Abstract
Sex differences in vocal communication are prevalent in both the animals and humans. The mechanism(s) mediating gender differences in human language are unknown, although, sex hormones, principally androgens, play a central role in the development of vocalizations in a wide variety of animal species. The discovery of FOXP2 has added an additional avenue for exploring the origins of language and animal communication. The FOXP2 gene is a member of the forkhead box P (FOXP) family of transcription factors. Prior to the prenatal androgen surge in male fetuses, we observed no sex difference for Foxp2 protein levels in cultured cells. In contrast, 24 hours after the onset of the androgen surge, we found a sex difference for Foxp2 protein levels in cultured cortical cells with males having higher levels than females. Furthermore, we observed the potent nonaromatizable androgen dihydrotestosterone altered not only Foxp2 mRNA and protein levels but also Foxp1. Androgen effects on both Foxp2 and Foxp1 were found to occur in the striatum, cerebellar vermis, and cortex. Immunofluorescence microscopy and coimmunoprecipitation demonstrate Foxp2 and the androgen receptor protein interact. Databases for transcription factor binding sites predict a consensus binding motif for androgen receptor on the Foxp2 promoter regions. We also observed a sex difference in rat pup vocalization with males vocalizing more than females and treatment of females with dihydrotestosterone eliminated the sex difference. We propose that androgens might be an upstream regulator of both Foxp2 and Foxp1 expression and signaling. This has important implications for language and communication as well as neuropsychiatric developmental disorders involving impairments in communication.
Language in humans and vocal communication in animals is a phenomenon that has intrigued scientists for more than a century (1). The discovery of the FOXP2 gene has added an additional avenue for exploring the origins of language and animal communication. The FOXP2 gene is a member of the forkhead box P (FOXP) family of transcription factors, which also includes FOXP1, FOXP3, and FOXP4 (for review see reference 2). Of these four FOXP genes, most is known about FOXP2 due to the identification of mutations more than a decade ago in the large intergenerational KE family (3). The affected members have a mutation in the DNA-binding domain of FOXP2 and exhibit profound deficits in cognition and language (4, 5). FOXP2 is expressed in several areas of the brain involved in motor control, sensory integration, and vocal communication (6–8). Furthermore, FOXP2 transcriptionally regulates genes involved in neuronal development, neurite outgrowth, dendritic branching, and axonal morphology (9, 10). A note about nomenclature is that nucleotide sequences are italicized, proteins are not, human forms are in uppercase letters (eg, FOXP2), murine forms are in lowercase letters (Foxp2) and those of other species, such as birds, contain both uppercase and lowercase letters (eg, FoxP2) (11).
Rodents communicate through the use of ultrasonic vocalizations (12, 13). Ultrasonic vocalizations (USVs) are produced during social encounters and function as a short-distance communication method (14, 15). It is hypothesized that USVs became the preferred mode of communication for rodents because many predators have a limited hearing capacity for ultrasonic tones. Thus, providing rodents an ideal method for communicating with intended receivers while minimizing the threat of predation (12, 15). USVs emerge early in infancy in many rodent species, (ie, within the first few days of life for mice and rats) (13, 16) and are triggered by aversive events such as maternal separation and hypothermia (17, 18). This specific type of USV (ie, maternal separation calls or pup isolation calls) is the most widely used method for eliciting and analyzing vocalizations in young rodents (19–21).
A number of Foxp2 mutant mice have been developed to investigate how this gene influences brain development and animal vocalizations (for a complete review, see references 6 and 22). Foxp2 mutant mice show a range of deficient neuronal and behavioral developments ranging from reductions in neurite outgrowth (23), decreased or atypical vocalizations (24, 25), and impairments in learning (26). FoxP2 has also been studied in the highly vocal bird with FoxP2 expressed in brain regions comparable with the mammalian expression pattern (27). FoxP2 levels change during song acquisition (28), further highlighting the important role for FoxP2 in learned vocal behavior. Together the research from both rodent and songbird emphasizes the vital role this transcription factor has on the development of neural circuitry underlying vocalization and behavior.
Sex differences in vocal communication are prevalent in both the animals (29) and human language (30–32). Several studies have found language processing in women involves more bilateral hemispheric activation, whereas men are more strongly lateralized to the left hemisphere in their language processing (33–36). There is also a consistent trend for girls to acquire language earlier than boys (31, 37), and girls begin to use gestural communication earlier than boys (38) as well as produce their first words (39) and first sentences (40) at a younger age than boys. Nonetheless, the existence of gender differences in human speech and language processing are highly controversial (for review see reference 41).
Potential mechanism(s) mediating gender differences in human language have not been identified, although sex hormones, principally androgens, play a central role in the development of vocalizations in a wide variety of animal species including primates (42–46). Interestingly, men with X chromosome polysomy have reduced androgen levels and often suffer speech impairments, which have recently been found to be improved by androgen treatment (47). Therefore, we tested the hypothesis that androgens would induce brain region specific changes in Foxp2 mRNA and protein levels as well as alter the USVs of rat pups. We found exogenous administration of the nonaromatizable androgen dihydrotestosterone (DHT) decreased the mRNA and protein levels for Foxp2 in the cortex and cerebellum but increased both mRNA and protein levels in the striatum. Moreover, DHT administration decreased the mRNA and protein levels for Foxp1 in the cerebellum, whereas only the mRNA levels in the cortex were decreased. Analogous to Foxp2, DHT administration increased Foxp1 protein and mRNA levels in the striatum. We also confirmed a previously observed sex difference in rat pup vocalization [Bowers et al (7)] and treatment of females with DHT eliminated that sex difference. Based on the data, we propose androgens might be an upstream regulator of both Foxp2 and Foxp1 expression. This has important implications for language and communication as well as neuropsychiatric developmental disorders involving impairments in communication.
Materials and Methods
Experimental design
There are three principal experiments performed in the paper. First, we assessed Foxp2 protein levels in cultured cells before and after the prenatal androgen surge in male fetuses. The goal of this experiment was to determine whether endogenous androgens influence Foxp2 levels in cultured cortical cells. Second, we quantified the relative protein and mRNA for Foxp2 and Foxp1. The goal of this experiment was to determine whether exogenous administration of androgens influence Foxp2 and Foxp1 levels. Lastly, we investigated the USV of male and female rat pups after treatment with androgens to determine whether there are identifiable sex differences in rat pup ultrasonic distress calls and whether these vocalizations are influenced by androgen treatment.
Animals
Pregnant Sprague Dawley rats raised in our breeding colony were allowed to deliver normally under standard laboratory conditions. The morning that pups were found in the nest was designated as the day of birth [postnatal day (PN) 0]. Male and female rat pups were then identified on PN0 and given an injection of India Ink into the footpad using a 30-gauge needle to determine the treatment condition. All animals were housed in polycarbonate cages (20 × 40 × 20 cm) with corncob bedding under a reverse 12-hour light, 12-hour dark cycle. Food and water were ad libitum. The University of Maryland, Baltimore, Institutional Animal Care and Use Committee approved all animal procedures.
Hormone treatment
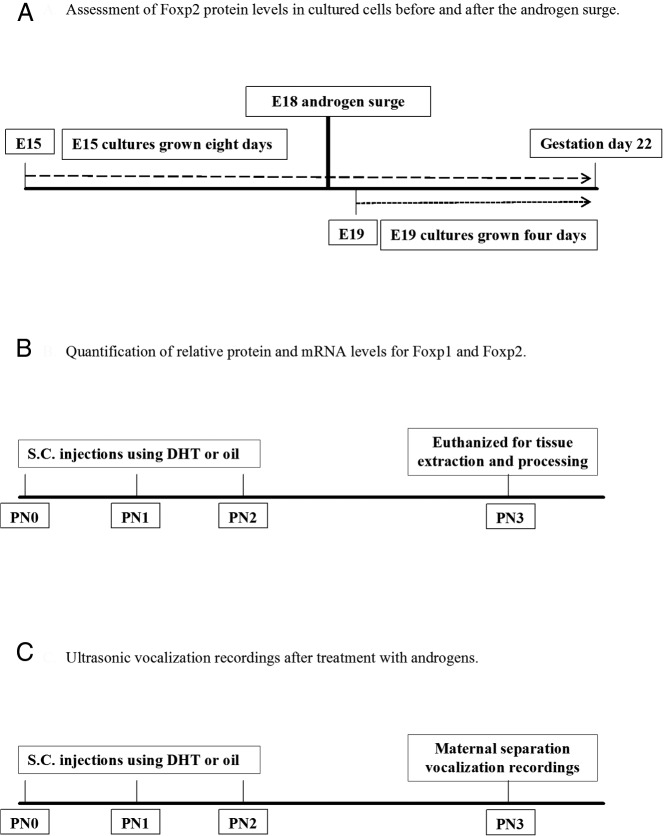
Starting at PN0, male and female rat pups were randomly distributed into different experimental groups and injected for 3 consecutive days with either DHT benzoate, the nonaromatizable form of androgen (Sigma; 100 μg per 0.1 mL in sesame oil, sc), or vehicle (sesame oil; see Figure 1 for the experimental time line). The benzoate moiety does not alter the DHT's biological activity but prolongs its bioavailability.
