton and lead to decreased red cell survival. In vitro reversibility of the damage by reducing agents suggests a possible new direction for prolonging the shelf life of stored blood.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Orringer E. P., Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982 May 13;306(19):1155–1161. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- Allen D. W., Cadman S., McCann S. R., Finkel B. Increased membrane binding of erythrocyte catalase in hereditary spherocytosis and in metabolically stressed normal cells. Blood. 1977 Jan;49(1):113–123. [PubMed] [Google Scholar]
- Becker P. S., Lux S. E. Hereditary spherocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):15–43. [PubMed] [Google Scholar]
- Becker P. S., Spiegel J. E., Wolfe L. C., Lux S. E. High yield purification of protein 4.1 from human erythrocyte membranes. Anal Biochem. 1983 Jul 1;132(1):195–201. doi: 10.1016/0003-2697(83)90447-5. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Beutler E., Villacorte D. Spectrin extractability in blood storage. Transfusion. 1981 Jan-Feb;21(1):96–99. doi: 10.1046/j.1537-2995.1981.21181127494.x. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F. Biochemical characterization of complex formation by human erythrocyte spectrin, protein 4.1, and actin. Biochemistry. 1984 Dec 4;23(25):6091–6098. doi: 10.1021/bi00320a029. [DOI] [PubMed] [Google Scholar]
- Dern R. J., Brewer G. J., Wiorkowski J. J. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J Lab Clin Med. 1967 Jun;69(6):968–978. [PubMed] [Google Scholar]
- Feuerstein H., Stibenz D. Banking-related decline of erythrocyte N-acetyl-neuraminic acid and phospholipids. Haematologia (Budap) 1982;15(2):205–209. [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. A., Casoria L. A., Eyster M. E. Identification of the molecular defect in the erythrocyte membrane skeleton of some kindreds with hereditary spherocytosis. Blood. 1982 Sep;60(3):772–784. [PubMed] [Google Scholar]
- Goodman S. R., Weidner S. A. Binding of spectrin alpha 2-beta 2 tetramers to human erythrocyte membranes. J Biol Chem. 1980 Sep 10;255(17):8082–8086. [PubMed] [Google Scholar]
- Haradin A. R., Weed R. I., Reed C. F. Changes in physical properties of stored erythrocytes relationship to survival in vivo. Transfusion. 1969 Sep-Oct;9(5):229–237. doi: 10.1111/j.1537-2995.1969.tb04929.x. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P. Auto-oxidation and a membrane-associated 'Fenton reagent': a possible explanation for development of membrane lesions in sickle erythrocytes. Clin Haematol. 1985 Feb;14(1):129–140. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H. U. Vesicles isolated from ATP-depleted erythrocytes and out of thrombocyte-rich plasma. J Supramol Struct. 1978;8(3):375–389. doi: 10.1002/jss.400080314. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Ukena T. E. Diminished spectrin extraction from ATP-depleted human erythrocytes. Evidence relating spectrin to changes in erythrocyte shape and deformability. J Clin Invest. 1978 Mar;61(3):815–827. doi: 10.1172/JCI108996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian V., Wolfe L. C., John K. M., Pinder J. C., Lux S. E., Gratzer W. B. Analysis of the ternary interaction of the red cell membrane skeletal proteins spectrin, actin, and 4.1. Biochemistry. 1984 Sep 11;23(19):4416–4420. doi: 10.1021/bi00314a027. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):45–87. [PubMed] [Google Scholar]
- Palek J., Liu S. C. Dependence of spectrin organization in red blood cell membranes on cell metabolism: implications for control of red cell shape, deformability, and surface area. Semin Hematol. 1979 Jan;16(1):75–93. [PubMed] [Google Scholar]
- Platt O. S., Falcone J. F., Lux S. E. Molecular defect in the sickle erythrocyte skeleton. Abnormal spectrin binding to sickle inside-our vesicles. J Clin Invest. 1985 Jan;75(1):266–271. doi: 10.1172/JCI111684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Schrier S. L., Hardy B., Bensch K., Junga I., Krueger J. Red blood cell membrane storage lesion. Transfusion. 1979 Mar-Apr;19(2):158–165. doi: 10.1046/j.1537-2995.1979.19279160285.x. [DOI] [PubMed] [Google Scholar]
- Schrier S. L., Sohmer P. R., Moore G. L., Ma L., Junga I. Red blood cell membrane abnormalities during storage: correlation with in vivo survival. Transfusion. 1982 Jul-Aug;22(4):261–265. doi: 10.1046/j.1537-2995.1982.22482251202.x. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C. The membrane and the lesions of storage in preserved red cells. Transfusion. 1985 May-Jun;25(3):185–203. doi: 10.1046/j.1537-2995.1985.25385219897.x. [DOI] [PubMed] [Google Scholar]
- Wood L., Beutler E. The viability of human blood stored in phosphate adenine media. Transfusion. 1967 Nov-Dec;7(6):401–408. doi: 10.1111/j.1537-2995.1967.tb04875.x. [DOI] [PubMed] [Google Scholar]