Figure 1.
Time line for experiments. A, Foxp2 protein levels were measured from male and female embryos on E15, prior to the androgen surge, with a separate group of male and female embryos measured on E19, after the androgen surge. B, Male and female pups were treated from birth to PN2 with androgens or oil vehicle and euthanized on PN3, with relative protein and mRNA levels for Foxp1, Foxp2, and AR measured. C, Vocal calls were recorded at PN3 from male and female pups treated from birth to PN2 with androgens or oil vehicle.
Western blot analysis
PN3 male and female rat pups were euthanized by rapid decapitation, and brain tissue was dissected and processed for Western blot analysis as previously reported (7). All membranes were blocked in 10% nonfat milk protein (Bio-Rad Laboratories) for 1.5 hours and then incubated in the primary antibody, rabbit anti-Foxp2 (1:2500; Abcam), rabbit anti-Foxp1 (1:3000; Abcam), or rabbit antiandrogen receptor (1:1000; Santa Cruz Biotechnology) for 24 hours at 4°C. After a 1-hour incubation in antirabbit horseradish peroxidase-linked secondary antibody (1:3000; Cell Signaling Technologies), the immunoreactive bands were detected using chemiluminesence (Phototope horseradish peroxidase Western blot detection system, LumiGLO Reagent and peroxide; Cell Signaling). The membranes were then incubated in a Ponceau S stain (Sigma), and the density of the largest band on the membrane, which includes more than 200 proteins, was used as a loading control for normalization The protein of interest was detected as a band with a relative molecular mass of approximately 80 KDa (Foxp2), approximately 75 KDa (Foxp1), and approximately 110 kDa (androgen receptor). Twenty micrograms of protein were used for the protein loading. The integrative grayscale pixel area density was captured using a CCD72 camera (MTI) and analyzed with National Institutes of Health image software. For relative quantification purposes, the protein of interest was divided by the Ponceau band for quantification. The choice of Foxp2 and Foxp1 antibodies was based on our previous publication [Bowers et al (7)] as well as other publications that have used these antibodies (23, 25, 48, 49, 50, 51). Likewise, the specificity of the androgen receptor antibody was also based on previous publications (50, 51).
Coimmunoprecipitation (Co-IP)
The striatum was collected from a PN0 pup and processed according to manufacturer protocol for co-IP using the Active Motif Universal Magnetic Co-IP kit. Tissue extracts (300 μg) were incubated for 4 hours using 2 μg of antirabbit androgen receptor (Abcam). After the incubations, 50 μL of protein A/G magnetic beads was added to the samples and then incubated for 24 hours. Beads were then washed with a co-IP buffers provided in the kit and then processed for Western blotting analysis (as stated above) using antirabbit Foxp2 (Abcam: 1:2500). Control input consisted of 10 μg of striatum tissue from a PN3 rat pup without the addition of androgen receptor antibody and a negative control consisted of rabbit IgG serum incubated with androgen receptor antibody.
Pup USV recordings
For the USV recordings, pups from three litters were used for analysis. To reduce the variability that might be caused from a disparity between sex ratios, we pooled and redistributed the male and female pups with one litter having 12 pups and the other two litters each having eight pups. The sample size for each group was seven pups. Vocal emissions were recorded for 3 minutes using PN3 pups that were isolated from the dam and immediately returned to the home cage after recording. PN3 pups were used for USV recording because this is the same age we were testing the effects of androgen treatment and is the earliest time frame for reliable elicitation of USV in rat pups (18). Ambient temperature was maintained at 22° ± 1°C. On the day of testing, all pups were placed in a handmade sound attenuating, Plexiglas container (width 15 cm; height 15 cm) and recorded using an UltraSoundGate condenser microphone (CM16; Avisoft Bioacoustics) placed at a fixed height of 20 cm above the pups. The microphone was connected via an Avisoft Ultra-SoundGate 416 USB audio device (Avisoft Bioacoustics) to a personal computer. Acoustic data were recorded with a sampling rate of 300 000 Hz in 16 bit format. For acoustical analysis, recordings were transferred to SASLab Pro (version 5.10; Avisoft Bioacoustics), and a fast Fourier transform (FFT) was conducted (512 FFT length, 100% frame, Hamming window, and 75% time window overlap). An automated threshold-based algorithm (threshold −50 dB) and a hold time mechanism (5 msec) were used to detect USVs. A high pass finite impulse response filter was used to eliminate background noise below 20 kHz. Calls were also inspected manually to ensure that all USVs detected using the software's automatic processing algorithms were legitimate calls. False signals were removed before statistical analysis was performed. Various call parameters were quantified, including fundamental mean frequency, fundamental mean amplitude, frequency bandwidth (maximum-minimum frequency), duration, and the number of calls emitted. The investigators conducting USV analyses were blind to the experimental condition and sex of the pups.
Quantitative RT-PCR (qRT-PCR)
RNA was prepared from frozen rat brain tissue samples using Qiazol and purified on RNAeasy columns following the manufacturer's protocols (QIAGEN). Using 0.1g tissue, RNA was transcribed to cDNA using the Transcriptor first-strand cDNA synthesis kit (Roche Applied Science) using both oligodeoxythymidine and random primers. A quantity of 1.2–5.0 mg was used in a reaction to synthesize cDNA with oligodeoxythymidine primers. Samples were analyzed by the UMB genomics core facility using a Bioanalyzer 2100. The RNA integrity number values were always greater than 9.0, generally ranging from 9.7 to 10.0. Quantitative real-time PCR was performed on an ABI ViiA 7 (Applied Biosystems) with ViiA 7 1.2 software. qRT-PCR was performed with an annealing temperature of 60°C, using Power SYBR Green master mix (Applied Biosystems). Primers specific for candidate genes (Foxp2 and Foxp1) and the control housekeeping gene (Rpl13a) were designed using PrimerBank 0.34. Primers specific for (SRY) were obtained from a previously published manuscript (52). Rat Foxp2 primers used were as follows: forward, 5′-TATGGAGCAGCCCTTAATGC-3′ and reverse, 5′-GGTTACTTAGCAAAGGCAAACTG-3′. Foxp1 primers used were as follows: forward, 5′-CGGCCTTTCTAAAACATCTCAAC-3′ and reverse, 5′-TGTACTCTACATTGAGCTGTGCTTCTATC-3′. Primers for the control gene used (Rpl13a) were as follows: forward, 5′-AGGCAAAGATCCATTACCG-3′ and reverse, 5′-GGCACAAACAGTCTTTA-3′. For sexing of cell cultures, we used primers targeting the Sry gene including the following: forward, 5′-GCGCCCCATGAATGCAT-3′ and reverse, 5′-TGGGATTCTGTTGAGCCAACT-3′.
Primary cell culture
On embryonic day (E) 15 or E19, sex-specific cell cultures were performed. Embryos from two dams were used for the E15 as well as two dams for the E19 cultures. The sex of the embryos was determined via PCR for Sry. We microdissected and placed cortical tissue into 2 mL of Hanks' balanced salt solution (Sigma) and digested with 500 μL of 0.25% Trypsin (Invitrogen) and 250 μL of 1% deoxyribonuclease for 15 minutes at 37°C. The supernatant was removed and cells washed twice with Hanks' balanced salt solution and then triturated in Neurobasal-A (Invitrogen), 5% fetal bovine serum (Invitrogen), and 1% antibiotic/antimycotic (Invitrogen) until dissociated. Cell density and viability were determined on a hemacytometer using Trypan blue and cells plated at 250 000 cell/slip on 2.5-cm round polylysine-treated glass coverslips in 3.5-cm petri dishes. After allowing cells to seed for 2 hours, cultures were fed with 2 mL of cell culture medium, which contained the following: Neurobasal-A, 0.1% L-glutamine (Sigma), 1% B-27 (Sigma), and 1% antibiotic/antimycotic. The E15 cultures were harvested for Western blot analysis on day in vitro 8. The E19 cells were harvested on day in vitro 4.
Fluorescent Immunohistochemistry
Free-floating tissue sections generated from PN3 pup brains, cryosectioned at 45 μm, were rinsed with 0.1 M PBS, incubated with 3% hydrogen peroxide in PBS for 30 minutes, and then rinsed. After the rinse, sections were incubated with 0.3 M glycine in 0.4% Triton X-100 (PBS-T) for 60 minutes and rinsed followed by coincubated with 10% BSA in 0.4% PBS-T for 60 minutes at room temperature and then for 24 hours at 4°C using a polyclonal antigoat antibody against Foxp2 in PBS-T (1:200; Abcam), antimouse Foxp1 (1: 500; Santa Cruz Biotechnology), and antirabbit androgen receptor (1: 50; Santa Cruz Biotechnology). Sections were then rinsed in PBS and coincubated with the fluorescent secondary: Alexa 488 antimouse (1:500; Invitrogen), Alexa 596 antirabbit (1:500, Invitrogen), and Alexa 633 antigoat (1:200; Invitrogen) in PBS-T for 60 minutes at room temperature. Sections were rinsed in PBS and then coverslipped using Vecto Vectashield (Vector Laboratories) with 4′,6′-diamino-2-phenylindole. The validation for the Foxp2 and Foxp1 antibodies used for the fluorescent staining was based on a comparison of their performance with the known antibodies we used for the Western blots as well as previous publications using them (53)
Statistical analyses
All data are expressed as means and SEMs. Data sets from the embryonic cultures were analyzed using an independent t test. Data sets from the Western blot, qRT-PCR, and the ultrasonic vocalizations were analyzed using a two-factor ANOVA with sex and treatment as fixed factors followed by post hoc pairwise comparisons. We also report squared-effect size calculations. All post hoc comparisons were performed using a Holm's sequential Bonferroni correction to control for family-wise error.
Results
A sex difference in Foxp2 levels appears after the prenatal androgen surge
To test the hypothesis that endogenous androgens influence Foxp2 levels, we generated embryonic sex-specific cultures before (E15) and after the prenatal androgen surge in males at E19. Cultured cortical cells were grown until they would have reached 22 days of gestation in situ. In male rats, T markedly surges during days 18–19 of gestation (54, 55) and again during the first few hours after parturition (56, 57). There was no statistical difference in Foxp2 levels in cortical cultures from males and females at E15 [t (22) = 0.976, P = .34, d = 0.41, Figure 2A], but males had significantly higher Foxp2 levels in cultures generated from E19 embryos than females [t (20) = 4.42, P = .007, d = 1.97 Figure 2B].
Figure 2.
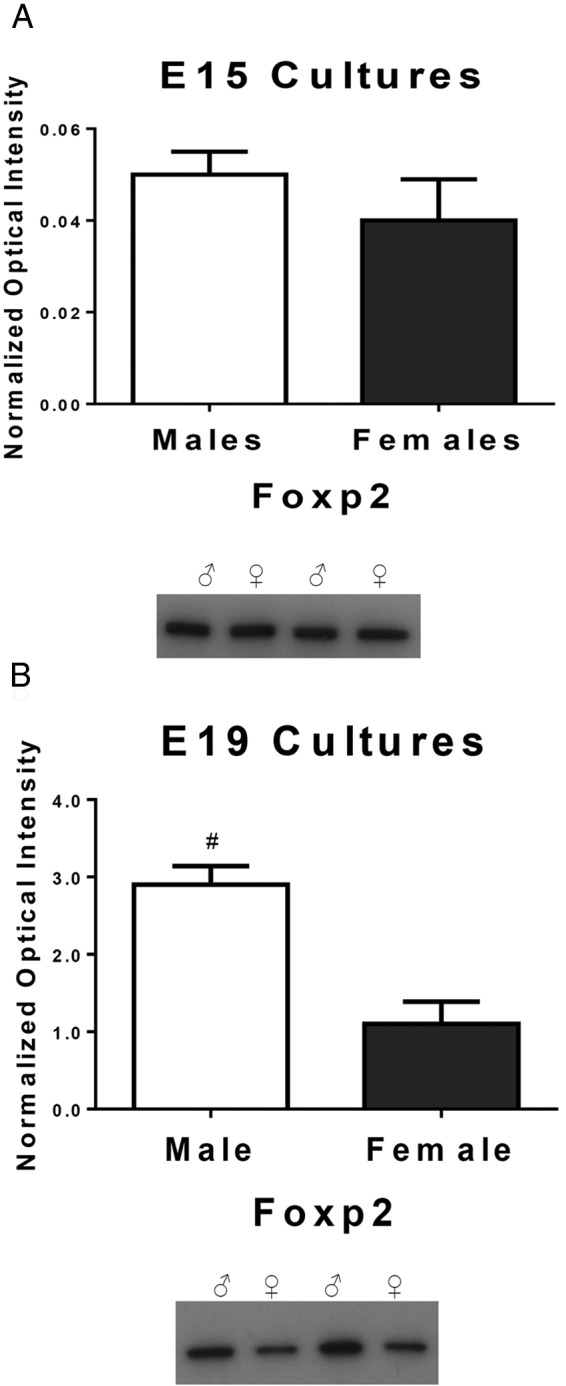
FOXP2 is differentially expressed in males vs females after the androgen surge. The relative amount of Foxp2 protein was quantified by Western blot. A, In cultured cortical cells prior to the androgen surge in males at E15, there is no sex difference in the Foxp2 level (t test; P > .05, n = 12 males and 12 females/group). B, After the onset of the male androgen surge at E18, cultured cortical cells from males at E19 had significantly higher levels of Foxp2 compared with the females. Data are expressed as mean (±SEM; n = 10 males and 12 females/group). *, A significant sex difference (t test; P < .05).
Exogenous administration of androgens modulates Foxp2 and Foxp1 protein levels
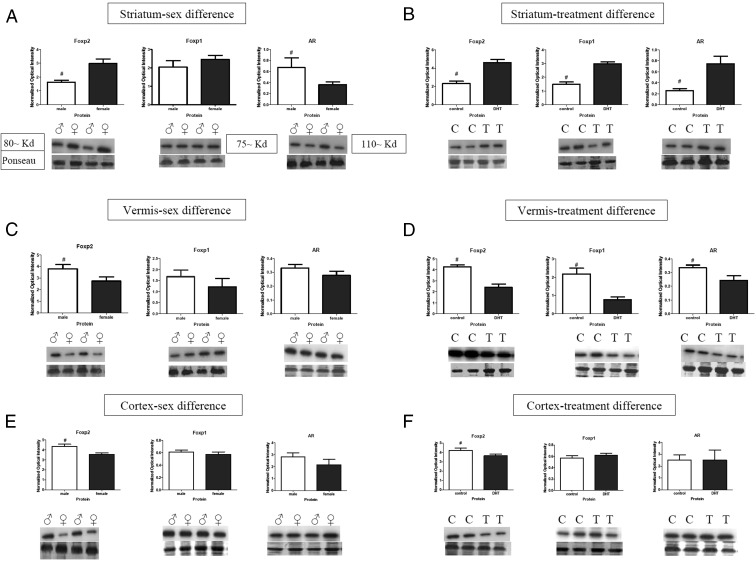
At PN3 there was a significant main effect for sex observed in the striatum. We found females to have higher protein levels for Foxp2 [F (1, 24) = 5.50, P = .03, η2 = 0.27] but no sex difference for Foxp1 protein levels [F's (1, 24) = 2.67, P = .12, η2 = 0.13]. Males had higher androgen receptor (AR) protein levels than females [F (1, 24) = 22.59, P < .001, η2 = 0.57, Figure 3A]. A significant main effect for DHT treatment was found with increased Foxp2 [F (1, 24) = 35.04, P < .001, η2 = 0.69], Foxp1 [F (1, 24) = 45.66, P < .001, η2 = 0.73], and AR protein levels [F (1, 24) = 66.05, P < .001, η2 = 0.80, Figure 3B].
Figure 3.
Androgens influence Foxp2, Foxp1, and AR protein levels differentially based on brain region. The relative amount of Foxp2 and Foxp1 protein was quantified in tissue collected from the striatum, vermis, and cortex at 3 days of age after treatment with the nonaromatizable androgen DHT on PN0 and PN1. A, Females had significantly more Foxp2 than males in the striatum, whereas males had higher levels of AR. B, DHT treatment significantly increased Foxp2, Foxp1, and AR levels in the striatum as compared with controls. C, In the vermis, males were found to have higher Foxp2 levels, in which no sex difference was found for Foxp1 or AR. D, DHT treatment decreased all three proteins: Foxp2, Foxp1, and AR levels in the vermis. E, In the cortex, males had significantly more Foxp2 than females, and no sex difference was found for Foxp1 or AR. F, DHT treatment decreased Foxp2 levels but had no significant effect on Foxp1 or AR protein levels. Data are expressed as mean (±SEM; n = 7/group). *, A significant sex difference (F-test; P < .05). Symbols on the graphs represent the following: C, control, ♀, female, ♂, male, T, DHT treatment.
The vermis and the hemispheres of the cerebellum were analyzed separately. In contrast to the striatum, there was a significant sex difference in Foxp2 protein levels for the vermis, with males having higher levels than females [F (1, 24) = 7.60, P = .01, η2 = 0.32] but no sex difference for Foxp1 or AR [F's < 1.0, P > .05, η2 < 0.09, Figure 3C]. In contrast to the pattern observed in the striatum, DHT treatment decreased Foxp2 [F (1, 24) = 31.37, P < .001, η2 = 0.66], Foxp1 [F (1, 24) = 15.06, P = .013, η2 = 0.49], and AR protein levels in the vermis [F (1, 24) = 5.34, P = .03, η2 = 0.25, Figure 3D]. There was no statistical significance found for the hemispheres (data not shown).
In the cortex, we found the main effect for sex, with Foxp2 protein levels being higher in males than in females [F (1, 24) = 8.78, P = .009, η2 = 0.35, Figure 3E]. However, our analyses did not show a significant difference for either Foxp1 or AR protein levels [F's < 1.0, P > .05, η2 < 0.09]. Similar to the pattern observed in the vermis, DHT treatment decreased Foxp2 [F (1, 24) = 4.29, P = .05, η2 = 0.21] but had no effect on the Foxp1 or AR protein levels [F's < 1.0, P > .05, η2 < 0.05, Figure 3F].
Exogenous administration of androgens modulates Foxp2 and Foxp1 mRNA levels
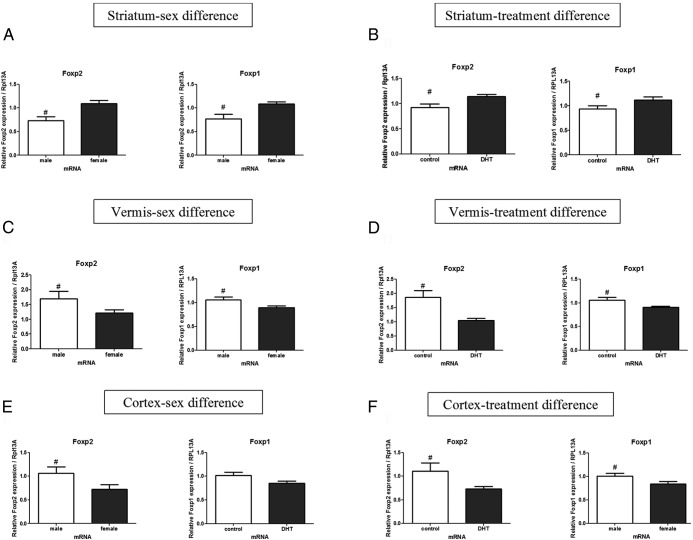
We also assessed the mRNA levels for Foxp2 and Foxp1 in the brains on PN3. In the striatum, a significant main effect of sex was observed for the Foxp2 and Foxp1 mRNA, with females having higher levels of mRNA than males [F (1, 30) = 8.87, P = .006, η2 = 0.22 and F (1, 30) = 4.21, P = .04, η2 = 0.11, Figure 4A], respectively. There was also a main effect of treatment with DHT increasing Foxp2 and Foxp1 mRNA levels, [F (1, 30) = 11.63, P = .002, η2 = 0.27, and F (1, 30) = 4.86, P = .03, η2 = 0.12, Figure 4B, respectively].
Figure 4.
Androgen treatment influences Foxp2 and Foxp1 mRNA production distinctly based on brain region. A, A sex difference for Foxp2 and Foxp1mRNA was found in the striatum, with females having higher mRNA levels than males. B, DHT treatment increased both Foxp2 and Foxp1 mRNA levels above controls for both male and female rat pups. C, In contrast to the higher mRNA levels for females in the striatum, in the vermis, males were found to have higher mRNA levels for Foxp2 and Foxp1 than females. D, Treatment with androgens decreased both Foxp2 and Foxp1 mRNA. E, In the cortex, a sex difference was found for Foxp2 but not Foxp1, with males having higher mRNA levels than females. F, Similar to the vermis, androgen treatment decreased both Foxp2 and Foxp1 mRNA in the cortex. Data are expressed as mean (±SEM; n = 10–12/group). #, A significant difference (F-test; P < .05).
In the vermis portion of the cerebellum, there was a significant main effect for sex, indicating male have higher mRNA levels than females for both Foxp2 and Foxp1 [F (1, 30) = 4.38, P = .04, η2 = 0.12 and F (1, 30) = 6.45, P = .01, η2 = 0.17, Figure 4C, respectively]. There was also a significant main effect for treatment condition, showing DHT administration reduced Foxp2 [F (1, 30) = 12.69, P = .001, η2 = 0.28) and Foxp1 [F (1, 30) = 5.40, P = .02, η2 = 0.14, Figure 4D] mRNA levels but no effect of DHT on the hemispheres of the cerebellum (data not shown).
In the cortex, the pattern was similar to the one observed in the vermis with a significant main effect for sex showing males had higher mRNA levels for both Foxp2 and Foxp1 [F (1, 30) = 4.83, P = .03, η2 = 0.15 and F (1, 30) = 3.93, P = .02, η2 = 0.12, Figure 4E, respectively]. Likewise, exogenous DHT administration reduced mRNA levels for both Foxp2 [F (1, 30) = 5.80, P = .02, η2 = 0.17] and Foxp1 [F (1, 30) = 4.00, P = .05, η2 = 0.13, Figure 4F].
Foxp2, Foxp1, and AR are coexpressed in the same cells and AR binding sites are predicted in the promoter region of Foxp2
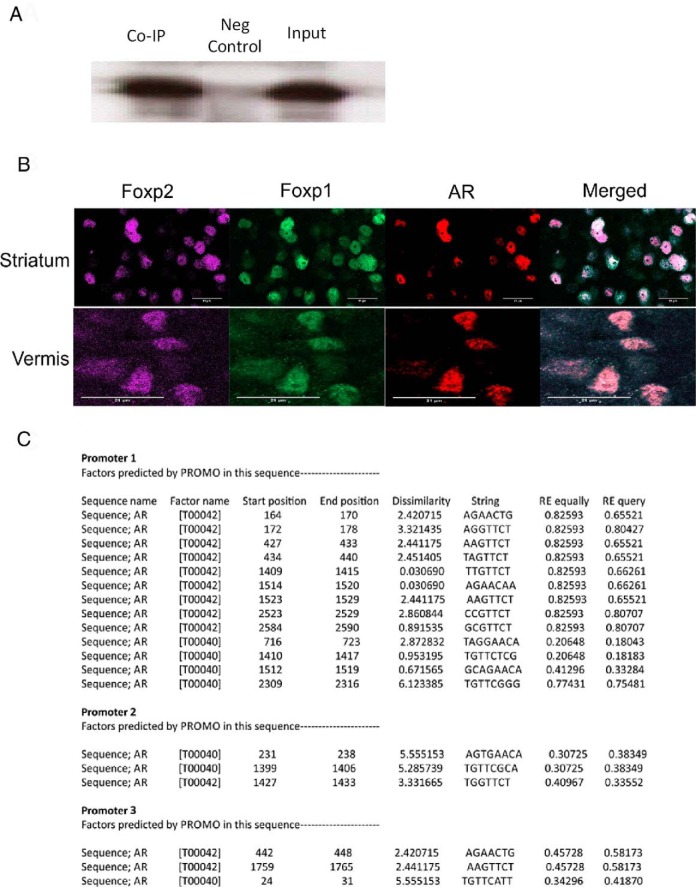
To elucidate the potential relationship between Foxp2 and AR, we investigated, whether Foxp2 and AR protein physically associate in vivo as has previously been reported for Foxp1 in a human prostate adenocarcinoma cell line (58). Using brain tissue lysate from the striatum of a PN0 rat pup, we coimmunoprecipitated AR and performed a Western blot with Foxp2. We found Foxp2 and AR proteins do interact (Figure 5A). We also conducted fluorescent immunohistochemistry to determine whether all three proteins were coexpressed in the same cells. The coexpression of Foxp2, Foxp1, and AR was evident when visualized by immunohistochemistry in both striatal and cerebellar Purkinje cells (Figure 5B). Two separate online transcription factor binding sites [1) TFSEARCH: http://www.cbrc.jp/research/db/TFSEARCH.html and 2) Promo: http://alggen.lsi.upc.es/recerca/frame-recerca.html (59)] indicated 19 putative transcription factor binding sites 2000 bp upstream and 1000 bp downstream of the start of the Foxp2 promoter, with 85% or greater similar rate to the predicted consensus binding elements for AR (Figure 5C).
Figure 5.
Cellular expression of Foxp2 and AR is confirmed by immunohistochemistry, co-IP, and predicted transcription binding. A, Protein-protein interaction of Foxp2, and AR was confirmed by co-IP of AR with Foxp2 in striatal tissue of a PN0 rat pup. B, The coexpression of Foxp2, Foxp1, and AR was confirmed when visualized by immunohistochemistry, as shown in the medium spiny neurons of the striatum and Purkinje neurons of the cerebellum. C, Table showing the potential binding sites for AR upstream of the Foxp2 promoter regions as assessed by the transcription factor binding site PROMO.
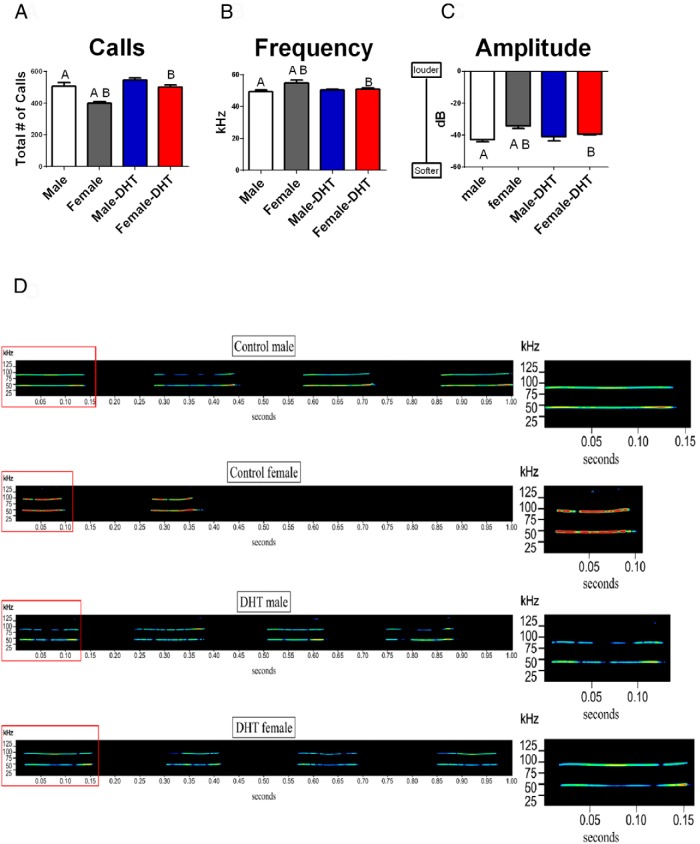
Sex differences in the ultrasonic vocalizations of rat pups are eliminated by androgens
To determine whether there are identifiable sex differences in rat pup ultrasonic distress calls and whether they are influenced by androgen treatment, we recorded and quantified the ultrasonic calls of single PN3 rat pups that were separated from the dam for 3 minutes. The two-way ANOVA indicated a significant interaction between treatment and sex [F (1, 24) = 4.43, P = 0.04, η2 = 0.16]. Post hoc pairwise comparison found control males produced more total vocalizations than control females, [t (12) = 4.47, P = .04], whereas DHT treatment increased the total number of calls produced by females [t (12) = 5.70, P < .001] but had no effect in males [t (12) = 1.51, P = .16, Figure 6A]. There was no difference between the sexes or hormonal modulation of call duration or interval [F's < 1.23, P > .05].
Figure 6.
Male pup ultrasonic vocalizations are different from female, and treatment with androgens reverses the sex difference. A, Treatment of 3-day-old pups with DHT or vehicle revealed males produced more distress USVs than females, but treatment with DHT significantly increased the total number of calls in females. B, The mean frequency of the calls was significantly lower in control males compared with their female littermates (F-test; P < .05). Treatment with DHT lowered the frequency of female calls but had no effect on male calls. C, The same pattern observed for frequency was found for amplitude (F-test; P < .05). D, Typical sonograms of the USV from a control male (top panels), control female, DHT-treated male, and DHT-treated female (bottom panels). The y-axis represents kilohertz and the x-axis is time in seconds. The inset denoted with the red box represents the first vocalization within the 1-second-long sonogram. Data are expressed as mean (±SEM; n = 7 /group). Groups with the same alphabet letter are significantly different from each other.
We also analyzed the fundamental mean frequency of the vocalizations. Again there was a significant interaction between sex and treatment [F (1, 24) = 5.57, P = 0.02, η2 = 0.20]. Post hoc pairwise testing found control males vocalized at a lower frequency than control females [post hoc pairwise test, t (12) = 2.77, P= 0.02, Figure 6B], and treatment with DHT reversed this pattern such that females treated with androgens produced calls that were lower in frequency than control females [post hoc pairwise test, t (12) = 2.30, P = 0.040, Figure 6B]. There was no effect of treatment on the frequency of the vocalizations between control males and treated males [post hoc pairwise test t (12) = 0.30, P > .05].
In addition to quantifying the total number of calls and mean frequency, we also analyzed the mean amplitude of each call produced. Again a significant interaction was observed [F (1, 24) = 4.63, P = 0.04, η2 = 0.17]. A post hoc pairwise test found control males vocalized at a lower amplitude than control females [t (12) = 4.38, P = .001], and DHT treatment reversed this pattern but only in the females, such that DHT-treated females produced vocalizations with lower amplitudes than control females [t (12) = 3.84, P > .002, Figure 6C]. Figure 6D displays typical sonograms for USVs from each group.
Discussion
In the developing brain, the expression pattern for Foxp2 has distinct but also overlapping patterns of expression with Foxp1 (8, 48). Foxp2 and Foxp1 are highly homologous, and evidence suggests they are both expressed in the developing brain and can either homodimerize (eg, FOXP2-FOXP2) or heterodimerize with each other (eg, FOXP2-FOXP1) through their zinc finger domains (60). Although there is clear evidence of FOXP2 involvement in language and animal communication more recently, individuals with FOXP1 mutations have also been found to have language impairments (61, 62). This suggests both FOXP1 and FOXP2 may be integral for the development of brain regions responsible for language.
Androgens are definitively involved in the expression and evolution of secondary sex characteristics (63), including vocalizations produced in the context of aggression and mate attraction (64, 65). In rodents, androgens modulate USVs produced in contextual encounters involving reproduction or aggression (65–68). Current knowledge for how androgens influence other facets of mammalian vocalizations are narrowly limited to variations in pitch and frequency (eg, human males castrated in the 17th to 18th centuries to preserve the prepubertal singing voice and adult men administered T therapy) (69, 70).
Given the intriguing parallel involvement of Foxp2 and androgens, we were interested to know whether androgens were modifying Foxp2 in in the brain and whether these modifications resulted in sex-specific changes in rat pup USVs. We found exogenous androgens influenced postnatal mRNA and protein levels of Foxp2, thereby demonstrating androgens have the capability to modify Foxp2 and Foxp1 activity in brain regions associated with vocalizations. Prior to the androgen surge at E18, there was no sex difference in Foxp2 levels for cultured cortical cells, whereas after the surge males had higher levels than females. Foxp2 is expressed in the rat brain starting at E13 (71) and Foxp1 begins expressing around E14 (71).
In the present study, we selected the cortex, striatum, and cerebellum to analyze because all these brain regions are known to express Foxp2 and are recognized to be important to language in humans (72, 73) and vocal production in animals (74, 75). Previously we reported a sex difference in cognitively associated brain regions for Foxp2, but not Foxp1 protein levels, with males having higher levels of Foxp2 than females (7). We found a similar pattern of sex differences with the exception of the striatum, which showed an opposite pattern with males having lower Foxp2 protein levels than females. In our previous work [Bowers et al (7)], we did not sample the striatum. Moreover, in the present study, we separated PN3 tissue from the cerebellum into the vermis and hemispheres, whereas the extraction of cortical tissue consisted of a single region immediately dorsal to the striatum. In our previous study, we sampled the entire cerebellum and cortex at PN4. Nonetheless, our findings replicate and expand on our previous findings by showing two important points: 1) both mRNA and protein for Foxp2 and Foxp1 are influenced by androgens but in a brain region-specific manner and 2) future studies should consider not only the sex of the animal but also the brain region specificity for the expression of both Foxp1 and Foxp2 proteins.
A surprising observation was the brain region-specific response of Foxp2 and Foxp1 to androgens for both sexes. In the striatum, androgens increased the mRNA and protein levels for both Foxp2 and Foxp1, whereas the same treatment down-regulated the mRNA and protein levels in the cerebellar vermis as well as in the cortex for Foxp2 and Foxp1. A possible variable determining these results is the maturation state of the specific brain regions. The cerebellum and the prefrontal cortex reach maturity later in development than the striatum (76, 77) and the cells of the cerebellar vermis, in general, are produced later than those of the hemispheres (78). In contrast, the striatum matures much earlier than the cerebellum or the cortex (ie, complete maturation by the end of the second postnatal week in rats) (79, 80). Moreover, the neocortex in all mammalian species comprises six layers of neurons, the architecture, connectivity, and chemistry, which are distinct, depending on their location. The neurons that will occupy the deepest layers V and VI are produced, or born, first, followed by the more superficially displaced neurons of cortical layers II-IV (81). Neurons in these deep cortical layers express Foxp2, whereas the more superficially layers express Foxp1 (71, 82). In the present study, we found androgens down-regulated both Foxp2 and Foxp1 mRNA in the cortex, yet only the Foxp2 protein was significantly down-regulated, whereas Foxp1 protein levels were unchanged. This is not the first report of Foxp proteins diverging from the mRNA levels (83). Regardless, it is plausible the distinct time frames for brain region maturation underlie the variations in the responses of Foxp2 and Foxp1 to the organizing effect of brain-sculpting sex hormones.
Cry vocalizations of humans and rat pups during the early postnatal period have biological and social significance critical to early development and survival. What is being communicated between the offspring and the mother has not been determined conclusively (for a review, see reference 84). It is plausible that the cry of an infant is initiated by its sensory experiences, such as hunger or pain, so that during the first couple of months, the cry of a human infant may be more similar to the distress vocalizations of other primates than it is to the form and function of subsequent human language (85). Therefore, rat pup vocalizations are a useful tool for exploring communication between the young and its mother.
Previously we found PN4 male rat pups produced more vocalizations that were lower in frequency and amplitude than females (7). Here we replicate that finding on PN3. Administration of exogenous androgens to females eliminated the sex difference with DHT-treated females producing more total vocalizations, which were at a lower frequency and amplitude than vehicle-treated females. An alternative interpretation of the data is that DHT increases vocalizations in females but not males. This is evident because the androgen-treated males did not differ from the control males, perhaps due to a ceiling effect. There is a limitation on the total number of vocalizations a pup can emit in a minute. In contrast, the acoustic signal changes (ie, lower frequency and amplitude) might be due to the masculinization of the vocal pathway by androgens. The vocal tract of rats, like humans, contains smooth muscle. Specifically, the laryngeal muscles contain myosin heavy chains, which are the motor proteins that power smooth muscle contraction (86). Exogenous androgens masculinize the myosin heavy chains in females, thereby modifying female vocalizations to be similar to males (46). Androgens also increase the muscle strength of the diaphragm, thereby allowing for increases in the volume of air that can be taken into the lungs during respiration (87).
Androgen and Foxp2 modifications impacting the USVs of rat pups can have a profound behavioral impact on the dam. In rats, sex-specific bias in maternal care is apparent in the higher rate of maternal anogenital grooming (88), a behavior that is driven by androgen-dependent olfactory cues from males. More recently we have shown alterations in USVs in rat pups influence the order of maternal retrieval such that males are retrieved first and females who sound like males are preferentially retrieved as opposed to normal vocalizing females (7). Therefore, the data support the notion that USVs serve a critically important communicative role between the dam and her pups, and these vocalizations are influenced by Foxp2, androgens, and potentially by Foxp1.
An intriguing divergence exists between the known gender difference for human language favoring girls to boys and our findings for a sex difference with male rat pups to be more vocal than females. Based on our present observations coupled with our previously published data (7), we postulate this dissimilarity might be due to the distinctive evolutionary pressures shaping human language, as compared with those influencing the vocal distress calls in rat pups. Furthermore, the findings reported here replicate not only our previous data but also Hamson et al (89), which reported a sex difference for Foxp2 expression in the cerebellum of adult rats. Here we confirmed higher Foxp2 in the cerebellum of neonatal males. Moreover, there is a wealth of literature surrounding the lateralization of language. To our knowledge, there are no published reports for lateralized expression for any of the Foxp family members. Thus, in the present study, we did not focus on left/right hemisphere differences in the expression of Foxp1/2. However, there are published reports for a left hemisphere advantage in the recognition of conspecific communicative calls in mice (90). Thus, there exists the potential for lateralization differences in Foxp1/2 expression in male and female rat pups.
Currently little is known about the upstream regulators of Foxp2 and Foxp1 gene expression. Our results implicate androgens as an upstream regulator of both Foxp2 and Foxp1 expression and signaling. The Centers for Disease Control and Prevention has identified the prevalence of autism spectrum disorders (ASDs) to be 1 in 68 children with a 5:1 ratio favoring males over females (91). Exposure to increased levels of androgens during prenatal development has been proposed to underpin the etiology of ASDs (92), although to date, no direct relationship between any autism associated gene, language, or sex hormones has been established. Studies in humans suggest a strong correlation between high levels of androgens and changes in the structure and function of cortical, striatal, and cerebellar brain regions, which are regions known to be atypical in children with ASDs (93–96). Abnormal development of these brain regions manifests itself in one of the most fundamental symptoms of autism, the disruption of language communication. FOXP2 regulates several autism-associated genes (eg, CNTNAP2, MET, and SRPX2) (97–99), implicating a role for FOXP2 in the pathophysiology of ASD. FOXP2 is distinct from other candidate genes because it is currently the only potential ASD gene for which a direct connection to human language has been established. Unlike FOXP2, a direct relationship between ASD and FOXP1 has been detected as mutations, deletions, or copy number of FOXP1 in individuals diagnosed with ASD (61, 100–102).
It is not known what molecules are specifically trigged by Foxp2 and Foxp1 that might have an impact on vocalization in animals and humans. Nevertheless, we postulate there is a potential connection with dopamine. It is known that dopaminergic signaling is critical for vocal production in birds and rats (103, 104) as well for human language production (105, 106). Androgen receptor activation can cause changes in dopaminergic signaling by altering gene expression (107). However, this is a purely speculative conclusion, which will require more investigation.
In summary, we found androgens can increase Foxp2 protein levels during embryonic development. We also found exogenous androgen treatment affects the levels of mRNA and protein, for both Foxp1 and Foxp2, in the developing brain, with these effects being brain region specific. Lastly, we found a basal sex difference in the USVs of rat pups, which is eliminated by androgen treatment, hence confirming hormonal responsiveness of this vitally important gene family.
Acknowledgments
This work was supported by National Institutes of Health Grants K99 MH101252 (to J.M.B.) and R01 NS050525 (to M.M.M.) and Consejo Nacional de Ciencia y Tecnologia Postdoctoral Fellowship Grant 207561 C000/649/13 (to M.P.-P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- ASD
- autism spectrum disorder
- Co-IP
- coimmunoprecipitation
- DHT
- dihydrotestosterone
- E
- embryonic day
- FOXP
- forkhead box P
- PBS-T
- glycine in Triton X-100
- PN
- postnatal day
- qRT-PCR
- quantitative RT-PCR
- USV
- ultrasonic vocalization.
References
- 1. Darwin C. The Expression of the Emotions in Man and Animals. London: J. Murray; 1872. [Google Scholar]
- 2. Bowers JM, Konopka G. The role of the FOXP family of transcription factors in ASD. Dis Markers. 2012;33(5):251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413(6855):519–523. [DOI] [PubMed] [Google Scholar]
- 4. Vargha-Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R. Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci USA. 1995;92(3):930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watkins KE, Dronkers NF, Vargha-Khadem F. Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002;125(Pt 3):452–464. [DOI] [PubMed] [Google Scholar]
- 6. Fisher SE, Scharff C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009;25(4):166–177. [DOI] [PubMed] [Google Scholar]
- 7. Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2013;33(8):3276–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460(2):266–279. [DOI] [PubMed] [Google Scholar]
- 9. Schulz SB, Haesler S, Scharff C, Rochefort C. Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 2010;9(7):732–740. [DOI] [PubMed] [Google Scholar]
- 10. Vernes SC, Oliver PL, Spiteri E, et al. Foxp2 regulates gene networks implicated in neurite outgrowth in the developing brain. PLoS Genet. 2011;7(7):e1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14(2):142–146. [PubMed] [Google Scholar]
- 12. Nyby J, Whitney G. Ultrasonic communication of adult myomorph rodents. Neurosci Biobehav Rev. 1978;2:1–14. [Google Scholar]
- 13. Sales GD, Pye D. Ultrasonic Communication by Animals. London: Chapman, Hall; 1974. [Google Scholar]
- 14. Whishaw IQ, Metz GA, Kolb B, Pellis SM. Accelerated nervous system development contributes to behavioral efficiency in the laboratory mouse: a behavioral review and theoretical proposal. Dev Psychobiol. 2001;39(3):151–170. [DOI] [PubMed] [Google Scholar]
- 15. Costantini F, D'Amato FR. Ultrasonic vocalizations in mice and rats: social contexts and functions. Acta Zool Sinica. 2006;52(4):619–633. [Google Scholar]
- 16. Noirot E. Ultrasounds in young rodents: II. Changes in age with albino rats. Anim Behav. 1968;16:129–134. [DOI] [PubMed] [Google Scholar]
- 17. Blumberg MS, Sokoloff G. Do infant rats cry? Psychol Rev. 2001;108(1):83–95. [DOI] [PubMed] [Google Scholar]
- 18. Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996;21(2):203–217. [DOI] [PubMed] [Google Scholar]
- 19. Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125(1–2):49–56. [DOI] [PubMed] [Google Scholar]
- 20. Ehret G. Infant rodent ultrasounds—a gate to the understanding of sound communication. Behav Genet. 2005;35(1):19–29. [DOI] [PubMed] [Google Scholar]
- 21. Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34(3):195–204. [DOI] [PubMed] [Google Scholar]
- 22. Bowers JM, Konopka G. ASD-relevant animal models of the Foxp family of transcription factors. Autism Open Access. 2012;Suppl 1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enard W, Gehre S, Hammerschmidt K, et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137(5):961–971. [DOI] [PubMed] [Google Scholar]
- 24. Shu W, Cho JY, Jiang Y, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA. 2005;102(27):9643–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fujita E, Tanabe Y, Shiota A, et al. Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc Natl Acad Sci USA. 2008;105(8):3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurt S, Fisher SE, Ehret G. Foxp2 mutations impair auditory-motor association learning. PLoS One. 2012;7(3):e33130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004;24(13):3152–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teramitsu I, Poopatanapong A, Torrisi S, White SA. Striatal FoxP2 is actively regulated during songbird sensorimotor learning. PLoS One. 2010;5(1):e8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelley DB, Bass AH. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Curr Opin Neurobiol. 2010;20(6):748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bleses D, Vach W, Slott M, et al. The Danish Communicative Developmental Inventories: validity and main developmental trends. J Child Lang. 2008;35(3):651–669. [DOI] [PubMed] [Google Scholar]
- 31. Eriksson M, Marschik PB, Tulviste T, et al. Differences between girls and boys in emerging language skills: evidence from 10 language communities. Br J Dev Psychol. 2012;30(Pt 2):326–343. [DOI] [PubMed] [Google Scholar]
- 32. Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16(4):323–334. [DOI] [PubMed] [Google Scholar]
- 33. Kana RK, Murdaugh DL, Wolfe KR, Kumar SL. Brain responses mediating idiom comprehension: gender and hemispheric differences. Brain Res. 2012;1467:18–26. [DOI] [PubMed] [Google Scholar]
- 34. Clements AM, Rimrodt SL, Abel JR, et al. Sex differences in cerebral laterality of language and visuospatial processing. Brain Lang. 2006;98(2):150–158. [DOI] [PubMed] [Google Scholar]
- 35. Baxter LC, Saykin AJ, Flashman LA, et al. Sex differences in semantic language processing: a functional MRI study. Brain Lang. 2003;84(2):264–272. [DOI] [PubMed] [Google Scholar]
- 36. Bitan T, Lifshitz A, Breznitz Z, Booth JR. Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. J Neurosci. 2010;30(35):11576–11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. Monogr Soc Res Child Dev. 1994;59(5):1–173; discussion 174–185. [PubMed] [Google Scholar]
- 38. Ozcaliskan S, Goldin-Meadow S. Sex differences in language first appear in gesture. Dev Sci. 2010;13(5):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maccoby E. The Development of Sex Differences. Stanford, CA: Stanford University Press; 1966. [Google Scholar]
- 40. Ramer A. Syntactic styles in emerging language. J Child Lang. 1976;3:49–62. [Google Scholar]
- 41. Wallentin M. Putative sex differences in verbal abilities and language cortex: a critical review. Brain Lang. 2009;108(3):175–183. [DOI] [PubMed] [Google Scholar]
- 42. Harding SM, Velotta JP. Comparing the relative amount of testosterone required to restore sexual arousal, motivation, and performance in male rats. Horm Behav. 2011;59(5):666–673. [DOI] [PubMed] [Google Scholar]
- 43. Kurvers RH, Roberts ML, McWilliams SR, Peters A. Experimental manipulation of testosterone and condition during molt affects activity and vocalizations of male blue tits. Horm Behav. 2008;54(2):263–269. [DOI] [PubMed] [Google Scholar]
- 44. Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53(5):659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gahr M. Sexual differentiation of the vocal control system of birds. Adv Genet. 2007;59:67–105. [DOI] [PubMed] [Google Scholar]
- 46. Potter KA, Bose T, Yamaguchi A. Androgen-induced vocal transformation in adult female African clawed frogs. J Neurophysiol. 2005;94(1):415–428. [DOI] [PubMed] [Google Scholar]
- 47. Samango-Sprouse CA, Sadeghin T, Mitchell FL, et al. Positive effects of short course androgen therapy on the neurodevelopmental outcome in boys with 47,XXY syndrome at 36 and 72 months of age. Am J Med Genet A. 2013;161A(3):501–508. [DOI] [PubMed] [Google Scholar]
- 48. Campbell P, Reep RL, Stoll ML, Ophir AG, Phelps SM. Conservation and diversity of Foxp2 expression in muroid rodents: functional implications. J Comp Neurol. 2009;512(1):84–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asprer JS, Lee B, Wu CS, et al. LMO4 functions as a co-activator of neurogenin 2 in the developing cortex. Development. 2011;138(13):2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang YC, Fu HC, Hsiao BL, et al. Androgen receptor inclusions acquire GRP78/BiP to ameliorate androgen-induced protein misfolding stress in embryonic stem cells. Cell Death Dis. 2013;4:e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meyer RP, Gehlhaus M, Schwab R, Burck C, Knoth R, Hagemeyer CE. Concordant up-regulation of cytochrome P450 Cyp3a11, testosterone oxidation and androgen receptor expression in mouse brain after xenobiotic treatment. J Neurochem. 2009;109(2):670–681. [DOI] [PubMed] [Google Scholar]
- 52. Turner ME, Martin C, Martins AS, et al. Genomic and expression analysis of multiple Sry loci from a single Rattus norvegicus Y chromosome. BMC Genet. 2007;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen XR, Heck N, Lohof AM, et al. Mature Purkinje cells require the retinoic acid-related orphan receptor-α (RORα) to maintain climbing fiber mono-innervation and other adult characteristics. J Neurosci. 2013;33(22):9546–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106(1):306–316. [DOI] [PubMed] [Google Scholar]
- 55. Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114(5):1635–1644. [DOI] [PubMed] [Google Scholar]
- 56. Baum MJ, Brand T, Ooms M, Vreeburg JT, Slob AK. Immediate postnatal rise in whole body androgen content in male rats: correlation with increased testicular content and reduced body clearance of testosterone. Biol Reprod. 1988;38(5):980–986. [DOI] [PubMed] [Google Scholar]
- 57. Lalau JD, Aubert ML, Carmignac DF, Gregoire I, Dupouy JP. Reduction in testicular function in rats. II. Reduction by dexamethasone in fetal and neonatal rats. Neuroendocrinology. 1990;51(3):289–293. [DOI] [PubMed] [Google Scholar]
- 58. Takayama K, Horie-Inoue K, Ikeda K, et al. FOXP1 is an androgen-responsive transcription factor that negatively regulates androgen receptor signaling in prostate cancer cells. Biochem Biophys Res Commun. 2008;374(2):388–393. [DOI] [PubMed] [Google Scholar]
- 59. Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18(2):333–334. [DOI] [PubMed] [Google Scholar]
- 60. Li S, Weidenfeld J, Morrisey EE. Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol Cell Biol. 2004;24(2):809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horn D, Kapeller J, Rivera-Brugues N, et al. Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum Mutat. 2010;31(11):E1851–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O'Roak BJ, Deriziotis P, Lee C, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43(6):585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Adkins-Regan E. Hormones and Animal Social Behavior. Princeton, NJ: Princeton University Press; 2005. [Google Scholar]
- 64. Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Ann NY Acad Sci. 2003;1007:211–231. [DOI] [PubMed] [Google Scholar]
- 65. Pasch B, George AS, Hamlin HJ, Guillette LJ, Jr, Phelps SM. Androgens modulate song effort and aggression in Neotropical singing mice. Horm Behav. 2011;59(1):90–97. [DOI] [PubMed] [Google Scholar]
- 66. Nyby J, Matochik JA, Barfield RJ. Intracranial androgenic and estrogenic stimulation of male-typical behaviors in house mice (Mus domesticus). Horm Behav. 1992;26(1):24–45. [DOI] [PubMed] [Google Scholar]
- 67. Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic 'songs' with approach behaviour. Biol Lett. 2009;5(5):589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. James PJ, Nyby JG, Saviolakis GA. Sexually stimulated testosterone release in male mice (Mus musculus): roles of genotype and sexual arousal. Horm Behav. 2006;50(3):424–431. [DOI] [PubMed] [Google Scholar]
- 69. Akcam T, Bolu E, Merati AL, Durmus C, Gerek M, Ozkaptan Y. Voice changes after androgen therapy for hypogonadotrophic hypogonadism. Laryngoscope. 2004;114(9):1587–1591. [DOI] [PubMed] [Google Scholar]
- 70. Jenkins JS. The voice of the castrato. Lancet. 1998;351(9119):1877–1880. [DOI] [PubMed] [Google Scholar]
- 71. Takahashi K, Liu FC, Hirokawa K, Takahashi H. Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. J Neurosci Res. 2003;73(1):61–72. [DOI] [PubMed] [Google Scholar]
- 72. van den Broek GS, Takashima A, Segers E, Fernandez G, Verhoeven L. Neural correlates of testing effects in vocabulary learning. Neuroimage. 2013;78:94–102. [DOI] [PubMed] [Google Scholar]
- 73. Hoogman M, Guadalupe T, Zwiers MP, Klarenbeek P, Francks C, Fisher SE. Assessing the effects of common variation in the FOXP2 gene on human brain structure. Front Hum Neurosci. 2014;8:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Garcia-Calero E, Bahamonde O, Martinez S. Differences in number and distribution of striatal calbindin medium spiny neurons between a vocal-learner (Melopsittacus undulatus) and a non-vocal learner bird (Colinus virginianus). Front Neuroanat. 2013;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Murugan M, Harward S, Scharff C, Mooney R. Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron. 2013;80(6):1464–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71(1):44–56. [DOI] [PubMed] [Google Scholar]
- 77. Altman J. Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granular layer. J Comp Neurol. 1972;145(4):465–513. [DOI] [PubMed] [Google Scholar]
- 78. Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985;231(1):42–65. [DOI] [PubMed] [Google Scholar]
- 79. Mengler L, Khmelinskii A, Diedenhofen M, et al. Brain maturation of the adolescent rat cortex and striatum: changes in volume and myelination. Neuroimage. 2014;84:35–44. [DOI] [PubMed] [Google Scholar]
- 80. Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. [DOI] [PubMed] [Google Scholar]
- 81. Rakic P. Less is more: progenitor death and cortical size. Nat Neurosci. 2005;8(8):981–982. [DOI] [PubMed] [Google Scholar]
- 82. Hisaoka T, Nakamura Y, Senba E, Morikawa Y. The forkhead transcription factors, Foxp1 and Foxp2, identify different subpopulations of projection neurons in the mouse cerebral cortex. Neuroscience. 2010;166(2):551–563. [DOI] [PubMed] [Google Scholar]
- 83. Miller JE, Spiteri E, Condro MC, Dosumu-Johnson RT, Geschwind DH, White SA. Birdsong decreases protein levels of FoxP2, a molecule required for human speech. J Neurophysiol. 2008;100(4):2015–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zeskind PS, McMurray MS, Garber KA, et al. Development of translational methods in spectral analysis of human infant crying and rat pup ultrasonic vocalizations for early neurobehavioral assessment. Front Psychiatry. 2011;2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lieberman P, Harris KS, Wolff P, Russell LH. Newborn infant cry and nonhuman primate vocalization. J Speech Hear Res. 1971;14(4):718–727. [DOI] [PubMed] [Google Scholar]
- 86. White SL, Zhou MY, Low RB, Periasamy M. Myosin heavy chain isoform expression in rat smooth muscle development. Am J Physiol. 1998;275(2 Pt 1):C581–C589. [DOI] [PubMed] [Google Scholar]
- 87. Padzys GS, Martrette JM, Tankosic C, Thornton SN, Trabalon M. Effects of short term forced oral breathing: physiological changes and structural adaptation of diaphragm and orofacial muscles in rats. Arch Oral Biol. 2011;56(12):1646–1654. [DOI] [PubMed] [Google Scholar]
- 88. Brouette-Lahlou I, Vernet-Maury E, Vigouroux M. Role of pups' ultrasonic calls in a particular maternal behavior in Wistar rat: pups' anogenital licking. Behav Brain Res. 1992;50(1–2):147–154. [DOI] [PubMed] [Google Scholar]
- 89. Hamson DK, Csupity AS, Gaspar JM, Watson NV. Analysis of Foxp2 expression in the cerebellum reveals a possible sex difference. Neuroreport. 2009;20(6):611–616. [DOI] [PubMed] [Google Scholar]
- 90. Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325(6101):249–251. [DOI] [PubMed] [Google Scholar]
- 91. CDC. Prevalence of autism spectrum disorder among children aged 8 years. Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. 2014. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6302a1.htm?s_cid=ss6302a1_w. [PubMed]
- 92. Whitehouse AJ, Maybery MT, Hart R, et al. Fetal androgen exposure and pragmatic language ability of girls in middle childhood: implications for the extreme male-brain theory of autism. Psychoneuroendocrinology. 2010;35(8):1259–1264. [DOI] [PubMed] [Google Scholar]
- 93. Lombardo MV, Ashwin E, Auyeung B, et al. Fetal programming effects of testosterone on the reward system and behavioral approach tendencies in humans. Biol Psychiatry. 2012;72(10):839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lombardo MV, Ashwin E, Auyeung B, et al. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci. 2012;32(2):674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Biamonte F, Assenza G, Marino R, et al. Interactions between neuroactive steroids and reelin haploinsufficiency in Purkinje cell survival. Neurobiol Dis. 2009;36(1):103–115. [DOI] [PubMed] [Google Scholar]
- 96. Mueller SC, Merke DP, Leschek EW, Fromm S, VanRyzin C, Ernst M. Increased medial temporal lobe and striatal grey-matter volume in a rare disorder of androgen excess: a voxel-based morphometry (VBM) study. Int J Neuropsychopharmacol. 2011;14(4):445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mukamel Z, Konopka G, Wexler E, et al. Regulation of MET by FOXP2, genes implicated in higher cognitive dysfunction and autism risk. J Neurosci. 2011;31(32):11437–11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vernes SC, Newbury DF, Abrahams BS, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359(22):2337–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Roll P, Vernes SC, Bruneau N, et al. Molecular networks implicated in speech-related disorders: FOXP2 regulates the SRPX2/uPAR complex. Hum Mol Genet. 2010;19(24):4848–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hamdan FF, Daoud H, Rochefort D, et al. De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet. 2010;87(5):671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pariani MJ, Spencer A, Graham JM, Jr, Rimoin DL. A 785kb deletion of 3p14.1p13, including the FOXP1 gene, associated with speech delay, contractures, hypertonia and blepharophimosis. Eur J Med Genet. 2009;52(2–3):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. O'Roak BJ, Vives L, Girirajan S, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behav Brain Res. 2007;182(2):284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen Q, Heston JB, Burkett ZD, White SA. Expression analysis of the speech-related genes FoxP1 and FoxP2 and their relation to singing behavior in two songbird species. J Exp Biol. 2013;216(Pt 19):3682–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stein CM, Truitt B, Deng F, et al. Association between AVPR1A, DRD2, and ASPM and endophenotypes of communication disorders. Psychiatr Genet. 2014;24(5):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wong PC, Ettlinger M, Zheng J. Linguistic grammar learning and DRD2-TAQ-IA polymorphism. PLoS One. 2013;8(5):e64983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Purves-Tyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS One. 2014;9(3):e91151. [DOI] [PMC free article] [PubMed] [Google Scholar]